Abstract
The prevalence of anti-sperm antibodies was assessed in 100 patients of male factor infertility. Majority of the patients were in 30-35 years age group. 18% of these patients had anti-sperm antibodies in their seminal fluid and 16% in their serum.
KEY WORDS: Agglutination, Anti-sperm antibodies, Infertility
Introduction
The spermatozoon with its complexity of antigens had always been an enigma. Though in as early as 1899, studies by Metalnikoff [1] demonstrated that sperm from one animal could elicit an antibody response when injected into another of the same or different species, and a later study by Adler [2] demonstrated that spermatozoa were auto-antigenic: guinea pigs developed antibodies when injected with their own sperms, it was only in the mid-fifties that scientists began relating antisperm antibodies to male infertility. In 1978, Beer and Neaves [3] established sperm autoimmunity as a cause of infertility in humans.
Antisperm antibodies may occur both in serum and seminal plasma. The local and systemic immune responses elicited by spermatozoa are independent of each other: antisperm antibodies may be present in the semen but not in the serum or vice versa. Thus the traditional tests for antisperm antibodies which have been performed only on the serum must be extended to the seminal plasma also.
The purpose of this study was to determine the incidence of antisperm antibodies both in the serum and semen of the patients reporting for infertility investigation.
Material and Methods
This study was conducted on 100 randomly chosen infertile male patients attending the outpatient department of the Infertility Clinic. History of mumps, orchitis, injury to testes, operation near the genitalia (like hernia, hydrocoele, varicocoele), extra marital sexual history and testicular biopsy/FNAC was taken. No patient had such history.
Semen of the patient was studied for semen parameters including morphology, motility, culture for Gram positive and negative organisms, ureaplasma and mycoplasma. Tray agglutination test (TAT) originally described by Freiberg [4] was modified to suit our purpose. Samples of patients serum were inactivated by incubation at 56°C for 30 minutes to destroy complement activity. They were then serially diluted with 0.01M phosphate buffered saline at pH 7.4 to produce dilution of 1:4, 1:8, 1:16 and 1:32. These dilutions were maintained at room temperature. Similarly seminal fluid of the patient was separated from the sperms by centrifugation at 1500 rpm for 5 minutes and was serially diluted with 0.01M phosphate buffered saline pH 7.2 to produce dilution of 1:4, 1:8, 1:16 and 1:32. A donor semen sample with a sperm concentration of more than 70x106/ml and motility 60% was incubated at 37° C for 20 min. for liquefaction. Then it was centrifuged at 1500 rpm for 5 minutes. The supernatant was separated and the sperm pellet was overlaid with Ham's F-10 nutrient media and was incubated for 30 min. The top layer into which a high percentage of motile spermatozoa had swum up, was then removed and sperm concentration was adjusted to between 30-40 × 106/ml. 1 microlitre of sperm suspension was added to 5 microlitres of the diluted serum and semen test samples under paraffin oil and mixed gently, in microchambers. Known positive and negative controls were included in the test. The trays were incubated at 37°C.
They were examined under inverted microscope after 1 and 2 hours and the following parameters were studied.
-
(1)
% agglutination.
-
(2)
Type of agglutination, (head to head, tail to tail, mixed)
-
(3)
Titre of serum/seminal fluid at which agglutination was seen. (a titre of more than 1:16 was taken as a positive result).
Results
Majority (64%) of the subfertile patients in our study were in the age group of 30-35 years. (Fig 1) The number of patients reporting for treatment of infertility decrease at the extreme of reproductive age. There were only 3% patients between the ages of 20 and 25 years and only 2% between the ages of 40-50 years. Most of the patients (83%) in our study had oligospermia. However two patients had high sperm count and still showed agglutination, which is normal for high count (Fig 2). 18% of our patients tested positive for agglutination in seminal fluid (as per the standard laid down in the methodology i.e. A titre of 1:16 dilutions). Of these majority (11 patients) were those who were having infertility for the past 8-12 years. (Fig 3).
Fig 1.
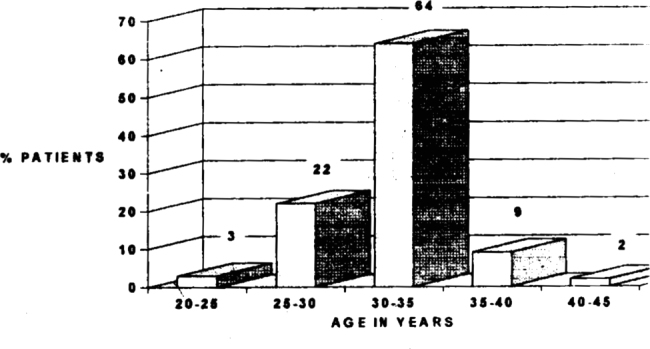
Incidence of infertility in relation to age of patient
Fig 2.
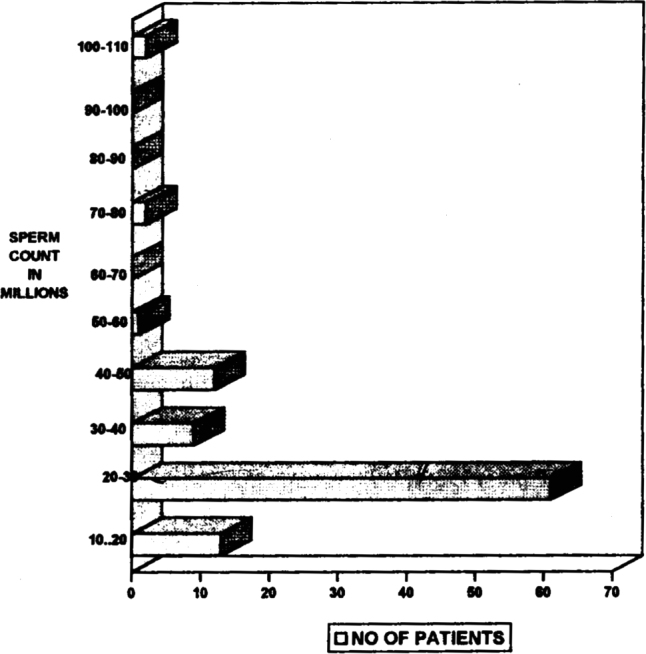
Incidence of infertility in relation to the sperm count of patient
Fig 3.
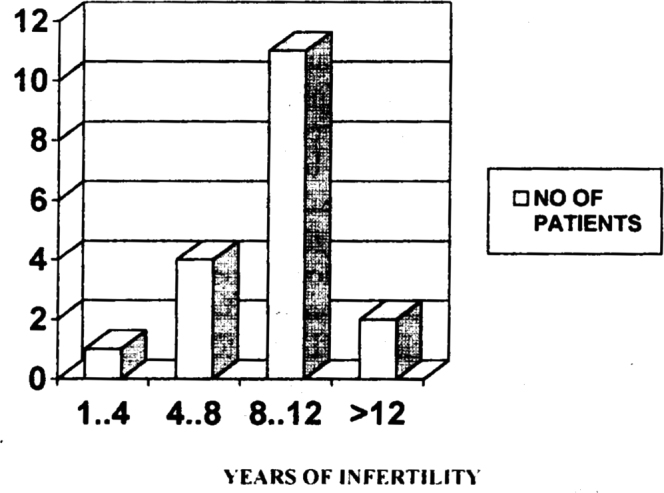
Incidence of agglutination in seminal fluid in relation to number of years of infertility
16% of our patients tested positive for agglutination in their serum. 10 of these patients had a history of 8-10 years of infertility (Fig 4).
Fig 4.
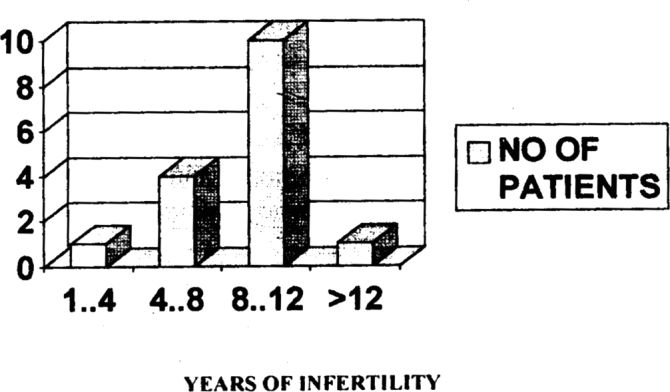
Incidence of agglutination in serum in relation to number of years of infertility
Another interesting finding in our study was that most of the agglutinations were of tail to tail type. 63% of the agglutinations in serum and 60% of those in semen were of the tail to tail type. Agglutinations of head to tail type were in the least common. Only 4% and 3% showed agglutination of this type in their serum and semen respectively (Table 1).
TABLE 1.
Type of agglutination
| Serum | Semen | |
|---|---|---|
| Head to head | 9% | 10% |
| Head to tail | 4% | 3% |
| Tail to tail | 63% | 60% |
| Mixed | 24% | 27% |
Discussion
Antisperm antibodies is the cause of subfertility in 3-20% of male patients [5]. Though the exact mechanism in which they cause a decrease in fertility is controversial, the following have been proposed.
-
1.
They cause agglutination of sperms in the semen [6].
-
2.
They prevent sperm migration in the cervical mucous by the binding of their Fc component with the glucoprotein micelles of cervical mucous causing ’shaking phenomenon’ [7, 8].
-
3.
They induce massive leucocytosis and sperm breakage within the uterus and isthmic region of the oviduct [9].
-
4.
Antibody coated sperms cannot bind to or penetrate through the zona pellucida [10, 11].
In our study 18% of the patients had anti sperm antibodies in their semen and 16% in their serum. The incidence of these antibodies was high in patients complaining of infertility for more than 8 years, probably due to increased incidence of sub-clinical infections. This was in conformity with the observation made by Henry et al [12].
The choice of treatment and prognosis are largely dependent on the class of immunoglobulins. Thus it becomes imperative for us, not only to detect the presence of antibodies to sperm but also to determine the immunoglobin class. This study using the tray agglutination test techniques has the advantage of distinguishing between the various class of antibodies through the type of agglutination. Tail to tail type is usually seen in IgG class and head to head is seen in IgM class. IgG is the commonest class and are regarded as transudatives from systemic circulation via the prostrate gland. IgA is exclusively found in semen and mostly secretory in type, suggesting intra testicular, epididymal synthesis. This study showed the maximum number of patients had tail to tail type of agglutination indicating that immunoglobulin of class G was the most common class found in the semen and serum.
Yet another advantage of TAT is that it can also be performed in patients with immotile and poorly motile sperms, as against immunobead test and mixed anti globulin reaction test.
Antisperm antibody production is mainly idiopathic. They are produced in a diversity of etiologies. These include damage or weakening of the blood testes barrier, obstruction of vas deferens and seminal tubules, diabetes mellitus, homosexuality, auto-immune disorders (rheumatoid arthritis, SLE), genetic predisposition and appendectomy.
Thus in conclusion, anti sperm antibody screening must be done in all subfertile male patients, since these antibodies produce an impairment in the sperm functions which is treatable. Moreover anti sperm antibodies are produced in a number of underlying pathologies, thus their detection can forewarn the physician of a potentially treatable disease.
REFERENCES
- 1.Metalnikoff E. Etude sur la spermatoxine. Ann L'Inst. Pasteru. 1900;14:577–578. [Google Scholar]
- 2.Shulman S. Reproduction and antibody response. Cleveland: CRC press. 1975:432–437. [Google Scholar]
- 3.Beer AE, Neaves WB. Antigenic status of semen from the view points of the male and female. Fertil Steril. 1978;29:3–7. [PubMed] [Google Scholar]
- 4.Freiberg J. A simple and sensitive micromethod for demonstration of sperm agglutinating antibodies in serum from infertile men and women. Acta Obstet Gynaecol Scand Supl. 1974;36:21–29. doi: 10.3109/00016347409156399. [DOI] [PubMed] [Google Scholar]
- 5.Bronson RA, Cooper GW. Roenfeld DL. Sperm antibodies: Their role in infertility. Fertil steril. 1984;38:171–173. [PubMed] [Google Scholar]
- 6.Jude Harris, Adege A. Male subfertility due to sperm antibodies: A clinical overview. Obs and Gyn survey. 1992;48:1–6. doi: 10.1097/00006254-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hass GG. The inhibitory effect of sperm associated immunoglobulins in cervical mucosal penetration. Fertil steril. 1986 a;46:334–337. doi: 10.1016/s0015-0282(16)49538-5. [DOI] [PubMed] [Google Scholar]
- 8.Jager S, Kremer J, Van Slochteren Draaisma T. A simple method of screening antibodies in human male. Int J Fertil. 1978;23:12–18. [PubMed] [Google Scholar]
- 9.Adeghe AJH, Barrett CLR, Cohen J. Principles and guidelines for antisperm antibody screening. In: Barratt CLR, Cooke ID, editors. Advances in Clinical Andrology Lanvcaster. MTP press; 1988. pp. 71–79. [Google Scholar]
- 10.Junk SM, Matson PL, Yovich JM. The fertilisation of human oocyte by spematozoa from men with antispermatozoal antibodies in semen. J In-vitro Fertil, Embryo Transfer. 1986;3:350–363. doi: 10.1007/BF01133246. [DOI] [PubMed] [Google Scholar]
- 11.Aitken RJ, Rudak EA, Richardson DW. The influence of anti-zona and anti-sperm antibodies on sperm-egg interaction. J Reprod Fertil. 1981;62:597–599. doi: 10.1530/jrf.0.0620597. [DOI] [PubMed] [Google Scholar]
- 12.Hendry WF. Treatment of anti-sperm antibodies. In: Hargreave TB, editor. Male Infertility. Springer-Verlag; New York: 1983. pp. 280–285. [Google Scholar]


