Abstract
Aims
Mental stress-induced myocardial ischemia (MSIMI) in patients with coronary artery disease (CAD) is associated with adverse cardiovascular outcomes. We aim to assess hemodynamic, neuro-hormonal, endothelial, vasomotor and vascular predictors of MSIMI.
Methods and Results
We subjected 660 patients with stable CAD to 99mTc sestamibi myocardial perfusion imaging at rest, with mental (speech task) and with conventional (exercise/pharmacological) stress. Endothelium-dependent flow-mediated dilation (FMD), microvascular reactivity [reactive hyperemia index (RHI)] and arterial stiffness [pulse wave velocity (PWV)] were measured at rest and 30-min after mental stress. The digital microvascular vasomotor response during mental stress was assessed using peripheral arterial tonometry (PAT). A total of 106(16.1%) patients had MSIMI. Mental stress was accompanied by significant increases in rate-pressure-product (heart rate x systolic blood pressure; RPP), epinephrine levels and PWV, and significant decreases in FMD and PAT ratio denoting microvascular constriction. In comparison to those with no MSIMI, patients with MSIMI had higher hemodynamic and digital vasoconstrictive responses (p<0.05 for both), but did not differ in epinephrine, endothelial or macrovascular responses. Only presence of ischemia during conventional stress (OR of 7.1, 95%CI of 4.2, 11.9), high hemodynamic response (OR for RPP response ≥ vs < ROC cutoff of 1.8, 95%CI of 1.1, 2.8), and high digital vasoconstriction (OR for PAT ratio < vs ≥ ROC cutoff of 2.1, 95%CI of 1.3, 3.3) were independent predictors of MSIMI.
Conclusion
Ischemia during conventional stress testing and hemodynamic and vasoconstrictive responses to mental stress can help predict subjects with CAD at greater risk of developing MSIMI.
Keywords: Peripheral arterial tonometry, vasoconstriction, mental stress, myocardial ischemia, catecholamines, endothelial dysfunction, arterial stiffness. Conventional stress test
INTRODUCTION
Emotional stress can trigger acute coronary syndromes in patients with coronary artery disease (CAD) as well as acute heart failure or sudden cardiac death.(1,2) In the laboratory, mental stress provocation can precipitate myocardial ischemia that can be diagnosed as reversible left ventricular dysfunction, transient electrocardiographic abnormalities or perfusion defects during nuclear imaging.(1–3) In comparison to ischemia with conventional exercise or pharmacological stress testing, mental stress-induced myocardial ischemia (MSIMI) is usually silent, occurs at a lower workload than exercise-induced ischemia and is independent of the underlying burden of CAD.(4–6) However, most previous studies on MSIMI were small, included select groups of patients, mostly those with demonstrated ischemia during conventional stress testing, and have reported a wide variation in the incidence of MSIMI ranging between 18 to 67%.(7–11) Although the presence of MSIMI portends increased risk of adverse cardiovascular outcomes, factors that predispose patients with CAD to MSIMI remain unclear.(3)
Mental stress activates the sympathetic nervous system with resulting hemodynamic changes that include an increase in heart rate, blood pressure and myocardial contractility.(12,13) Mental stress also induces coronary and peripheral vasoconstriction, transient endothelial dysfunction and arterial stiffening.(13–18) However, little is known about whether these hemodynamic and vascular changes predispose patients to MSIMI, as previous studies were small and selected, and did not address a comprehensive set of vascular parameters.(19–21) In patients with stable CAD with and without conventional myocardial ischemia, we investigated differences in the hemodynamic, macrovascular, microvascular, endothelial and neuro-hormonal parameters both at baseline and after mental stress in patients with and without MSIMI. Our hypothesis was that MSIMI is more likely to develop in those with a greater hemodynamic, adrenergic and vasoconstrictor response to mental stress, as well as those with greater endothelial dysfunction and arterial stiffness during mental stress.
METHODS
Study Population
The study design has been published previously.(22) Briefly, patients were enrolled into the Mental Stress Ischemia Prognosis Study (MIPS), a prospective study that recruited 695 patients with stable CAD between June 2011 and August 2014 at Emory University affiliated hospitals. The presence of CAD was defined by an abnormal coronary angiogram demonstrating evidence of atherosclerosis with at least luminal irregularities, documented previous percutaneous or surgical coronary revascularization, documented myocardial infarction, or a positive nuclear stress test. Patients with an acute coronary syndrome or with decompensated heart failure during the previous two months, end stage renal disease, or unstable psychiatric conditions were excluded. Clinical information including previous CAD events, CAD risk factors, coronary angiography results and current medications were documented using standardized questionnaires and chart reviews. The research protocol was approved by the Institutional Review Board and all participants provided informed consent. Patients were tested in the morning after a 12-hour fast. Anti-anginal medications (beta-blockers, calcium-channel blockers, and long-acting nitrates), xanthine derivatives and caffeine-containing products were withheld for 24 hours prior to stress testing. Current and lifetime diagnosis of major depression and other psychiatric diagnoses were assessed with the Structured Clinical Interview for DSM IV.(23)
Mental Stress Procedure
In a quiet dimly lit, temperature controlled (21–23 °C) room, after a 30-minute rest period, vital signs were measured and mental stress was induced by a standardized public speaking task.(22) Briefly, patients were asked to imagine a situation in which a close relative had been mistreated in a nursing home. Patients were given two minutes to prepare and three minutes to deliver a speech in front of an evaluative audience. Blood pressure and heart rate were recorded throughout. mental stress testing was performed by trained and experienced staff to standardize the psychophysiological stress-provoking elements of the test. During mental stress testing, 20–30 mCi of Tc99m radioisotope was given at one minute into the speech.
Conventional Stress Testing
On a separate day, and within one week of the mental stress test, conventional stress testing was performed using the Bruce Protocol (68% of the patients), or, when indicated, pharmacologic testing with regadenoson (32% of the patients). The radioisotope injection was given at peak exertion during the exercise test or immediately after the regadenoson injection. The exercise was continued for at least one minute after the injection.
Myocardial Perfusion Imaging and SPECT images interpretation
Myocardial perfusion imaging with 99m-Tc-sestamibi single-photon emission computed tomography (SPECT) was performed at rest and 30–60 minutes after mental and conventional (exercise/pharmacologic) stress according to standard protocols.(22) Studies were interpreted by two experienced readers blinded to the stressor (mental or exercise/pharmacological) and without prior knowledge of the severity of CAD or other patient medical history. Discrepancies in the interpretation of SPECT images were resolved by consensus. Rest and stress images were visually compared for number and severity of perfusion defects using a 17-segment model. Each segment was scored from 0 to 4, with 0 being normal uptake, and 4 no uptake. Ischemia was defined as a new impairment with a score of ≥2 in any segment, or as worsening of a pre-existing impairment by at least 2 points if in a single segment, or by at least 1 point if in two or more contiguous segments.(24) In addition to individual segment scores, we calculated the magnitude of the ischemic myocardium in a conventional fashion, as summed difference scores / 68 X100, as previously described. (24,25)
Hemodynamic Monitoring
Hemodynamic parameters, including the systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR), were recorded using IntelliSense Professional Digital Blood Pressure Monitor, HEM-907XL, OMRON, Japan. Rate-pressure product was calculated as SBP x HR. Hemodynamic parameters were recorded every 5 minutes during the resting period, every 1 minute during the mental stress, and every 5 minutes during the recovery period. Hemodynamic responses to mental stress were calculated as the difference between the maximum value of each hemodynamic parameter during the speech minus the minimum resting value during the rest period.
Epinephrine Measurements
Heparinized plasma samples were obtained at rest and 5 min after the mental stress test (n=540 and 519 respectively) for measurement of epinephrine levels (EIA Kit; 2-CAT ELISA, Labor Diagnostika Nord). This assay has an analytical sensitivity of 7 pg/mL.
Digital Blood Flow Measurement Using Finger Plethysmography
Digital pulse wave amplitude was continuously measured during rest and mental stress using peripheral arterial tonometry (PAT; Itamar-Medical), as previously described.(13,26) Analyzable data free of artifact were available in 548 (81%) patients. Briefly, the device, which uses a modified form of plethysmography was applied to the index finger. Registered pressure changes were fed into a personal computer where the signal was filtered, amplified, stored and analyzed in an operator-independent manner. The baseline pulse wave amplitude (PWA) was determined by averaging the last 3 minutes of recording that preceded the mental stress test. The stress amplitude was determined as the lowest PWA during the speaking period. The PAT ratio was then calculated as the ratio of PWA during the speaking task over the resting baseline, with a ratio < 1 signifying a vasoconstrictive response. The decrease in PWA signifies microvascular constriction.(26,27)
Vascular and Endothelial Function Measurements
All patients underwent peripheral vascular function measurements including brachial artery flow-mediated vasodilation (FMD) for endothelial function assessment, pulse wave velocity (PWV) for assessment of arterial stiffness, and digital reactive hyperemia index (RHI) measurement for assessment of microvascular dysfunction, before and 30 minutes after the mental stress test.(28) Analyzable data were available for most patients at baseline (560 with FMD, 580 with arterial stiffness and 528 with microvascular function data) and 30-minutes after the mental stress (554, 501 and 503, respectively). Detailed methodology is included in the supplement.
Statistical Analyses
To examine the differences between assigned groups (MSIMI positive vs negative), we used the two sample Student’s t test for continuous variables and the chi-square test for categorical variables. We present normally distributed data as mean ± SD and non-normally distributed data as median (interquartile range). We used Pearson and Spearman correlations to investigate the correlations among PAT ratio, epinephrine, hemodynamic response and vascular function tests. To accurately assess the response in FMD independent of the baseline diameter, we allometrically scaled FMD by using the log-transformed difference between hyperemic diameter and baseline diameter scaled against the log-transformed baseline diameter.(29) Linear regression models for repeated measures were used to investigate hemodynamic and vascular changes during mental stress. Logistic regression analysis was used to derive odds ratios for the association between hemodynamics, epinephrine levels, PAT ratio, FMD, PWV, RHI, and MSIMI. Logistic regression models were also used to determine which variables predicted MSIMI including age, gender, black race, body mass index, diabetes, hypertension, myocardial infarction, beta blocker use, resting RPP and presence of ischemia during conventional stress.
For PAT ratio and RPP response, we used receiver operator characteristic (ROC) curve analyses with 5-fold cross-validation to determine the optimal cutoffs. For a given cutoff, we used a logistic regression model to estimate the risk of MSIMI. The 5-fold cross-validation divides the data into 5 approximately equally sized portions. A logistic regression model is trained on 4 parts of the data and then estimates the risk of MSIMI in the fifth part. This is repeated for each of the 5 parts. We calculated the area under the curve with the estimated risk. The optimal cutoff is chosen to maximize area under the curve values.
RESULTS
Of the 695 patients enrolled into MIPS, a few patients had missing or poor quality SPECT scans and were excluded from the analysis. A total 680 patients completed scans at rest and after mental stress, 666 patients completed scans at rest and after conventional stress testing, and 660 patients had all scans completed with good imaging quality. Among 660 patients with data during both stress tests, 106 (16.1%) developed MSIMI and 229 (34.7%) had conventional stress induced myocardial ischemia (CSIMI). Baseline demographics, CAD risk factors, history of previous MI and medication use were similar in those with and without MSIMI. Patients with MSIMI had slightly lower ejection fraction and a higher incidence of CSIMI (Table 1).
Table 1.
Basic demographics of study population.
| MSIMI status | Total | No MSIMI | MSIMI | p value* |
|---|---|---|---|---|
| Number | 660 | 554 | 106 | |
| Age, years | 62.9 ± 9.1 | 63 ± 9.1 | 62.6 ± 9.2 | 0.67 |
| Male, % | 73 | 72 | 76 | 0.35 |
| African American, % | 30 | 29 | 36 | 0.18 |
| BMI, Kg/m2 | 29.6 ± 5.2 | 29.5 ± 5.2 | 30.3 ± 5.4 | 0.19 |
| Smoking, % | 59 | 58 | 63 | 0.33 |
| Depression, % | 26 | 28 | 20 | 0.11 |
| Diabetes, % | 32 | 31 | 39 | 0.13 |
| Hypertension, % | 76 | 75 | 78 | 0.51 |
| Dyslipidemia, % | 81 | 81 | 82 | 0.84 |
| Myocardial infarction, % | 37 | 36 | 43 | 0.20 |
| Heart Failure, % | 14 | 14 | 15 | 0.78 |
| Ejection fraction, % | 68.5 ± 13.7 | 69.1 ± 13.5 | 65.4 ± 13.9 | 0.01 |
| CSIMI, % | 35 | 27 | 74 | <0.001 |
| Medications | ||||
| Aspirin, % | 87 | 87 | 86 | 0.83 |
| Beta Blocker, % | 74 | 74 | 76 | 0.75 |
| ACE Inhibitors, % | 45 | 44 | 53 | 0.09 |
| Anti-Depressant, % | 23 | 23 | 19 | 0.33 |
| Statins, % | 85 | 85 | 86 | 0.86 |
| Resting hemodynamics | ||||
| SBP, mmHg | 135 ± 18 | 135 ± 18 | 136 ± 17 | 0.67 |
| DBP, mmHg | 79 ± 10 | 79 ± 10 | 79 ± 10 | 0.98 |
| HR, beat/min | 63 ± 11 | 63 ± 11 | 65 ± 13 | 0.03 |
| RPP, beat x mmHg/min | 8586 ± 1945 | 8528 ± 1926 | 8892 ± 2025 | 0.08 |
| Catecholamines | ||||
| Epinephrine, pg/mL | 18.4 ± 2.4 | 18.6 ± 2.3 | 17.7 ± 2.4 | 0.66 |
| Vascular function | ||||
| RHI | 2.1 ± 0.6 | 2.1 ± 0.6 | 2.1 ± 0.6 | 0.56 |
| Brachial Artery Diameter, mm | 3.9 ± 0.7 | 3.9 ± 0.7 | 3.8 ± 0.7 | 0.27 |
| Absolute FMD, mm | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.26 |
| FMD% | 4.6 ± 3.6 | 4.6 ± 4.0 | 4.9 ± 9.5 | 0.39 |
| Allometrically-scaled FMD | 4.6 ± 3.6 | 4.6 ± 4.0 | 4.9 ± 9.5 | 0.39 |
| Hyperemic VTI, cm | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.53 |
| PWV, m/s | 8.1 ± 2.1 | 8.1 ± 2.2 | 8.0 ± 2.0 | 0.71 |
| PAT ratio† | 0.72 ± 0.33 | 0.74 ± 0.35 | 0.63 ± 0.29 | 0.01 |
p values were derived from chi square and t tests for categorical and continuous variables, respectively.
PAT ratio was calculated as the ratio of lowest pulse wave amplitude during the speech to the average pulse wave amplitude during the last 3 minutes of rest prior to mental stress test.
Abbreviations: BMI: body mass index. CSIMI: Conventional (exercise/pharmacological) stress induced myocardial ischemia. ACE: angiotensin converting enzyme. SBP: Systolic blood pressure. DBP: Diastolic blood pressure. HT: Heart rate. RPP: rate pressure product (SBP x HR). RHI = reactive hyperemia index, PWV = pulse wave velocity, FMD = brachial artery flow-mediated dilation, VTI = volume time integral during brachial artery flow-mediated dilation. PAT: peripheral arterial tonometry.
Changes during and after mental stress
Hemodynamic and catecholamine response to mental stress
The mental stress test resulted in a significant increase in SBP (26±16 mmHg; 32±15% increase), DBP (13±9 mmHg; 34±17% increase), HR (11±9 beat/min; 29%±20% increase), RPP (4988±2706 mmHg x beat/min; 64±37% increase) and epinephrine levels (+75(12–155)%), p<0.001 for all (Online Figure 1A, B). The change in epinephrine level correlated with the hemodynamic changes (r=0.24, 0.19, 0.35 and 0.34 for SBP, DBP, HR and RPP, respectively, p<0.001).
As expected, exercise stress test resulted in a significant increase in systolic blood pressure, heart rate, and rate pressure product, but in a very minimal increase in diastolic blood pressure (Online Figure 2). The increases in these hemodynamic parameters during exercise are much higher than the increases observed during mental stress testing (eg RPP increased during Stage 1 and 2 Bruce protocol by 83%, and 142%, respectively, vs 64% increase during maximum mental stress testing). Pharmacological stress testing resulted in a transient increase in heart rate, but no change in systolic or diastolic blood pressure (Data not shown).
Digital microvascular response during mental stress
The mean PAT ratio during mental stress, was 0.71 ± 0.34, indicating an average 29% vasoconstriction of the digital microcirculation during mental stress. The PAT ratio was weakly and inversely correlated with the change in DBP (r=−0.09, p=0.03) and positively correlated with the change in epinephrine level (r=0.18, p <0.001) with mental stress.
Systemic vascular changes after mental stress
Thirty minutes after mental stress testing, there was a significant decrease in FMD of −0.8 ± 3.1 % (p=0.003), allometrically corrected FMD of −0.9 ± 0.3% (p<0.001), and an increase in PWV [0.3 (−0.8–1.2) m/s; +3 (−10–17)% increase, p=0.025], (Online Figure 1C). Microvascular vasodilator function remained unchanged; RHI change of 0.1 (−0.3–0.4); 4 (−14 – 21)%, p=0.21](Online Figure 1C).
Hemodynamic, Epinephrine, Vasomotor and Vascular Responses in Patients with and without MSIMI
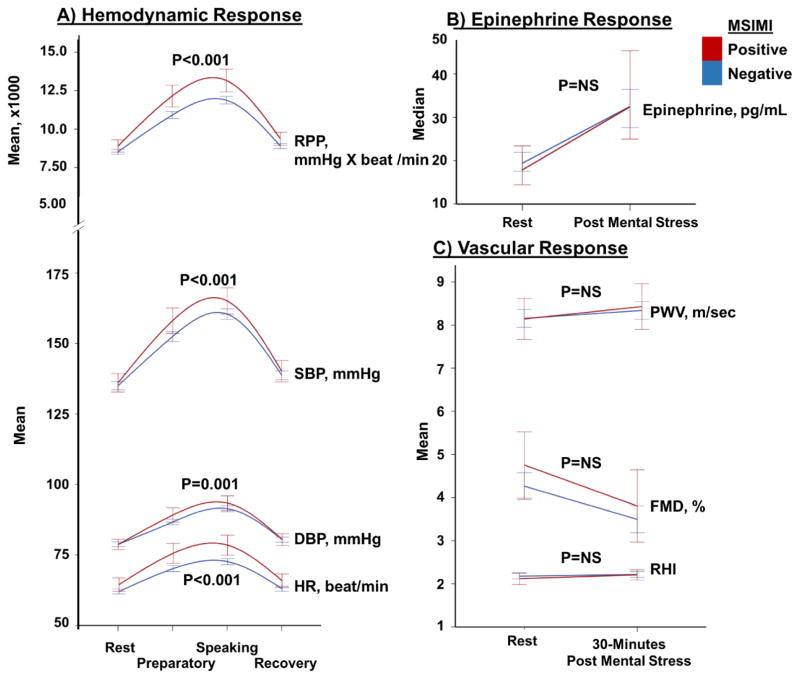
When compared to those without ischemia during mental stress, patients with MSIMI had a 3%, 6%, 5% and 11% (all p<0.05) higher SBP, DBP, HR and RPP responses to mental stress (Figure 1A). Patients with MSIMI also had greater digital vasoconstriction in response to mental stress in comparison to those without ischemia; PAT ratio of 0.62 ± 0.3 vs 0.73 ± 0.3, p=0.007. However, the circulating epinephrine levels and vascular functional measurements (FMD, PWV and RHI) either at baseline or their change with mental stress were not significantly different between patients with and without MSIMI (Figure 1B & 1C, Table 2).
Figure 1.
Comparison between hemodynamic, catecholamine, and vascular responses to mental stress in patients with and without MSIMI. A) Hemodynamic changes measured as Heart rate (HR), diastolic blood pressure (DBP), systolic blood pressure (SBP), and rate pressure product (RPP) in response to mental stress in patients with and without MSIMI. B) epinephrine changes in response to mental stress in patients with and without MSIMI; and C) Vascular changes measured as endothelial function using flow mediated dilation (FMD), arterial stiffness using pulse wave velocity (PWV), and microvascular function using reactive hypremia index (RHI), in response to mental stress in patients with and without MSIMI. P values represent the interaction with time.
Table 2.
Predictors of MSIMI using univariate logistic regression.
| OR (95%CI) | P value* | |
|---|---|---|
| Hemodynamics | ||
| Resting RPP, per 1000 increase | 1.1 (1 – 1.2) | 0.09 |
| RPP response†, per 1000 increase | 1.13 (1.1 – 1.2) | 0.001‡ |
| Vascular function tests | ||
| PAT ratio, as a continuous variable | 0.34 (0.2 – 0.7) | 0.008 |
| Resting Absolute FMD, per mm change § | 2.55 (0.5 – 13) | 0.26 |
| Absolute FMD response†, per mm change § | 0.62 (0.1 – 3.9) | 0.61‡ |
| Resting FMD, per % change § | 1.03 (1 – 1.1) | 0.39 |
| FMD response†, per % change | 0.98 (0.9 – 1.1) | 0.69‡ |
| Allometrically scaled FMD, per % change | 1.14 (0.89 – 1.46) | 0.30 |
| Allometrically scaled FMD response, per % change‡ | 0.53 (0.1 – 2.2) | 0.38 |
| Resting RHI | 0.62 (0.1 – 3.9) | 0.61 |
| RHI response† | 1.05 (0.7 – 1.6) | 0.83‡ |
| Resting PWV, m/s | 0.98 (0.9 – 1.1) | 0.71 |
| PWV response† | 1.03 (0.9 – 1.2) | 0.66‡ |
| Ischemia during conventional stress | ||
| CSIMI | 7.43 (4.6 – 11.9) | <0.001 |
p values were derived from univariate logistic regression analyses.
Response was calculated as the difference between post mental stress and resting values.
Adjusted for resting values.
Adjusted for resting basal brachial artery diameter. Abbreviations: OR: Odds ratio. CI: Confidence interval. RPP: rate-pressure product. PAT: peripheral arterial tonometry. FMD: flow-mediated dilation. PWV: pulse wave velocity. RHI: reactive hyperemia index. AI: Augmentation index. CSIMI: Conventional stress induced myocardial ischemia
In contrast to MSIMI, patients with and without CSIMI had similar hemodynamic responses during exercise stress testing (Online Figure 3), or pharmacological stress test (data not shown). There was also no significant difference in hemodynamic response during exercise stress test between patients with and without MSIMI (Data not shown).
Patients with a higher hemodynamic and greater vasoconstrictive responses to mental stress had a greater magnitude of MSIMI expressed as % ischemic myocardium (Spearman correlation of 0.12 and −0.13 for RPP and PAT responses, respectively, p<0.001 for both). No correlation was observed between the magnitude of myocardial ischemia and the epinephrine, endothelial, vascular or microvascular responses to mental stress.
Determinants of MSIMI
In bivariate analyses, only the PAT ratio, RPP change with mental stress, and the presence of CSIMI were significant predictors of MSIMI (Table 2). Thus, patients with a higher RPP response to mental stress, those with greater digital microvascular constriction, and those with CSIMI had a greater risk of MSIMI (Table 2).
We dichotomized the RPP response and PAT ratio using their receiver operator curve cutoff values. After adjustment for age, gender, race, body mass index, history of MI, resting RPP and beta blocker use, a higher hemodynamic response (RPP response ≥ 4402 beat x mmHg / min; ie ~52% increase from baseline) and greater vasoconstriction (PAT ratio < 0.68; ie 32% vasoconstriction of the digital microcirculation) in response to mental stress were both independent predictors of MSIMI with OR of 1.8 (95%CI, 1.1–2.8) and 2.1 (95% CI, 1.3–3.3), respectively (Table 3). Furthermore, patients with both a greater hemodynamic response and vasoconstriction had an incidence of MSIMI of 27%, corresponding to a 3.3 (1.7–6.7)-fold higher risk (adjusted p=0.001) compared to those with both a lower vasoconstrictor and hemodynamic responses (incidence of MSIMI 10%) (Online Figure 4A). Statistical interaction term between PAT ratio and RPP response to mental stress was not significant.
Table 3.
Odds of MSIMI using PAT ratio, hemodynamic reactivity, and RPP.
| OR (95%CI)* | p value* | |
|---|---|---|
| Bivariate analyses | ||
| RPP response, ≥ vs < cutoff† | 1.8 (1.1 – 2.8) | <0.001 |
| PAT ratio, < vs ≥ cutoff‡ | 2.1 (1.3 – 3.3) | 0.003 |
| CSIMI | 7.1 (4.2 – 11.9) | <0.001 |
| Multivariate analysis 1 | ||
| RPP response, ≥ vs < cutoff† | 2.0 (1.2 – 3.4) | 0.007 |
| PAT ratio, < vs ≥ cutoff‡ | 2.0 (1.2 – 3.3) | 0.011 |
| CSIMI | 7.2 (4.2 – 12.3) | <0.001 |
| Multivariate analysis 2 | ||
| RPP response, ≥ vs < cutoff† | 2.2 (1.3 – 3.8) | 0.005 |
| PAT ratio, < vs ≥ cutoff‡ | 1.8 (1.1 – 3.1) | 0.026 |
| CSIMI | 7.3 (4.2 – 12.7) | <0.001 |
OR and P values were derived from logistic regression analyses.
RPP response cutoff is 4402 beat x mmHg / min.
PAT ratio cutoff is 0.68.
Multivariate analysis 1: RPP response, PAT ratio and CSIMI were all included in one model as predictors of MSIMI.
Multivariate analysis 2: Multivariate analysis 1 + adjustment for age, gender, race, body mass index, diabetes, hypertension, myocardial infarction, beta blocker use and resting RPP.
Abbreviations: OR: odds ratio. CI: confidence interval. RPP: rate pressure product. PAT: peripheral arterial tonometry. CSIMI: Conventional stress induced myocardial ischemia.
Presence of CSIMI was also a strong independent predictor of MSIMI; 75% of subjects with MSIMI also had CSIMI (Table 3). Thus, whereas the incidence of MSIMI was 54% in those with CSIMI and both greater hemodynamic and vasoconstrictor responses to mental stress, it was 3% in those without CSIMI and lower hemodynamic and vasoconstrictor responses to mental stress (p<0.001) (Online Figure 4B).
Discrimination Testing
The C-statistic for prediction of MSIMI significantly increased from 0.60 (base model with conventional risk factors) to 0.638, p=0.006 after adding the PAT ratio; to 0.635, p=0.0098 after adding the RPP change; to 0.764, p<0.001 after adding CSIMI. When the PAT ratio, RPP change and CSIMI positivity were added to the model, the C-statistic improved to 0.784, p<0.001 (Online Table 1).
DISCUSSION
In the largest and most comprehensive study to date to assess hemodynamic, neuro-hormonal, vasomotor and vascular responses to acute mental stress in patients with CAD and their relationships with MSIMI, we found that acute mental stress was associated with an increase in HR and BP, with epinephrine release, peripheral microvascular constriction, arterial stiffness, and a transient decrease in endothelial nitric oxide bioavailability. The changes in HR, BP and digital microvascular tone were proportional to changes in circulating epinephrine levels during mental stress. The hemodynamic response to mental stress test was lower than exercise stress test.
We also assessed the determinants of MSIMI, measured as a reversible perfusion deficit during mental stress. The independent predictors of MSIMI were a) presence of ischemia during conventional stress testing, b) a greater hemodynamic response, measured as the RPP change, and c) a greater degree of peripheral digital vasoconstriction during mental stress. However, we found no significant associations between the presence of MSIMI and either arterial stiffness, arterial conductance or microvascular endothelial function. These results suggest that a combination of decreased coronary perfusion due to generalized vasoconstriction and increased myocardial demand, particularly in patients with significant CAD and ischemia during conventional stress testing, increases the likelihood of MSIMI. Both mental stress and MSIMI are associated with adverse long term outcomes, and it should therefore be further investigated if specific therapies that alter these responses to mental stress will result in decreased risk of MSIMI.(14,30–32)
Mental stress induced hemodynamic, epinephrine and vascular responses
We found that acute mental stress worsened endothelial function and increased arterial stiffness in a group of patients with known CAD. Consistent with our findings, several small studies, mainly in young healthy populations, showed a significant reduction in endothelial nitric oxide release and increased arterial stiffness that persisted for several hours after mental stress.(14–17,32) However, the extent of these changes was greater than in our study, possibly due to variation in timing of the measurements after mental stress, and due to enrollment of healthy subjects with preserved baseline vascular function. These changed in arterial function could be due to mental stress induced sympathetic nervous system stimulation with release of catecholamines, cortisol, endothelin-1,(33,34) and mediators of systemic inflammation(35), factors that contribute to worsening of arterial function.(17,36,37)
Predictors of MSIMI
Despite these robust hemodynamic, epinephrine and vascular responses that accompany mental stress, only a higher hemodynamic response, greater vasoconstriction, and presence of CSIMI were independent predictors of MSIMI. Increased myocardial oxygen demand in a setting of decreased myocardial perfusion are two key determinants of myocardial ischemia.(38) However, the relationship between the hemodynamic response to mental stress and the risk of MSIMI has remained controversial to date. While some studies, including our preliminary report of 225 patients of this cohort,(13) showed no association,(39–43) others are consistent with our findings.(12,44) Whereas mental stress is associated with peripheral and coronary vasoconstriction and an increase in peripheral vascular resistance,(40,41) exercise stress in largely accompanied by peripheral vasodilatation.(12,38,44) The increase in peripheral vascular resistance during mental stress results in a significant increase in left ventricular afterload, strain, and myocardial wall tension that further compromises endocardial perfusion.(38) These factors may explain why MSIMI occurs at lower workloads in comparison to exercise stress-induced myocardial ischemia.(12) Furthermore, significant epicardial coronary arterial vasoconstriction has been observed in response to mental stress in coronary segments with underlying atherosclerosis.(18,45–47) Consistent with this, we found that patients with CSIMI who have stronger hemodynamic and vasoconstrictor responses to mental stress are at highest risk of developing MSIMI. Whether the hemodynamic and vasoconstrictor responses to mental stress have prognostic implications above and beyond MSIMI needs to be further investigated.
Although epinephrine changes contributed to the magnitude of the hemodynamic responses during mental stress, they were not independently associated with the risk of MSIMI, a finding that is in agreement with previous reports.(12,48) We also found that neither the baseline vascular function, measured as the endothelial function, arterial stiffness and microvascular vasodilator function (RHI), nor its change after an episode of acute mental stress were predictors of MSIMI. Only one small study in post-menopausal women without CAD has shown that the presence of endothelial dysfunction was associated with MSIMI.(49) Our population had CAD and presumably all had endothelial dysfunction at baseline.
Strengths of this study are its large size and comprehensive nature of measurements made prospectively to investigate the effects of mental stress and their impact on MSIMI. The diversity of the population studied and use of state-of-the-art myocardial perfusion imaging are also important strengths. Limitations include the relatively modest incidence of MSIMI in our population and a lack of long term follow-up data although the collection of these data is currently underway. We have not enrolled healthy subjects to compare whether hemodynamic, catecholamine, or vascular responses are different than patients with CAD. Finally, we have not assessed catecholamine, PAT ratio or vascular functions after conventional stress testing.
Clinical Implications
Although there is increasing awareness that MSIMI is associated with adverse long term outcomes, the mechanisms and predisposing factors for MSIMI in patients with CAD remain unknown. Previous studies were small and were conducted in subgroups with and without CSIMI and consequently reported wide variations in the incidence of MSIMI.(7–10) In one of the largest studies conducted to date in CAD patients, we found that CAD patients with CSIMI who have the greatest hemodynamic and vasoconstrictive response to mental stress are at highest risk for MSIMI. Whether the hemodynamic and vasoconstrictive responses among patients with CSIMI will serve to identify subjects at higher risk without having the need for expensive ischemia detection needs to be determined with long term follow-up. Moreover, interventions to reduce the hemodynamic and vasoconstrictive responses to mental stress using both psychosomatic and pharmacologic approaches need to be further investigated.
Conclusion
Mental stress is associated with increased myocardial oxygen demand, neurohormonal activation, endothelial dysfunction, arterial stiffness, and microvascular vasoconstriction. The magnitude of the increase in RPP, digital vasoconstriction and presence of ischemia during conventional stress are independent predictors of MSIMI in patients with CAD.
Supplementary Material
PERSPECTIVES.
Core Clinical Competencies
Mental stress is associated with increased myocardial oxygen demand, neurohormonal activation, endothelial dysfunction, arterial stiffness, and microvascular vasoconstriction.
The magnitude of the increase in hemodynamic parameters, digital vasoconstriction and presence of ischemia during conventional stress are independent predictors of MSIMI in patients with CAD.
Translational Outlook implications
Further studies are needed to explore if interventions to reduce the hemodynamic and vasoconstrictive responses to mental stress, using both psychosomatic and pharmacologic approaches, can reduce the risk of MSIMI, and ultimately improve outcomes.
Acknowledgments
Sources of Funding: Dr. Vaccarino, Dr. Quyyumi, Dr.Bremner, Dr.Shah, Dr.Sheps, and Dr.O’Neal report research support from NIH. Dr. Garcia receives royalties from the sale of the Emory Cardiac Toolbox, used for some analyses in this study. This work was supported by the NIH (P01 HL101398, P20HL113451-01, P01HL086773-06A1, R56HL126558-01, R01 HL109413, R01HL109413-02S1, UL1TR000454, KL2TR000455, K24HL077506, and K24 MH076955). The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations
- MSIMI
Mental stress induced myocardial ischemia
- CSIMI
Conventional stress induced myocardial ischeia
- CAD
coronary artery disease
- PWV
Pulse wave velocity
- RHI
Reactive hyperemia index
- PAT
Peripheral arterial tonometry
- FMD
Flow mediated dilation
- RPP
Rate pressure product
- SBP
Systolic blood pressure
- DBP
diastolic blood pressure
- HR
Heart rate
Footnotes
Conflicts of Interest
None of the other authors report conflict of interest relevant to this article. Dr.Hammadah: none. Dr.Alkhoder: none. Dr.Al Mheid: none. Dr.Wilmot: none. Ms.Sullivan: none. Dr.Samman Tahhan: none. Dr.Isakadze: none. Dr.Abdulhadi: none. Dr.Mohamed Kelli: none. Dr.Ramadan: none. Dr.Pimple: none. Dr.Sandesara: none. Dr.Obideen: none. Ms.Ward: none. Ms.Ko: none. Dr.Sun:none. Ms.Chou: none. Dr.Uphoff: none. Dr.Pearce: none. Dr.Kutner: none. Dr.Esteves: none. Dr.Raggi: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaccarino V. Mental Stress-Induced Myocardial Ischemia. In: Baune TB, Tully JP, editors. Cardiovascular Diseases and Depression: Treatment and Prevention in Psychocardiology. Cham: Springer International Publishing; 2016. pp. 105–121. [Google Scholar]
- 2.Burg MM, Soufer R. Psychological Stress and Induced Ischemic Syndromes. Current Cardiovascular Risk Reports. 2014;8:1–6. doi: 10.1007/s12170-014-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J, Rooks C, Ramadan R, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. The American journal of cardiology. 2014;114:187–92. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepine CJ, Petersen JW, Bairey Merz CN. A Microvascular-Myocardial Diastolic Dysfunctional State and Risk for Mental Stress IschemiaA Revised Concept of Ischemia During Daily Life. JACC: Cardiovascular Imaging. 2014;7:362–365. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Ersbøll M, Al Enezi F, Samad Z, et al. Impaired Resting Myocardial Annular Velocities Are Independently Associated With Mental Stress–Induced Ischemia in Coronary Heart Disease. JACC: Cardiovascular Imaging. 2014;7:351–361. doi: 10.1016/j.jcmg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W, Babyak M, Krantz DS, et al. Mental stress--induced myocardial ischemia and cardiac events. Jama. 1996;275:1651–6. doi: 10.1001/jama.275.21.1651. [DOI] [PubMed] [Google Scholar]
- 7.Jain D, Burg M, Soufer R, Zaret BL. Prognostic Implications of Mental Stress-Induced Silent Loft Ventricular Dysfunction in Patients With Stable Angina Pectoris. The American Journal of Cardiology. 1995;76:31–35. doi: 10.1016/s0002-9149(99)80796-6. [DOI] [PubMed] [Google Scholar]
- 8.Krantz DS, Santiago HT, Kop WJ, Merz CNB, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. The American Journal of Cardiology. 1999;84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 9.Sheps DS, McMahon RP, Becker L, et al. Mental Stress—Induced Ischemia and All-Cause Mortality in Patients With Coronary Artery Disease: Results From the Psychophysiological Investigations of Myocardial Ischemia Study. Circulation. 2002;105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 10.Babyak MA, Blumenthal JA, Hinderliter A, et al. Prognosis After Change in Left Ventricular Ejection Fraction During Mental Stress Testing in Patients With Stable Coronary Artery Disease. The American Journal of Cardiology. 2010;105:25–28. doi: 10.1016/j.amjcard.2009.08.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burg MM, Vashist A, Soufer R. Mental stress ischemia: present status and future goals. Journal of Nuclear Cardiology. 2005;12:523–529. doi: 10.1016/j.nuclcard.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, Hemodynamic, and Neurohormonal Responses to Mental and Exercise Stress: Experience From the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–2409. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 13.Ramadan R, Sheps D, Esteves F, et al. Myocardial Ischemia During Mental Stress: Role of Coronary Artery Disease Burden and Vasomotion. Journal of the American Heart Association. 2013:2. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiadoni L, Donald AE, Cropley M, et al. Mental Stress Induces Transient Endothelial Dysfunction in Humans. Circulation. 2000;102:2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 15.Lipman RD, Grossman P, Bridges SE, Hamner JW, Taylor JA. Mental Stress Response, Arterial Stiffness, and Baroreflex Sensitivity in Healthy Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57:B279–B284. doi: 10.1093/gerona/57.7.b279. [DOI] [PubMed] [Google Scholar]
- 16.Vlachopoulos C, Xaplanteris P, Alexopoulos N, et al. Divergent effects of laughter and mental stress on arterial stiffness and central hemodynamics. Psychosomatic medicine. 2009;71:446–53. doi: 10.1097/PSY.0b013e318198dcd4. [DOI] [PubMed] [Google Scholar]
- 17.Xue Y-T, Tan Q-w, Li P, et al. Investigating the role of acute mental stress on endothelial dysfunction: a systematic review and meta-analysis. Clinical Research in Cardiology. 2015;104:310–319. doi: 10.1007/s00392-014-0782-3. [DOI] [PubMed] [Google Scholar]
- 18.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO., III Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. American Journal of Cardiology. 76:125–130. doi: 10.1016/s0002-9149(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandruni S, Fillingim RB, McGorray SP, et al. Mental Stress Provokes Ischemia in Coronary Artery Disease Subjects Without Exercise- or Adenosine-Induced Ischemia. Journal of the American College of Cardiology. 2006;47:987–991. doi: 10.1016/j.jacc.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 20.Krantz DS, Burg MM. Current perspective on mental stress-induced myocardial ischemia. Psychosomatic medicine. 2014;76:168–70. doi: 10.1097/PSY.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W. Emotional Triggering of Cardiac Dysfunction: The Present and Future. Current Cardiology Reports. 2015;17:91. doi: 10.1007/s11886-015-0635-3. [DOI] [PubMed] [Google Scholar]
- 22.Hammadah M, Al Mheid I, Wilmot K, et al. The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosomatic medicine. 2016 doi: 10.1097/PSY.0000000000000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 24.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2010;17:941–73. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 25.Vaccarino V, Wilmot K, Al Mheid I, et al. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan M, York KM, Li H, et al. Usefulness of Peripheral Arterial Tonometry in the Detection of Mental Stress-Induced Myocardial Ischemia. Clinical Cardiology. 2009;32:E1–E6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction--a new marker of obstructive sleep apnea. Sleep. 1999;22:939–46. [PubMed] [Google Scholar]
- 28.Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–92. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis. 226:425–427. doi: 10.1016/j.atherosclerosis.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biological psychiatry. 2010;68:133–9. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Dietz LJ, Matthews KA. Depressive symptoms and subclinical markers of cardiovascular disease in adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011;48:579–84. doi: 10.1016/j.jadohealth.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosomatic medicine. 2006;68:231–7. doi: 10.1097/01.psy.0000203171.33348.72. [DOI] [PubMed] [Google Scholar]
- 33.Noll G, Wenzel RR, Schneider M, et al. Increased Activation of Sympathetic Nervous System and Endothelin by Mental Stress in Normotensive Offspring of Hypertensive Parents. Circulation. 1996;93:866–869. doi: 10.1161/01.cir.93.5.866. [DOI] [PubMed] [Google Scholar]
- 34.Spieker LE, Hürlimann D, Ruschitzka F, et al. Mental Stress Induces Prolonged Endothelial Dysfunction via Endothelin-A Receptors. Circulation. 2002;105:2817–2820. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 35.STEPTOE A, WILLEMSEN G, OWEN N, FLOWER L, MOHAMED-ALI V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clinical Science. 2001;101:185–192. [PubMed] [Google Scholar]
- 36.Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. International journal of cardiology. 2013;166:30–7. doi: 10.1016/j.ijcard.2012.03.165. [DOI] [PubMed] [Google Scholar]
- 37.Poitras VJ, Pyke KE. The impact of acute mental stress on vascular endothelial function: Evidence, mechanisms and importance. International Journal of Psychophysiology. 2013;88:124–135. doi: 10.1016/j.ijpsycho.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Arri SS, Ryan M, Redwood SR, Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016 doi: 10.1136/heartjnl-2014-307306. [DOI] [PubMed] [Google Scholar]
- 39.Holmes SD, Krantz DS, Kop WJ, Del Negro A, Karasik P, Gottdiener JS. Mental Stress Hemodynamic Responses and Myocardial Ischemia: Does Left Ventricular Dysfunction Alter These Relationships? Psychosomatic medicine. 2007;69:495–500. doi: 10.1097/PSY.0b013e3180cabc73. [DOI] [PubMed] [Google Scholar]
- 40.Jain D, Shaker SM, Burg M, Wackers FJ, Soufer R, Zaret BL. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314–22. doi: 10.1016/s0735-1097(98)00092-8. [DOI] [PubMed] [Google Scholar]
- 41.Burg MM, Graeber B, Vashist A, et al. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosomatic medicine. 2009;71:14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang W, Samad Z, Boyle S, et al. Prevalence and Clinical Characteristics of Mental Stress–Induced Myocardial Ischemia in Patients With Coronary Heart Disease. Journal of the American College of Cardiology. 2013;61:714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes SD, Krantz DS, Kop WJ, Del Negro A, Karasik P, Gottdiener JS. Mental stress hemodynamic responses and myocardial ischemia: does left ventricular dysfunction alter these relationships? Psychosomatic medicine. 2007;69:495–500. doi: 10.1097/PSY.0b013e3180cabc73. [DOI] [PubMed] [Google Scholar]
- 44.Stepanovic J, Ostojic M, Beleslin B, et al. Mental Stress–Induced Ischemia in Patients With Coronary Artery Disease: Echocardiographic Characteristics and Relation to Exercise-Induced Ischemia. Psychosomatic medicine. 2012;74:766–772. doi: 10.1097/PSY.0b013e3182689441. [DOI] [PubMed] [Google Scholar]
- 45.Yeung AC, Vekshtein VI, Krantz DS, et al. The Effect of Atherosclerosis on the Vasomotor Response of Coronary Arteries to Mental Stress. New England Journal of Medicine. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 46.Kop WJ, Krantz DS, Howell RH, et al. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359–66. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 47.Lacy CR, Contrada RJ, Robbins ML, et al. Coronary vasoconstriction induced by mental stress (simulated public speaking) The American journal of cardiology. 1995;75:503–5. doi: 10.1016/s0002-9149(99)80590-6. [DOI] [PubMed] [Google Scholar]
- 48.Schoder H, Silverman DH, Campisi R, et al. Effect of mental stress on myocardial blood flow and vasomotion in patients with coronary artery disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2000;41:11–6. [PubMed] [Google Scholar]
- 49.Peix A, Ponce F, Valiente J, et al. Mental stress-induced myocardial ischemia in women with angina and normal coronary angiograms. Journal of Nuclear Cardiology. 2006;13:507–513. doi: 10.1016/j.nuclcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.