Abstract
Seminal fluids of 197 males with complaints of involuntary infertility were examined for spermatozoal counts, morphological changes in the spermatozoa and cultured for ureaplasmas and mycoplasmas. In 12, no spermatozoa were present, 29 had a count of less than one million and 156 had more than one million spermatozoa per mL of the seminal fluid. Various morphological changes were detected in the spermatozoa in some cases. U urealyticum and M hominis were grown in 43.15% and 16.75% in comparison to control figures of 15.9% and 11.4% respectively. There was no correlation between growth of ureaplasmas and the spermatozoal count. Among the morphological changes, presence of coiled tails, presence of a fuzzy coat around the tail and microcolonies were highly specific for culture positivity (98.2, 98.2 and 97.35% respectively) but of low sensitivity (55.2%, 14.1% and 8.2% respectively).
KEY WORDS: Mycoplasma, Spermatozoa, Ureaplasma
Introduction
There has been intense interest to define the role played by ureaplasmas in the diseases of man, particularly in the pathogenesis of genital tract diseases. Considerable data is available to support the hypothesis that ureaplasmas are the causative agents in some cases of nongonococcal urethritis [1, 2, 3, 4]. The contributory role of these organisms in the other diseases of the genital tract and in involuntary infertility is yet to be proved conclusively, though numerous reports are available that record the isolation of these organisms from infertile persons and the results of their eradication with antibiotics [5], the doubt over the commensal versus pathogenic status continues. Hence the study to evaluate the prevalence of mycoplasmas in infertile persons and their correlation with the morphological changes of the spermatozoa was undertaken.
Material and Methods
Patients attending the infertility clinic of Command Hospital (SC), Pune were taken up for the study. Their particulars were noted. Those with a history of antibiotic use over the previous month were excluded from the study.
Ejaculated seminal fluid, after 3 days of abstinence, was collected at the laboratory and 100ul of the seminal fluid was inoculated into mycoplasma transport medium. Mycoplasma and ureaplasma broths were subsequently inoculated from the transport medium. The broths were incubated at 37°C aerobically. A colour change from yellow to pink in ureaplasma broth and amber to red in mycoplasma broth was taken as alkaline shift and growth of mycoplasmas were suspected. The broths were subcultured to respective agar media immediately on colour change and blindly after overnight incubation. The plates were further processed and the growth identified as per the laid down procedures [6].
Few smears were prepared from the seminal fluid, fixed and stained with Giemsa and PAP stain for studying the morphological characters. The spermatozoa were also counted.
Results
Study population included 197 males with complaints of infertility, 129 had primary infertility and 68 secondary infertility. None of them had any obvious cause for the infertility. The study population belonged to middle socio-economic group and were of the age group of 23 to 42 years.
On the basis of spermatozoa counts per ml of the seminal fluid the cases were divided into three groups. Group A consisted of patients with no spermatozoa, group B with less than 1 million spermatozoa and group C with more than 1 milion spermatozoa per ml of the seminal fluid. Number of cases in each group were as follows - Group A : 12, Group B : 29, Group C : 156.
Several morphological abnormalities were detected in the stained smears of the spermatozoa obtained from these cases (Table-1).
TABLE 1.
Morphological abnormalities noted in spermatozoa
| Portion of spermatozoa affected | Morphological abnormalities |
|---|---|
| Tail | Presence of fuzzy/granular coat |
| Short tails | |
| Absence of tail | |
| Coiled tails | |
| Sharp angulation of the tails | |
| Head | Double head appearance |
| Miscellaneous | Deposition of microcolonies |
The ejaculated seminal fluid was cultured for mycoplasmas and ureaplasmas. U urealyticum was isolated from 85 (43.1%) and M hominis from 33 (16.8%) seminal fluid samples. In 17 cases amongst these there was a mixed growth of both mycoplasmas and ureaplasmas.
Several studies in mycoplasmas have suffered because of the difficulty in obtaining suitable controls [7]. We also faced similar problem in getting the seminal fluids of normal fertile males as control. However the presence of ureaplasma in the genital tract of fertile females can be taken as control since there is a high correlation between positive vaginal or cervical U urealyticum cultures and the presence of these organisms in the seminal fluid of their male partners [5]. We have studied the vaginal flora of 44 fertile women attending the OPD for noninfectious causes and found the prevalence of U urealyticum in 15.9% and M hominis in 11.36%. They were used as study controls. The rate of prevalence of U urealyticum in the infertile males was significantly higher than these controls.
The culture results of U urealyticum between three groups were compared (Table 2). There was no relationship between spermatozoa counts in different groups and culture positivity. Comparison of the growths of U urealyticum in groups A and B, B and C, and C and A did not reveal any statistical difference (values being 0.81, 0.64 and 0.79 respectively). Comparison of the growth of ureaplasmas in cases and control population revealed a statistically significant difference (p < 0.05).
TABLE 2.
Correlation of mycoplasmas cultures with spermatozoa counts
| Groups | ‘n’ | Presence of growth of |
|
|---|---|---|---|
| U urealyticum | M hominis | ||
| A | 12 | 6 (50) | 2 (16.7) |
| B | 29 | 1 (48.2) | 7 (24.1) |
| C | 156 | 65 (41.7) | 24 (15.4) |
| Overall | 197 | 85 (43.1) | 33 (16.8) |
| Controls | 44 | 7 (15.9) | 5 (11.36) |
On comparing the growth of M hominis in all the groups no statistically significant difference was noted (p values being 0.91, 0.37, 0.76 respectively). There was also no statistically significant difference in the growth of M hominis in the cases of infertility and the controls (p=0.51).
Certain morphological abnormalities were detected to be more frequent in culture positive cases than in culture negative cases. Spermatozoa with short tails were found to be present in 39 (45.9%) (Fig 1) whereas in culture negative cases they were found in 34 (30.4%) cases. Thus it had a sensitivity of 45.9% and specificity of 69.4%.
Fig. 1.
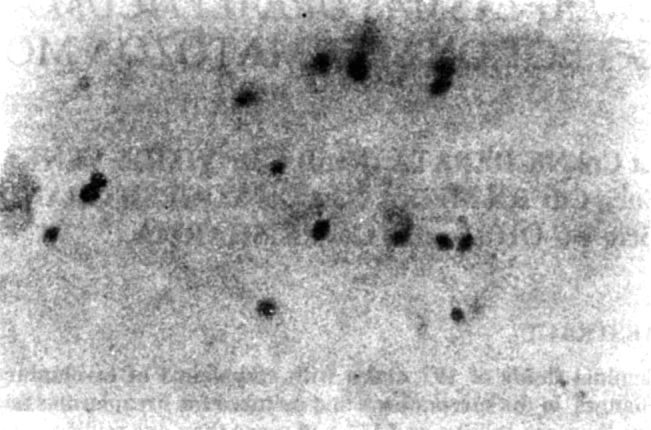
Spermatozoa with short tail
In some cases there were depositions which covered either the whole tail or segments of it. This type of fuzzy coat when present showed a granular appearance (Fig 2). This was not a very common finding though 12 culture positive cases showed presence of such coat whereas only 2 of the culture negative cases had this abnormality. It had a sensitivity of 14.1% but a specificity of 98.2%.
Fig. 2.
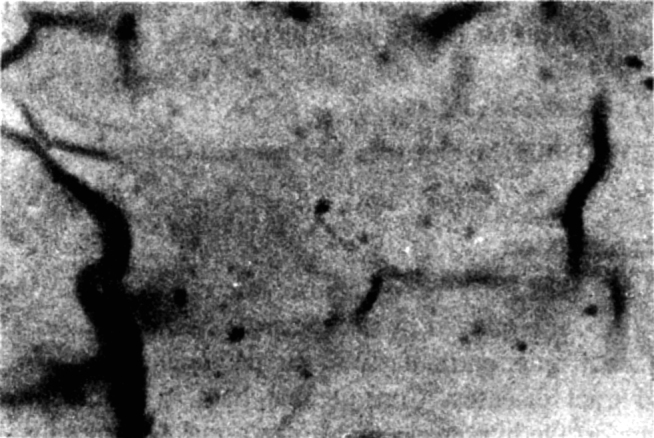
Spermatozoa with short tail and with fuzzy coat
In others the tails of the spermatozoa were coiled (Fig 3). The coiled tails appeared as rings next to or around the head. Tails showing coiling were thicker and appeared to have a granular appearance. Coiling when present was seen in large number of the spermatozoa on a slide. Some of the tails showed sharp angulation (Fig 4) of the tail. They were also included in counting for coiled tails. Such coiling or angulation of the tails was present more significantly in culture positive cases than in culture negative cases. It had a sensitivity of 55.3% but a specificity of 98.2%. Only 3(1.5%) of the culture positive cases showed bicephalic/enlarged cephalic appearance.
Fig. 3.
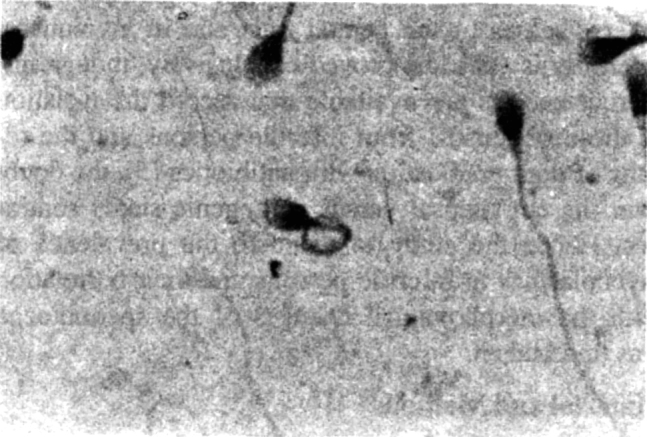
Coiling of tail of spermatozoa
Fig. 4.
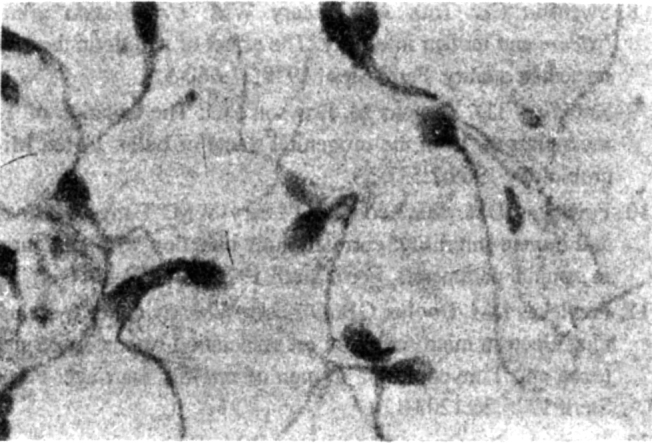
Sharp angulation of tails of spermatozoa
In some cases dense globular deposits were found adherent to the (Fig 5) spermatozoa either to head or to the tail. Such deposits were noted in 7 of the culture positive cases whereas only in 3 cases of culture negative, these were noted. It had a sensitivity of 8.2% but a specificity of 97.2% (Table 3).
Fig. 5.
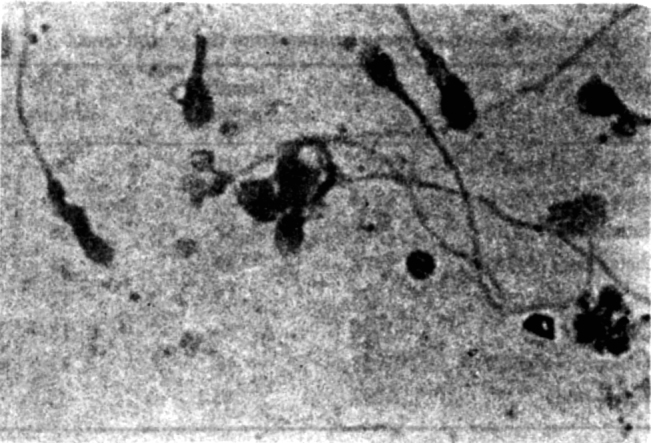
Deposition of microcolonies
TABLE 3.
Sensitivity and specificity of the morphological changes
| Morphological changes | ‘n’ | Sensitivity% | Specificity % |
|---|---|---|---|
| Short tail | 73 | 45.9 | 69.4 |
| Coiled tails | 49 | 55.2 | 98.2 |
| Fuzzy coat | 14 | 14.1 | 98.2 |
| Presence of microcolonies | 10 | 8.2 | 98.2 |
Discussion
Studies in the past on the seminal fluids of infertile males have shown sperm numbers, morphology and motility to deviate from normal, more among study subjects with positive cultures for mycoplasmas [5].
Several investigations have implicated ureaplasma as an etiologic agent of human infertility. Reports of isolation of ureaplasmas from infertile patients and the effect of their treatment have also been reported [8]. However the importance of association of ureaplasmas in the seminal fluids of infertile men is still debated. Bovine semen samples have a high fertilising capacity despite the fact that practically all samples contain ureaplasma [9].
Several morphologic abnormalities have been noted in the spermatozoa of infertile males with U urealyticum infection [10]. Changes in the spermatozoa have been noted by the scanning electron microscope [11]. Morphological changes can be detected by light microscopy and the changes so detected have been used as a predictive indicator for infection by urealyticum by some workers [12].
Since the ureaplasmas were suggested as cause of infertility, studies have been conducted in defined population to assess the rate of colonisation of genital mycoplasmas in such group of population. The findings have been controversial.
The first study conducted in 1972 found 91% of women and 85% of men with unexplained infertility were colonised by U urealyticum as compared to controls where only 23% of pregnant women and 26% of male partners of pregnant women were found to harbor the organism [13]. A similar study in 1973 reported higher incidence of M hominis in the cervices of infertile women (19%) than in healthy non-pregnant women (4%) and pregnant women (10%) [14]. These articles with indictment of mycoplasma species in the etilogy of infertility stimulated a number of other epidemiological studies.
Some workers in the subsequent years could not find any significant difference in the prevalence of mycoplasma species in the genital tract of fertile or infertile women [15, 16, 17]. However many other studies in the subsequent years have reported higher rates of isolation of U urealyticum from infertile couples than in fertile couples [18, 19, 20]. The rates of isolation in these studies showed a wide variation (Table-4) and probably is affected by factors peculiar to each place.
TABLE 4.
Percentage of isolation of U urealyticum, from the genital tracts
| Presence | Year | Infertile persons | Fertile persons |
|---|---|---|---|
| Gnarpe and Friberg (13) | 1972 | 91 | 23 |
| DeLouvois et al (16) | 1974 | 52 | 55 |
| Mathew et al (17) | 1975 | 52 | 66 |
| Khatamee et al (18) | 1978 | 59 | 68 |
| Idris et al (19) | 1978 | 55 | 12 |
| Koren et al (20) | 1978 | 59 | 12 |
| Upadhyaya et al (21) | 1983 | 48 | 33 |
| Present study | 1997 | 43.1 | 15.9 |
The present study was carried out on the seminal fluid of men with history of infertility. U urealyticum was isolated from 43.15% of our study population. This is comparable with that of Upadhaya et all [20] and Idris et al [18].
Only few studies in the past have described the cytological abnormalities associated with U urealyticum infection of the seminal fluid of infertile males.
Of the cytological abnormalities detected presence of short tails was more sensitive (45.88%) but not very specific (69.64%). More specific findings were presence of fuzzy coat (98.2%), deposition of microcolonies (97.32%) and presence of coiled tails (98.2%). Tooth et al also had similar observations. Thus a careful cytological examination of a well prepared smear of the seminal fluid can to some extent predict the infection by U urealyticum.
REFERENCES
- 1.Robinson TD, Csonka GW. Prentice MS. Human intraurethral inoculation of ureaplasmas. Q J Med. 1977;46:309–326. [PubMed] [Google Scholar]
- 2.Brown MB, Cassell GH, Robinson TD, Shephard MC. Measurement of antibody to Ureaplasma urealyticum by an enzyme linked immunosorbent assay and detection of antibody responses in patients with nongonococcal urethritis. J Clin Microbiol. 1983;17:288–295. doi: 10.1128/jcm.17.2.288-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csonka GW, Williams REO, Corse J. T-strain mycoplasma in nongonoccal urethritis. Lancet. 1966;1:1292–1295. doi: 10.1016/s0140-6736(66)91201-3. [DOI] [PubMed] [Google Scholar]
- 4.Oriel JD. Role of genital mycoplasmas in non-gonocoal ureathritis and prostatitis. Sex Transm Dis. 1983;10:8263. [PubMed] [Google Scholar]
- 5.Styler M, Shapiro SS. Mollicutes (mycoplasma) in infertility. Fertil Steril. 1985;44:1–12. doi: 10.1016/s0015-0282(16)48668-1. [DOI] [PubMed] [Google Scholar]
- 6.Debata NK, Venkatesh V. Ohri VC. Prevalence of mycoplasmas in the female genital tract. Ind J Med Microbiol. 1997;15(1):25–27. [Google Scholar]
- 7.Dunlop EMC, Hare MJ, Jones BR. Robinson TD. Mycoplasmas and nonspecific genital infections: II clinical aspects. Brit Jr Vener Dis. 1969;45:274–281. doi: 10.1136/sti.45.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenson CE, Toth A, O'Leary WM. Ureaplasma urealyticum and human infertility. The effect of antibiotic therapy on semen quality. Fertil Steril. 1979;31:660–665. doi: 10.1016/s0015-0282(16)44057-4. [DOI] [PubMed] [Google Scholar]
- 9.Robinson TD, Thomas M. Dawson PLJ. The isolation of T-mycoplasmas from the urogenital tracts of bulls. J Med Microbiology. 1969;2:527–533. doi: 10.1099/00222615-2-4-527. [DOI] [PubMed] [Google Scholar]
- 10.Fowlekes DM, MacLeod J. O'Leary WM. T-mycoplasmas and human infertility: correlation of infection with alteration in seminal parameters. Fertil Steril. 1975;26:1212–1218. doi: 10.1016/s0015-0282(16)41537-2. [DOI] [PubMed] [Google Scholar]
- 11.Fowlekes DM, Dooher GB. O'Leary WM. Evidence by scanning electron microscopy for an association between spermatozoa and T-mycoplasma in men of infertile marriage. Fertil Steril. 1975;26:1203–1211. [PubMed] [Google Scholar]
- 12.Tooth A, Swenson CE. O'Leary WM. Light microscopy as an aid in predicting ureaplasma infection in human semen. Fertil steril. 1978;30:586–591. [PubMed] [Google Scholar]
- 13.Gnarpe H, Friberg J. Mycoplasma and human reproductive failure. The occurrence of different mycoplasmas in couples with reproductive failure. Am J Obstet Gynaecol. 1972;114:727–731. [PubMed] [Google Scholar]
- 14.Love W, Jones M, Andrews B, Thomas M. Mycoplasmas in human infertility. Lancet. 1973;1:1130–1131. doi: 10.1016/s0140-6736(73)90450-9. [DOI] [PubMed] [Google Scholar]
- 15.Gnarpe H, Friberg J. T-mycoplasma as a possible cause of reproductive failure. Nature. 1973;242:120–121. doi: 10.1038/242120a0. [DOI] [PubMed] [Google Scholar]
- 16.DeLouvois J, Blades M, Harrison RF, Hurley R. Stanley VC. Frequency of mycoplasma in fertile and infertile couples. Lancet. 1974;1:1073–1075. doi: 10.1016/s0140-6736(74)90554-6. [DOI] [PubMed] [Google Scholar]
- 17.Mathews CD, Elmslie RG, Clapp KH. Svigos JM. The frequency of genital mycoplasma in human infertility. Fertil Steril. 1975;26:988–990. doi: 10.1016/s0015-0282(16)41412-3. [DOI] [PubMed] [Google Scholar]
- 18.Khatamee MA. Decker WH. Recovery of genital mycoplasma using New York city medium. Infertility. 1978;1:155–158. [Google Scholar]
- 19.Idris WM, Patton WC, Taymor ML. On etiologic role of Ureaplasma urealyticum (T-mycoplasma) infection in infertility. Fertil Steril. 1978;30:229–300. doi: 10.1016/s0015-0282(16)43514-4. [DOI] [PubMed] [Google Scholar]
- 20.Koren Z, Spigland I. Irrigation technique for detection of mycoplasma intruterine infection in infertile patients. Obstet Gynaecol. 1978;52:588–590. [PubMed] [Google Scholar]
Uncited reference
- 21.Upadhyaya M, Hibbard BM. Walker SM. The role of mycoplasmas in reproduction. Fertil Steril. 1983;39:814–818. doi: 10.1016/s0015-0282(16)47122-0. [DOI] [PubMed] [Google Scholar]


