Abstract
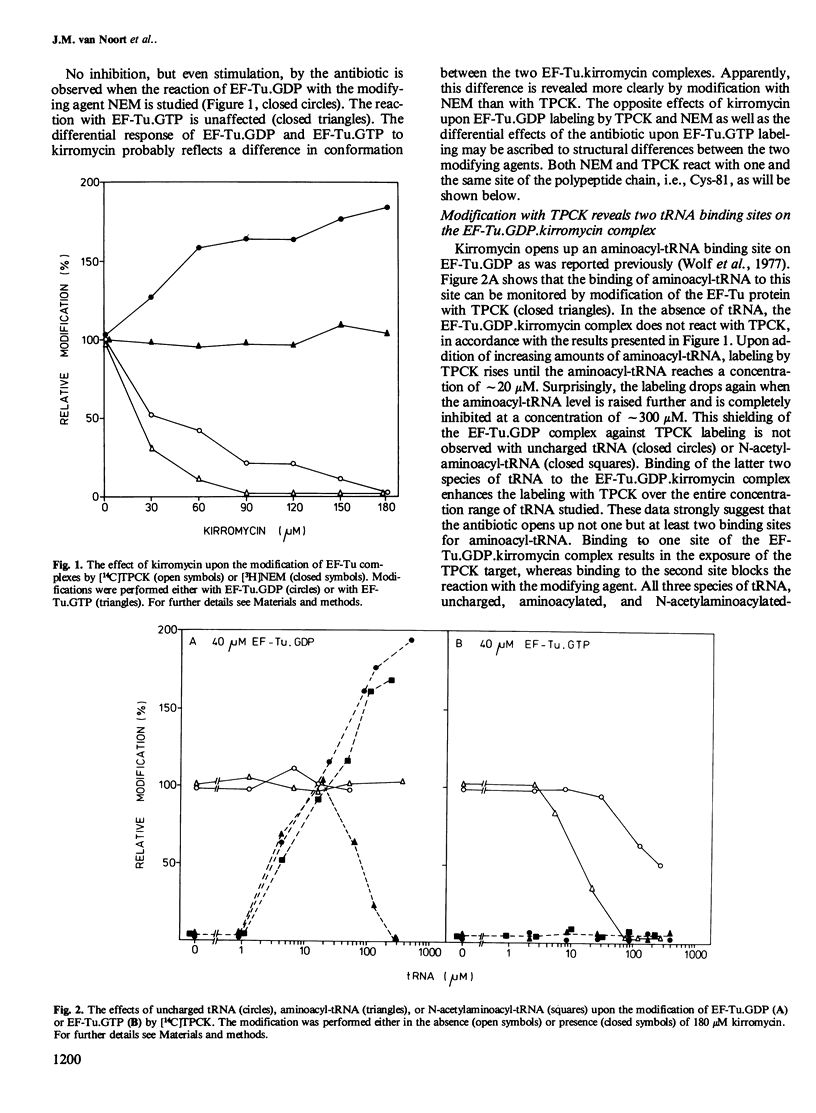
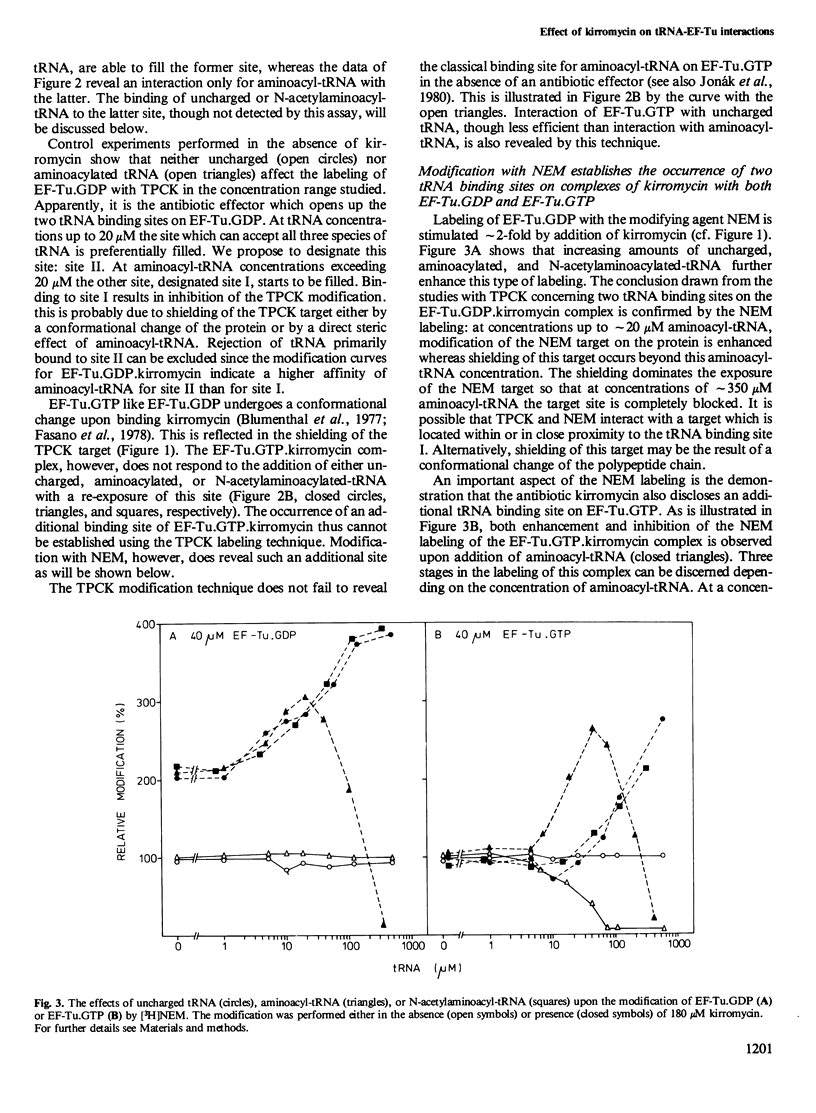
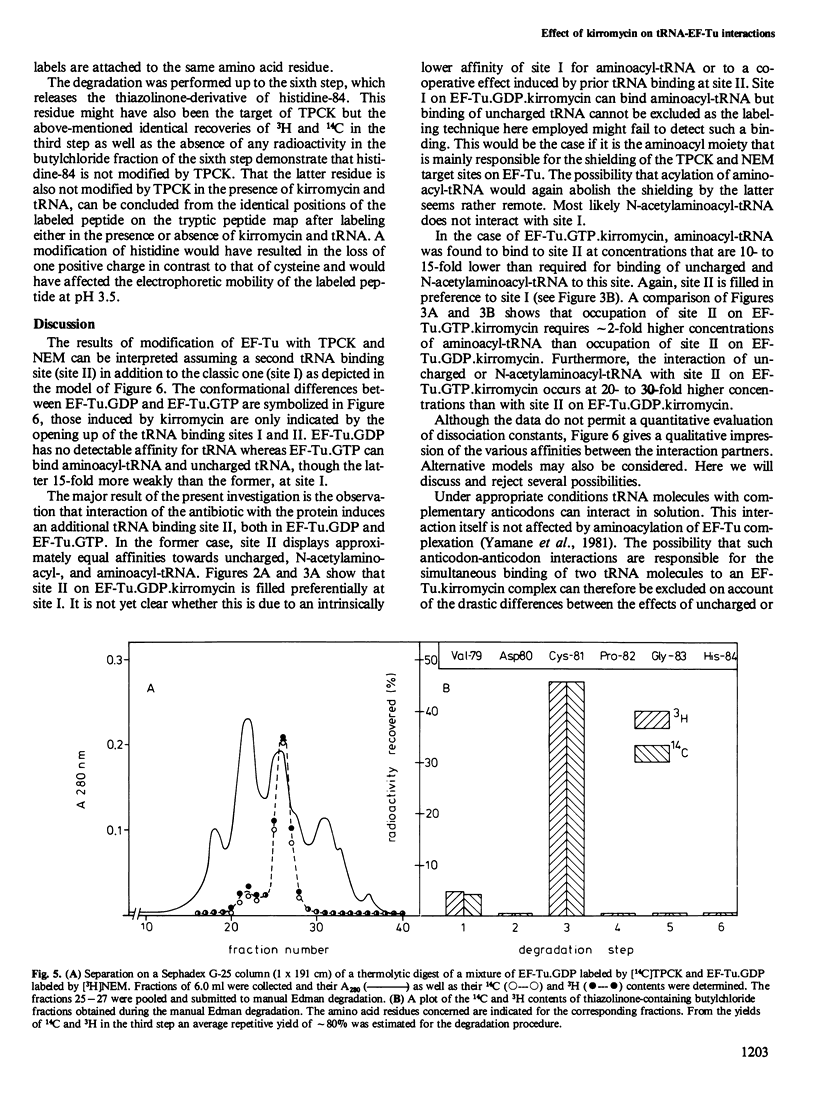
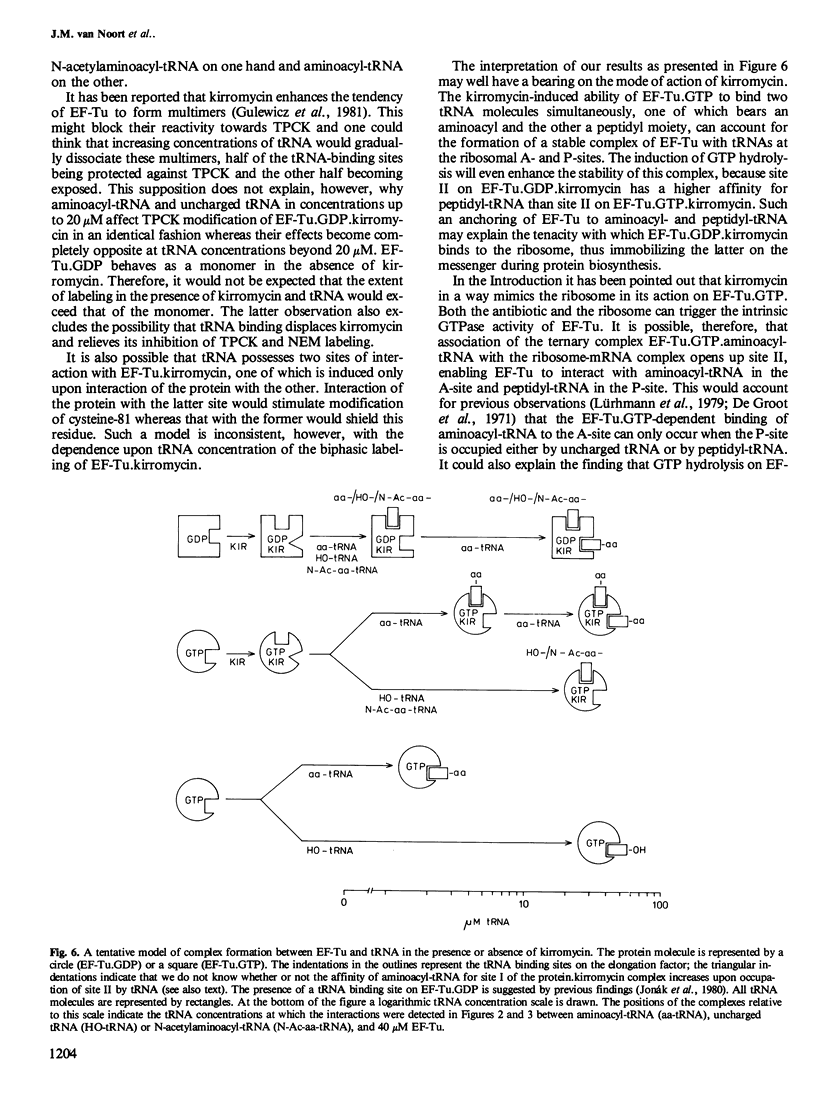
The interaction of the polypeptide chain elongation factor Tu (EF-Tu) with the antibiotic kirromycin and tRNA has been studied by measuring the extent of protein modification with N-tosyl-L-phenylalanine chloromethylketone (TPCK) and N-ethylmaleimide (NEM). Kirromycin protects both EF-Tu.GDP and EF-Tu.GTP against modification with TPCK. Binding of aminoacyl-tRNA added at increasing concentrations to a solution of 40 microM EF-Tu.GDP.kirromycin complex re-exposes the TPCK target site on the protein. However, when the aminoacyl-tRNA concentration is raised beyond 20 microM, TPCK labeling drops again and is blocked completely at approximately 300 microM aminoacyl-tRNA. By contrast, addition of uncharged tRNA or N- acetylaminoacyl -tRNA enhances TPCK labeling of the protein over the entire tRNA concentration range studied. These data strongly suggest that kirromycin induces in EF-Tu.GDP an additional tRNA binding site that can bind uncharged tRNA, aminoacyl-tRNA, and N- acetylaminoacyl -tRNA. Support for this assumption is provided by measuring the modification of EF-Tu.GDP with the sulfhydryl reagent NEM. Moreover, NEM modification also indicates an additional tRNA binding site on EF-Tu.GTP.kirromycin, which could not be detected with TPCK. Mapping of the tryptic peptides of EF-Tu.GDP labeled with [14C]TPCK revealed only one target site for this agent, i.e., cysteine-81. Modification occurred at the same site in the presence and in the absence of kirromycin and uncharged tRNA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Kawakita M., Nakamura S., Ishikawa K., Kaziro Y. Studies on the polypeptide elongation factors form E. coli. VI. Characterization of sulfhydryl groups in EF-Tu and EF-Ts. J Biochem. 1974 Sep;76(3):523–534. doi: 10.1093/oxfordjournals.jbchem.a130596. [DOI] [PubMed] [Google Scholar]
- Beck B. D., Arscott P. G., Jacobson A. Novel properties of bacterial elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1250–1254. doi: 10.1073/pnas.75.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Douglass J., Smith D. Conformational alteration of protein synthesis elongation factor EF-Tu by EF-Ts and by kirromycin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3264–3267. doi: 10.1073/pnas.74.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Effects of antibiotics, N-acetylaminoacyl-tRNA and other agents on the elongation-factor-Tu dependent and ribosome-dependent GTP hydrolysis promoted by 2'(3')-O-L-phenylalanyladenosine. Eur J Biochem. 1981 Jun;117(1):27–31. doi: 10.1111/j.1432-1033.1981.tb06298.x. [DOI] [PubMed] [Google Scholar]
- De Groot N., Panet A., Lapidot Y. The binding of purified Phe-tRNA and peptidyl-tRNA Phe to Escherichia coli ribosomes. Eur J Biochem. 1971 Dec 10;23(3):523–527. doi: 10.1111/j.1432-1033.1971.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Duisterwinkel F. J., de Graaf J. M., Kraal B., Bosch L. A kirromycin resistant elongation factor EF-Tu from Escherichia coli contains a threonine instead of an alanine residue in position 375. FEBS Lett. 1981 Aug 17;131(1):89–93. doi: 10.1016/0014-5793(81)80894-0. [DOI] [PubMed] [Google Scholar]
- FRAZER A. C., FLETCHER R. F., ROSS C. A., SHAW B., SAMMONS H. G., SCHNEIDER R. Gluten-induced enteropathy: the effect of partially digested gluten. Lancet. 1959 Sep 5;2(7097):252–255. doi: 10.1016/s0140-6736(59)92051-3. [DOI] [PubMed] [Google Scholar]
- Fasano O., Bruns W., Crechet J. B., Sander G., Parmeggiani A. Modification of elongation-factor-Tu . guanine-nucleotide interaction by kirromycin. A comparison with the effect of aminoacyl-tRNA and elongation factor Ts. Eur J Biochem. 1978 Sep 1;89(2):557–565. doi: 10.1111/j.1432-1033.1978.tb12560.x. [DOI] [PubMed] [Google Scholar]
- Gulewicz K., Faulhammer H. G., Sprinzl M. Properties of native and nicked elongation factor Tu from Thermus thermophilus HB 8. Eur J Biochem. 1981 Dec;121(1):155–162. doi: 10.1111/j.1432-1033.1981.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Hamel E., Cashel M. Guanine nucleotides in protein synthesis. Utilization of pppGpp and dGTP by initiation factor 2 and elongation factor Tu. Arch Biochem Biophys. 1974 May;162(1):293–300. doi: 10.1016/0003-9861(74)90128-3. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Takacs B. J., Rosenbusch J. P. Properties of a major protein released from Escherichia coli by osmotic shock. Biochemistry. 1976 Jun 1;15(11):2297–2303. doi: 10.1021/bi00656a008. [DOI] [PubMed] [Google Scholar]
- Jonák J., Rychlík I., Smrt J., Holý A. The binding site for the 3'-terminus of aminoacyl-tRNA in the molecule of elongation factor Tu from Escherichia coli. FEBS Lett. 1979 Feb 15;98(2):329–332. doi: 10.1016/0014-5793(79)80210-0. [DOI] [PubMed] [Google Scholar]
- Jonák J., Sedlácek J., Rychlík I. Mode of action of N-tosyl-L-phenylalanylchloromethane on the elongation protein-synthesizing S 3 factor from Bacillus stearothermophilus. Biochim Biophys Acta. 1973 Jan 19;294(2):322–328. doi: 10.1016/0005-2787(73)90304-3. [DOI] [PubMed] [Google Scholar]
- Jonák J., Smrt J., Holý A., Rychlík I. Interaction of Escherichia coli EF-Tu.GTP and EF-Tu.GDP with analogues of the 3' terminus of aminoacyl-tRNA. Eur J Biochem. 1980 Apr;105(2):315–320. doi: 10.1111/j.1432-1033.1980.tb04503.x. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Eckhardt H., Stöffler G. Codon-anticodon interaction at the ribosomal peptidyl-site. Nature. 1979 Aug 2;280(5721):423–425. doi: 10.1038/280423a0. [DOI] [PubMed] [Google Scholar]
- Sedlácek J., Jonák J., Rychlík I. Inactivation of protein-synthesizing T-factor by N-tosyl-L-phenylalanyl chloromethane. Biochim Biophys Acta. 1971 Dec 30;254(3):478–480. [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Verhoef N. J., Kraal B., Bosch L. The binding of aminoacyl-tRNA to complexes of Escherichia coli ribosomes and plant viral RNA. Biochim Biophys Acta. 1968 Feb 26;155(2):456–464. doi: 10.1016/0005-2787(68)90191-3. [DOI] [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor Tu. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4910–4914. doi: 10.1073/pnas.71.12.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. Mechanism of the inhibition of protein synthesis by kirromycin. Role of elongation factor Tu and ribosomes. Eur J Biochem. 1977 May 2;75(1):67–75. doi: 10.1111/j.1432-1033.1977.tb11504.x. [DOI] [PubMed] [Google Scholar]
- Yamane T., Miller D. L., Hopfield J. J. Interaction of elongation factor Tu with the aminoacyl transfer ribonucleic acid dimer Phe-tRNA-Glu-tRNA. Biochemistry. 1981 Jan 20;20(2):449–452. doi: 10.1021/bi00505a034. [DOI] [PubMed] [Google Scholar]



