Abstract
Objective
Circulating levels of cardiotonic steroids (CTS) are elevated in various chronic inflammatory conditions, but the role of CTS in inflammation remains largely unknown. We have previously shown that the CTS ouabain stimulates pro-inflammatory responses in murine macrophages. In this study, we aim to explore the mechanism how CTS induce pro-inflammatory responses in primary murine and human macrophages.
Approach and Results
Using both murine peritoneal macrophages and human monocyte-derived macrophages, we demonstrated that ouabain activated NF-κB, leading to pro-inflammatory cytokine (e.g. MCP-1, TNF-α, IL-1β and IL-6) production. By applying siRNA techniques as well as murine peritoneal macrophages isolated from genetically modified mice, we showed that macrophages partially deficient in Na/K-ATPase, the receptor for CTS or fully deficient in the scavenger receptor CD36 or TLR4 were resistant to ouabain-induced NF-κB activation, suggesting an indispensible role of these three receptors in this pathway. Mechanistically, this effect of ouabain was independent of the ion transport function of the Na/K-ATPase. Instead, ouabain stimulated a signaling complex including Na/K-ATPase, CD36 and TLR4. Subsequently, TLR4 recruited MyD88 adaptor protein for NF-κB activation. Furthermore, intraperitoneal injection of ouabain into mice specifically recruited Ly6C+CCR2+ monocyte subtypes to the peritoneal cavities, indicating that the CTS ouabain triggers inflammation in vivo.
Conclusions
CTS activate NF-κB leading to pro-inflammatory cytokine production in primary macrophages through a signaling complex including CD36, TLR4 and Na/K-ATPase. These findings warrant further studies on endogenous CTS in chronic inflammatory diseases such as atherosclerosis.
Keywords: Na/K-ATPase, NF-κB, CD36, TLR4, macrophage
Subject codes: Signal Transduction, Inflammation, Vascular Disease
Introduction
NF-κB is a transcription factor expressed in most cell types. It mediates expression of many genes involved in inflammation and is considered a master regulator of immunity1. In macrophages the NF-κB pathway is activated in response to microbial infection when pathogen-associated molecular patterns are recognized by pattern recognition receptors, particularly of the TLR family2. This leads to a portfolio of pro-inflammatory responses, including reactive oxygen species generation, pro-inflammatory cytokine production and attraction of more immune cells to the infection sites1. NF-κB can also be activated in the absence of pathogens and abnormal activation/termination of this pathway is involved in various chronic inflammatory diseases, including atherosclerosis3, rheumatoid arthritis4, and diabetes5. The mechanisms underlying NF-κB activation in these settings remain incompletely understood.
Cardiotonic steroids (CTS), such as ouabain and digitalis, are naturally occurring plant alkaloids with biological activities based on their ability to bind the α subunit of the plasma membrane ion channel, Na/K-ATPase (NKA), and thereby modulate its transport function6. NKA actively transports Na+ and K+ across the plasma membrane using energy from ATP6 and is essential for normal cellular hemostasis. The NKA modulating activity of CTS has been exploited pharmacologically for decades related to its positive cardiac inotropic effect. The therapeutic window is narrow, however, and CTS have also been used as potent neurotoxins. CTS have been shown to stimulate signaling cascades through NKA at much lower concentrations than needed to impact ion transport7–12. These signaling functions are mediated by localized intracellular activation of Src-family kinases (SFK) and are independent of ion transport; because of the differences in dose response it is possible to activate NKA-mediated SFK signaling without altering Na/K homeostasis. This may have pathologic significance in humans as an analogue of ouabain has been detected in human plasma13 and levels of so-called “endogenous CTS” are elevated in diseases associated with chronic inflammation14–17.
We previously showed that NKA interacts structurally and functionally in macrophages with the type 2 scavenger receptor CD36, and that this interaction is enhanced by oxidized LDL (oxLDL). OxLDL/CD36 mediated pro-inflammatory, pro-atherogenic signaling in macrophages was diminished by knocking down NKA expression and NKA knockdown in bone marrow cells partially protected mice from diet-induced atherosclerosis18. In the current study we used human monocyte-derived macrophages to demonstrate that the CTS ouabain activates the NF-κB pathway leading to pro-inflammatory cytokine production. Mechanistically, ouabain at concentrations that did not impact Na/K transport, enhanced NKA/CD36 complex formation and led to recruitment of TLR4 and MyD88 to the signaling complex. Moreover, injection of ouabain into mice induced an inflammatory response in vivo that specifically attracted monocytes/macrophages to the peritoneal cavity. This study linking CTS to NF-κB activation through NKA and CD36 signaling sheds light on a potential role of endogenous CTS in chronic inflammatory disorders, and may also explain some of the unexpected negative clinical outcomes associated with chronic CTS use in humans.
Materials and Methods
available in the online-only Data Supplement
Results
Ouabain activates NF-κB and stimulates pro-inflammatory cytokine production in human macrophages
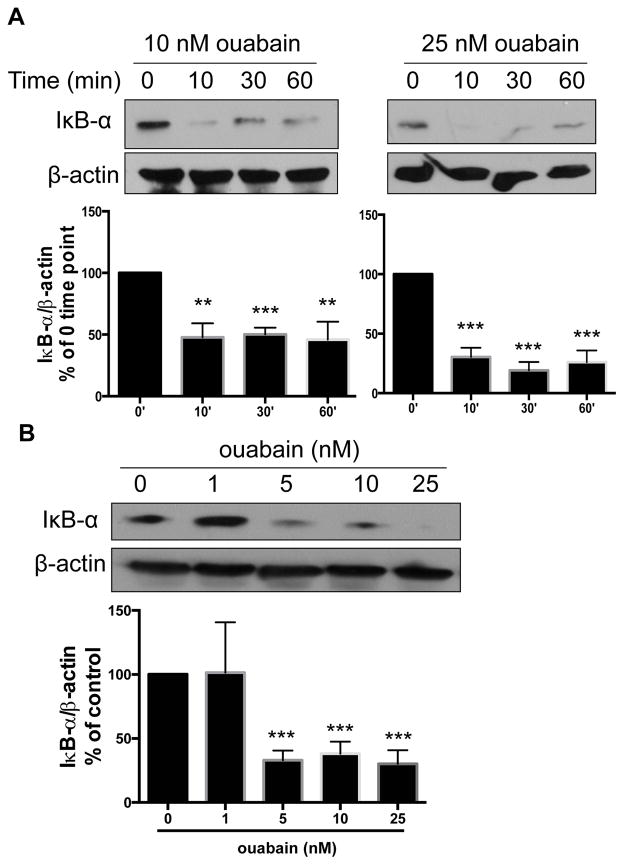
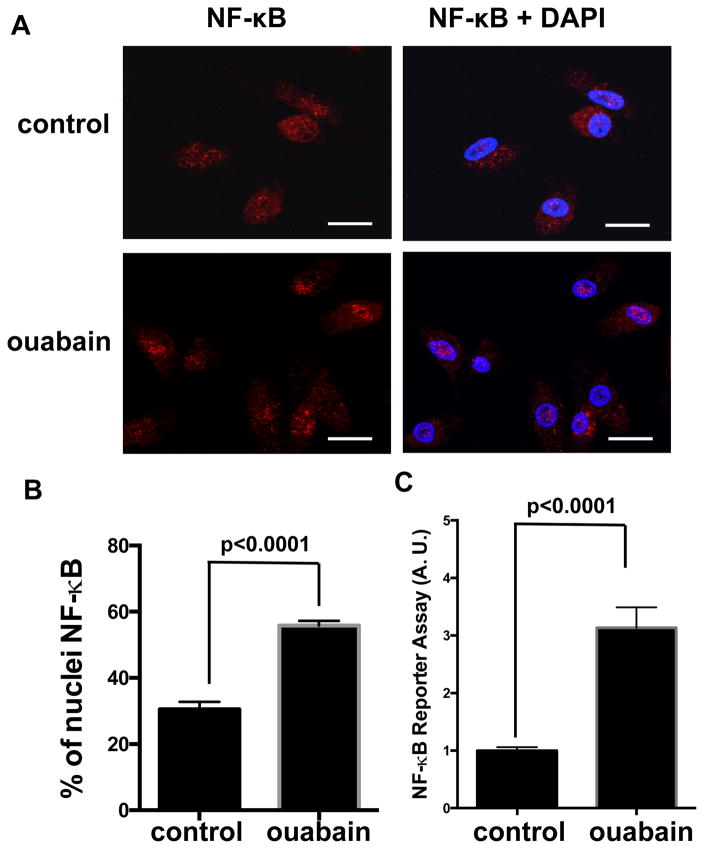
Human monocyte-derived macrophages (HMDM) incubated with the CTS ouabain exhibited a rapid and sustained decrement in IκB-α levels, as shown by western blot (Figure 1A). The effect was dose dependent with significant decreases seen at concentrations as low as 5 nM and maximum effect seen at 25 nM (Figure 1B). LPS contamination in ouabain preparations could be responsible for NF-κB activation, but LPS was not detected in any samples using an assay sensitive to 1–10 pg/ml (Supplemental Figure IA) and low doses of LPS (1 pg/ml) did not stimulate IκB-α degradation in our system (Supplemental Figure IB). Since NF-κB translocates to the nucleus following IκB-α degradation we also performed immunofluorescence staining with anti-NF-κB antibody and showed directly that ouabain-treated cells had increased nuclear localization compared to control (Figure 2A) with approximately 2 times more nuclei staining for NF-κB (Figure 2B; p<0.0001). To test NF-κB transcriptional activity directly we used a commercially available reporter system (THP1-Dual cells19) and found that 25nM ouabain increased NF-κB-dependent transcriptional activity by up 3 fold (Figure 2C; p < 0.0001).
Figure 1. Ouabain induces IκB-α degradation.
(A) HMDMs treated with ouabain for indicated time periods were immunoblotted for IκB-α and β-actin (internal control). Representative Western blot images were shown. Quantified data from five separate experiments were combined and shown in the bar graph below the images. (B) HMDMs treated with indicated doses of ouabain for 10 min were immunoblotted for IκB-α and β-actin. Representative Western blot images were shown. Quantified data from four separate experiments were combined and shown in the bar graph below the images. **, p<0.01. ***, p<0.001.
Figure 2. Ouabain leads to activation of NF-κB.
(A) HMDMs treated with ouabain for 20 min were fixed and immunostained by anti-NF-κB. Nuclei were counter stained by DAPI. Representative images from three separate experiments are shown. Scale bar: 20 μm. (B) Percentage of the nuclei NF-κB signals were quantified (n=30 for vehicle-treated control cells and n=35 for ouabain-treated cells), combined and shown in the bar graph. (C) THP-1 Dual cells were incubated with 25 nM ouabain for 24 h and medium was collected for NF-κB reporter assay. Data from eight separate experiments were combined and shown in the bar graph.
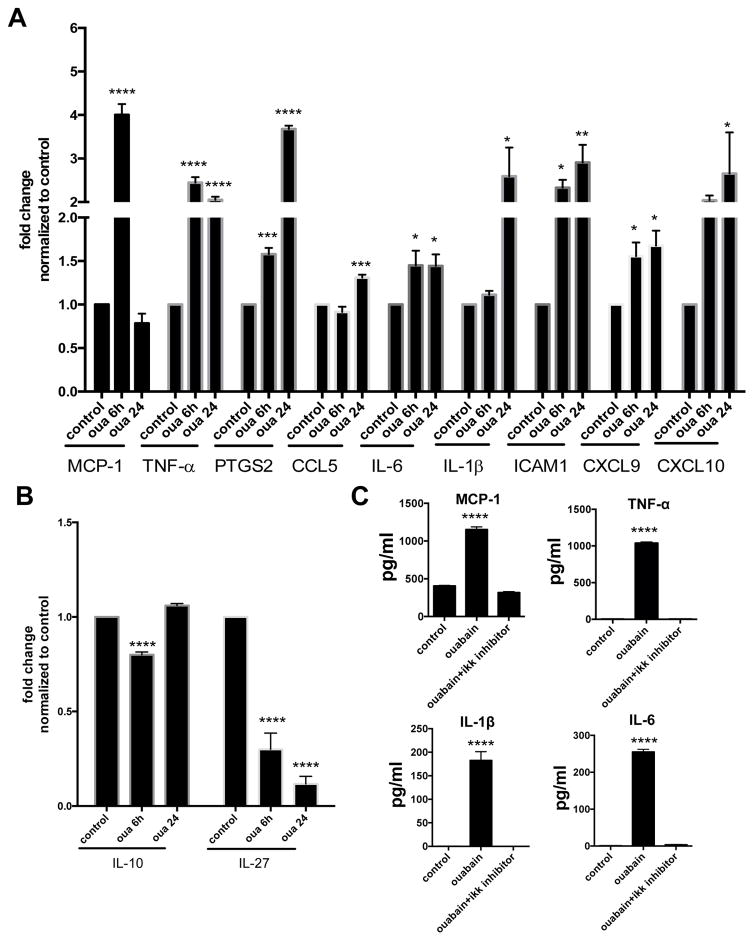
Consistent with the well-known role of NF-κB in regulating pro-inflammatory genes we used quantitative RT-PCR to demonstrate that ouabain significantly increased expression of MCP-1, TNF-α, PTGS2, CCL5, IL-6, IL-1β, ICAM1, CXCL9 and CXCL10 in HMDM (Figure 3A). Expression of the anti-inflammatory gene IL-27 was decreased by 90% after ouabain treatment (Figure 3B; p < 0.0001) while expression of IL-10, another anti-inflammatory gene, was minimally impacted. To confirm the mRNA results we measured cytokine protein levels in post-culture media by ELISA and found that ouabain treatment also led to significantly increased levels of MCP-1, TNF-α, IL-1β, and IL-6 (Figure 3C; p<0.0001). Cytokine expression was fully abolished by a specific pharmacologic IκB kinase inhibitor, Wedelolactone (Figure 3C). These data indicate that ouabain stimulated human macrophage pro-inflammatory cytokine production through the NF-κB pathway.
Figure 3. Ouabain stimulates pro-inflammatory cytokine expression through NF-κB.
RNA isolated from HMDMs were subjected to quantitative RT-PCR. (A) Pro-inflammatory and (B) Anti-inflammatory gene expression data from four separate experiments were combined and shown. (C) HMDMs were incubated with ouabain or ouabain plus 5 μM IKK inhibitor for 24 h. Cytokines (MCP-1, TNF-α, IL-1β and IL-6) in post-culture media were determined by ELISA assay. Data from three separate experiments were combined and shown. *, P<0.05. **, p<0.01. ***, p<0.001. ****, p<0.0001.
Ouabain activation of NF-κB in HMDM is not due to ion transport and requires both NKA and CD36
Previous studies showed that ouabain modulation of Na/K transport mobilized intracellular Ca2+ in renal proximal tubule cells which resulted in NF-κB activation20. Surprisingly, ouabain at concentrations up to 0.1mM did not affect intracellular Ca2+ levels in HMDM as detected with the fluorescent Ca2+ indicator Fluo-4 AM (Supplemental Figure IC). Moreover, while treatment with the Ca2+ ionophore ionomycin led, as expected, to increased intracellular Ca2+ levels, the sodium ionophore monensin did not, suggesting that increasing intracellular Na+ levels is not sufficient to raise cytosolic Ca2+ levels in human macrophages (Supplemental Figure IC). Monensin did not induce significant degradation of IκB-α (Supplemental Figure ID). These data strongly suggest that the effect of ouabain on NF-κB activation in HMDM was not due to ion shifts and subsequent Ca2+ mobilization.
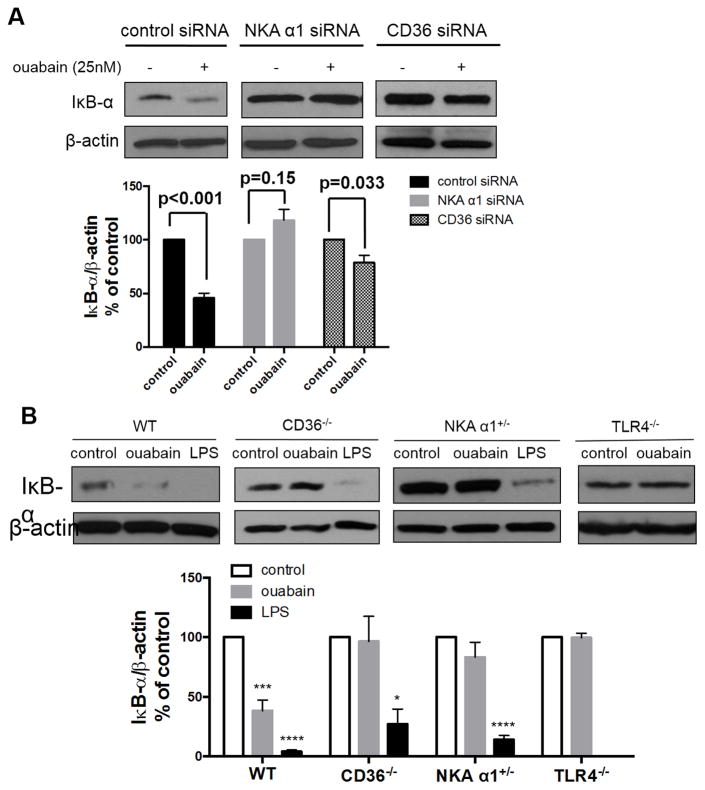
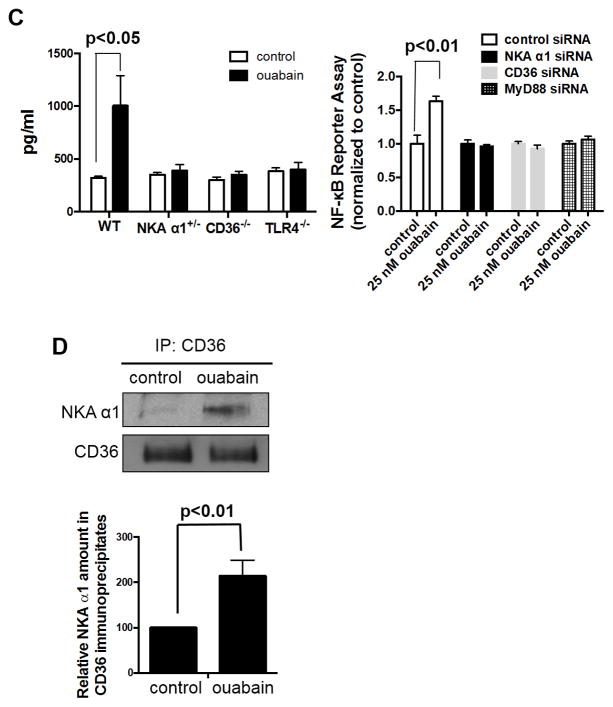
To determine the role of NKA signaling in ouabain-induced NF-κB activation, we used siRNA to knock down expression of the NKA α1 subunit by 80% in HMDM (Supplemental Figure IIA) and showed that this abolished ouabain-induced IκB-α degradation (Figure 4A). Since expression of the LPS receptor TLR4 was not affected by NKA siRNA (Supplemental Figure IIA), this provides further evidence that LPS contamination was not responsible for NF-κB activation by ouabain. Moreover, knockdown of CD36 by siRNA (Supplemental Figure IIB) attenuated ouabain-induced IκB-α degradation (Figure 4A). To confirm these findings, we also studied peritoneal macrophages isolated from cd36 null and atp1a1 heterozygous null mice (NKA α1+/−). The latter express 60% less NKA α1 in peritoneal macrophages compared to cells from wild type mice21. While macrophages from NKA α1+/− and CD36−/− mice responded normally to LPS in terms of IκB-α degradation, they did not respond to ouabain (Figure 4B). Ouabain-induced MCP-1 production was also abolished in NKA α1+/− and CD36−/− macrophages (Figure 4C left panel). Furthermore, siRNA-mediated knockdown of either NKA α1 or CD36 in THP1-Dual cells (Supplemental Figure III) prevented ouabain-induced NF-κB transcriptional activity (Figure 4C right panel). These data indicate that both NKA α1 and CD36 are both required for ouabain-induced NF-κB activation.
Figure 4. Ouabain activation of NF-κB pathway is dependent on CD36/NKA complex.
(A) HMDMs transfected with control, NKA α1 or CD36 siRNA for 48h were treated with ouabain and cell lysates were immunoblotted for IκB-α and β-actin. Representative Western blot images were shown. Quantified data from three separate experiments were combined and shown. (B) Peritoneal macrophages from wild type, CD36−/−, NKA α1+/− or TLR4−/− mice were treated with 100 μM ouabain or 100 ng/ml LPS for 1 h and cell lysates were immunoblotted for IκB-α and β-actin. Representative Western blot images were shown. Quantified data from three separate experiments were combined and shown below the images. *, p<0.05, ***, p<0.001, ****, P<0.0001. (C) Left panel: peritoneal macrophages were treated with 50 μM ouabain for 24 h. MCP-1 levels in the post-culture medium were determined by ELISA assay. Combined data from six separate experiments were shown. Right panel: siRNA-transfected THP-1 Dual cells were treated with ouabain for 24 h. Culture medium was collected for NF-κB reporter assay. Data from eight separate experiments were combined and shown. (D) CD36 immunoprecipitates from HMDMs were immunoblotted for NKA α1 and CD36. Representative blot images were shown and quantified data were combined from four separate experiments and shown in the bar graph
These observations prompted us to hypothesize that ouabain-induced pro-inflammatory signaling in macrophages was mediated by NKA α1-CD36 interactions. Using co-immunoprecipitation we showed (Figure 4D) that anti-CD36 immunoprecipitates from ouabain-treated cells contained ~ 2-fold more NKA α1 than from control cells, suggesting that ouabain stimulates formation of NKA/CD36 signaling complexes.
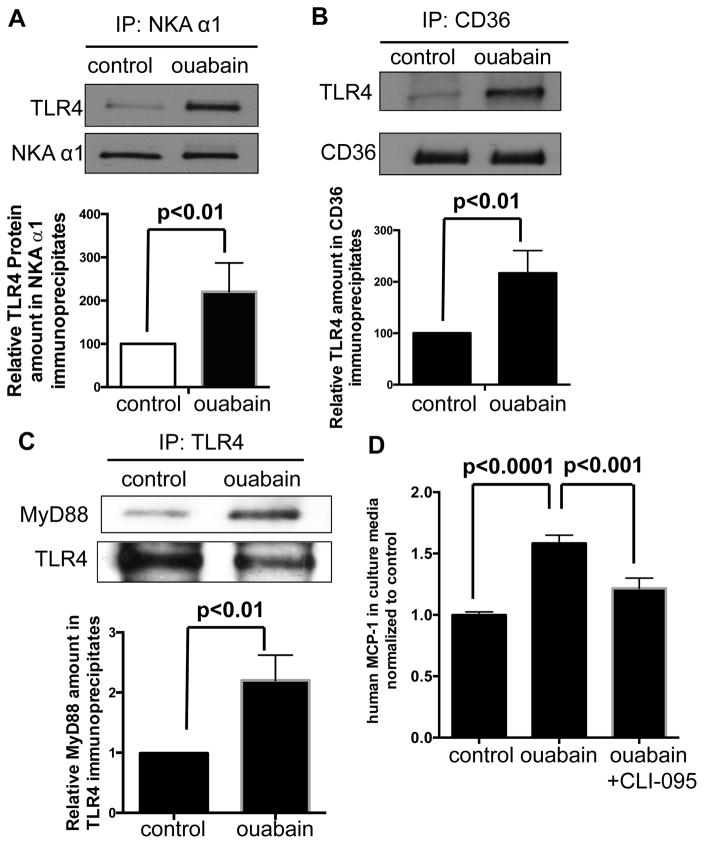
The NKA/CD36 signaling complex recruits TLR4, which is necessary for the ouabain effects
To explore signaling downstream of NKA/CD36 in the ouabain response, we tested the role of TLR4, which is known to interact with CD36 in oxLDL-mediated NF-κB activation in macrophages22. Immunoprecipitation with anti-NKA α1 from HMDM lysates revealed that TLR4 co-precipitated with NKA α1 under basal conditions and that treatment with ouabain resulted in ~2-fold more TLR4 in the precipitates (Figure 5A). Similarly, immunoprecipitation with anti-CD36 also showed ~2-fold more TLR4 in the precipitates from ouabain-treated cells (Figure 5B). Additionally, ouabain failed to induce IκB-α degradation (Figure 4B) and MCP-1 production (Figure 4C left panel) in tlr4 null (TLR4−/−) macrophages, indicating that TLR4 is required in this pathway. Furthermore, Figure 5C shows that ouabain stimulated recruitment of the adaptor protein MyD88 to TLR4 immunoprecipitates, consistent with activation of the TLR4 pathway. Using siRNA targeting MyD88 (Supplemental Figure III), we found that knockdown of MyD88 blocked ouabain-induced NF-κB activity (Figure 4C right panel). CLI-095, a small molecular weight chemical inhibitor that specifically binds to the cytosolic domain of TLR4 and blocks recruitment of MyD8823, 24, significantly decreased MCP-1 levels in the post-culture media from ouabain-treated HMDM (Figure 5D; p<0.001). In sum, these results indicate that the CTS ouabain activates NF-κB in HMDM through a TLR4/MyD88 pathway.
Figure 5. Ouabain induces recruitment of TLR4 to CD36/NKA complexes for NF-κB activation.
(A) NKA α1 immunoprecipitates (B) CD36 immunoprecipitates (C) TLR4 immunoprecipitates from HMDMs were immunoblotted for indicated proteins. Representative blot images were shown and quantified data were combined from four separate experiments and shown in the bar graph below blot images. (D) HMDMs were incubated with ouabain or ouabain plus 1 μg/ml CLI-095 for 24 h. MCP-1 levels in cell culture media were determined by ELISA assay. Data from twelve separate experiments were combined and shown in the bar graph.
Ouabain elicits NKA/CD36-dependent inflammation in vivo
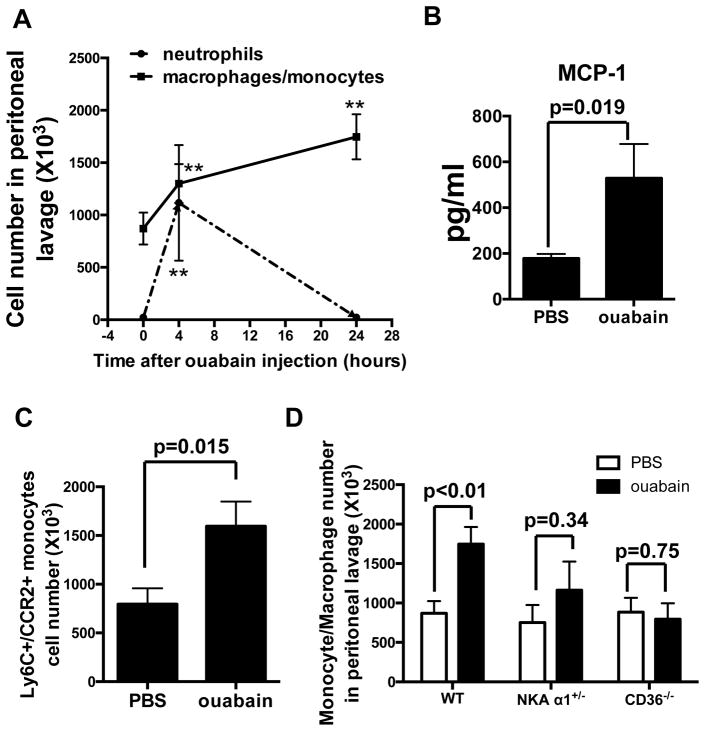
To determine the in vivo relevance of these findings we injected mice I.P. with ouabain and 4h and 24h later collected peritoneal cells by lavage with PBS. Cellular content was quantified and analyzed by flow cytometry using a panel of cell specific antibodies. The gating strategy for all genotypes and quantification of different white blood cell types is shown in Supplemental Figure IV. Figure 6A shows that compared to PBS (vehicle)-injected mice, mice injected with ouabain for 4h showed ~60% increase in CD11c−/CD11b+/F4/80+ cells (defined as monocytes/macrophages) in the peritoneal lavages. In addition, peritoneal CD11c−/CD11b+/GR1high cells (defined as neutrophils) were also significantly elevated, strongly indicating an inflammatory response to ouabain injection. Considering the time frame, we reasoned that those CD11c−/CD11b+/F4/80+ cells were most likely monocytes attracted from the circulation instead of local proliferated macrophages. Consistently, we detected elevated levels of MCP-1 in peritoneal fluid 4h after ouabain injection (Figure 6B). 24h after ouabain injection, we detected a further increase in the monocytes/macrophage population (Figure 6A and Supplemental Figure VA) while neutrophils dropped to the basal level. In addition, we observed a 50% decrease in expression levels of IκB-α in peritoneal cells after ouabain injection, indicating activation of NF-κB pathway by ouabain in vivo (Supplemental Figure VB). There are two major subsets of mouse monocytes based on differential expression of inflammatory monocyte marker, Ly6C (Ly6C+ subtype and Ly6C− subtype)25. Subtype analysis revealed that in all conditions more than 90% of the CD11c−/CD11b+/F4/80+ cells were Ly6C+CCR2+ (Supplemental Figure IV) and this subpopulation doubled 24h after ouabain injection (Figure 6C). This is consistent with in vitro and in vivo data that ouabain stimulated MCP-1 production and secretion (Figure. 3 and Figure. 6), as CCR2 is the receptor for MCP-1 and typically highly expressed on Ly6C+ monocyte surface26. Contrary to monocytes, the levels of CD4+ and CD8+ T cells were not affected by ouabain injection 24h later (Supplemental Figure VIA). Furthermore, numbers of resident peritoneal monocytes/macrophages and neutrophils as well as F4/80 expression levels were comparable among WT, NKA α1+/− and CD36−/− mice (Supplemental Figure VIB and VIC), ouabain injection to NKA α1+/− or CD36−/− mice did not lead to increased monocytes recruitment (Figure 6D). These in vivo data are consistent with the in vitro data that ouabain pro-inflammatory signaling was mediated by a NKA/CD36 complex (Figure 4).
Figure 6. Ouabain elicits NKA/CD36-dependent inflammation in vivo.
(A) Ouabain was I.P. injected (0.5 mg/Kg body weight) into peritoneal cavities of wild type mice. At indicated time points after injection, peritoneal cells were lavaged with PBS, stained with a panel of cell specific monoclonal antibody markers, and analyzed by flow cytometry. The CD11c−/CD11b+/F4/80+ subpopulation of cells were counted as monocytes/macrophages while the CD11c−/CD11b+/GR1high subpopulation was counted as neutrophils. Data were combined from ten mice in each group and shown. **, p<0.01 compared to control group. (B) Peritoneal fluid was collected 4h after injection. MCP-1 levels were determined by ELISA assay. Data were combined from six mice in each condition. (C) Monocytes/macrophages from 24h-injected mice were further stained with anti-CCR2 and anti-Ly6C antibodies and counted. Data were combined from ten mice in each group and shown in the bar graph. (D) Ouabain was I.P. injected into peritoneal cavities of wild type, NKA α1+/− or CD36−/− mice. 24h later, peritoneal cells were lavaged and processed as in (A). Peritoneal monocytes/macrophages were quantified and combined from ten mice in each condition.
Discussion
Detectable levels of circulating endogenous CTS are well documented in humans13, 27 and elevated levels into the nanomolar range have been reported in various diseases including hypertension14, 15, congestive heart failure16, 28 and type 2 diabetes17, all of which are associated with the chronic inflammatory disease atherosclerosis. However, the effect of CTS on immune cell functions as well as the underlying molecular mechanisms remain poorly understood. In this study, we discovered that the CTS ouabain induced assembly of a multi-protein receptor complex in macrophages made up of CD36, TLR4 and NKA, and that this triggered activation of NF-κB. Additionally, other CTS compounds, including digoxin and marinobufagenin showed similar NF-κB stimulating effects in human macrophages (Supplemental Figure VII), suggesting this is a common feature of CTS.
Previous studies reported that ouabain at pharmacologic doses activated NF-κB in rat renal proximal tubular cells via a mechanism dependent on mobilization of intracellular Ca2+, presumably related to the ion transport functions of NKA20. By inhibiting NKA pump activity CTS treatment leads to intracellular Na+ elevation and then via reversed Na/Ca exchange activity27, Na+ moves out of the cells in exchange for Ca2+. NKA was also shown to interact with the IP3 receptor stimulating Ca2+ release from intracellular stores29, 30 in a ouabain-dependent manner. Our studies, however, suggest that the mechanism of NF-κB activation in macrophages by CTS is different. The dose of ouabain used in our studies (25nM) did not lead to elevation of intracellular Ca2+ levels, and doses 4000 fold higher (0.1mM) that inhibit NKA pumping activity did not increase intracellular Ca2+ levels in HMDM. Moreover, the Na+ ionophore monensin neither increased intracellular Ca2+ levels nor activated NF-κB. Knocking down NKA α1 expression using siRNA abolished the ouabain effect, rather than promoting it, which is also consistent with a mechanism independent of NKA transport inhibition and Ca2+ signaling.
Our data reveal a novel mechanism by which ouabain stimulates formation of a multi-protein complex including NKA, CD36, and TLR4, which then triggers NF-κB activation. An important question is how CTS stimulate assembly of the CD36/NKA/TLR4 complex. Both the NKA ligand ouabain and the CD36 ligand oxLDL are capable of enhancing the NKA/CD36 interaction in macrophages18, 21. Additionally, both NKA and CD36 are known to be concentrated in specific cholesterol-rich membrane microdomains31, 32, placing the two plasma membrane receptors in close proximity. It is thus possible that their specific ligands induce conformational changes, that facilitate protein-protein interactions between the two receptors. This model explains why CD36−/− macrophages showed much lower response to ouabain, as CD36 plays a role as an essential adaptor protein linking TLR4 signaling to CTS.
NF-κB is a master regulator of inflammation1 and its signaling is implicated in all stages of atherosclerosis3. In agreement with a pro-atherogenic role of CTS, we found that CTS activation of NF-κB led to pro-inflammatory cytokine production and secretion including MCP-1, TNF-α, IL-1β and IL-6, all of which are highly pro-atherogenic33–36. We reported previously that plasma CTS levels were significantly elevated in apoe null mice fed a high fat diet18, a widely used atherosclerotic animal model37. Furthermore, we recently showed that downregulation of the CTS receptor NKA in apoe null mice significantly reduced macrophage recruitment to atherosclerotic plaque and protected mice from diet-induced atherosclerosis. Thus, our studies suggest that elevated endogenous CTS, by stimulating chronic NKA signaling and NF-κB pathway activation could promote chronic inflammation and thereby promote atherosclerosis.
In macrophages, CD36 cooperates with TLR4 to mediate inflammatory responses to exogenous and endogenous danger signals, including pro-atherogenic oxLDL22. Our findings that CTS, similarly to oxLDL, also stimulated assembly of a CD36/TLR4 complex leading to NF-κB activation suggest CTS may represent a previously unrecognized group of endogenous danger signals that mediate sterile inflammation in vivo. On the other hand, we have reported previously on the pro-atherogenic functions of CD36 such as uptake of oxLDL leading to foam cell formation38, inhibition of cell migration39, and reactive oxygen species generation39, 40. It will be of high interest to test whether CTS/NKA signaling also promotes these CD36-mediated functions.
Taken together, we have defined a novel mechanistic connection between the pro-atherogenic innate immune response and CTS/NKA signaling. Since CTS antagonists such as pNaKitde41 and rostafuroxin42 have been developed, it will be interesting to test whether they are effective against atherosclerosis in vivo and therefore provide a novel therapeutic strategy. Finally, our findings may also provide a mechanistic basis for recent clinical reports of increased mortality rates in patients taking digoxin in the setting of chronic heart diseases43–45.
Supplementary Material
Highlights.
CTS stimulate NF-κB pathway in primary macrophages leading to pro-atherogenic cytokine production
The NF-κB activating effect of CTS requires formation of a plasma membrane signaling complex including CD36, TLR4 and NKA
CTS stimulate peritonitis in mice through NKA and CD36
Acknowledgments
We thank Gloria R. Yuan and Marie Schulte for excellent technical support.
Sources of Funding: This work was supported by NIH grants P01 HL087018 (to R.L.S.) and R01 HL109015 (to Z.X.) and American Heart Association grant 13POST14800034 (to Y.C.).
Disclosures: none
Abbreviations
- CTS
Cardiotonic steroids
- HMDM
Human monocyte-derive macrophages
- NKA
Na/K-ATPase
- oxLDL
oxidized LDL
- SEAP
secreted embryonic alkaline phosphatase
Footnotes
Graphic Abstract
See the supplemental file.
References
- 1.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, Alvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289:2457–68. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 4.Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001;3:200–6. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61:595–603. doi: 10.1016/s1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- 6.Lingrel JB, Kuntzweiler T. Na+, K(+)-ATPase. J Biol Chem. 1994;269:19659–62. [PubMed] [Google Scholar]
- 7.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–7. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–9. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 10.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–26. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Akkuratov EE, Bai Y, Gaskill CM, Askari A, Liu L. Cell signaling associated with Na(+)/K(+)-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry. 2013;52:9059–67. doi: 10.1021/bi4011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketchem CJ, Conner CD, Murray RD, DuPlessis M, Lederer ED, Wilkey D, Merchant M, Khundmiri SJ. Low dose ouabain stimulates NaK ATPase alpha1 subunit association with angiotensin II type 1 receptor in renal proximal tubule cells. Biochim Biophys Acta. 2016;1863:2624–2636. doi: 10.1016/j.bbamcr.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88:6259–63. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manunta P, Maillard M, Tantardini C, Simonini M, Lanzani C, Citterio L, Stella P, Casamassima N, Burnier M, Hamlyn JM, Bianchi G. Relationships among endogenous ouabain, alpha-adducin polymorphisms and renal sodium handling in primary hypertension. J Hypertens. 2008;26:914–20. doi: 10.1097/HJH.0b013e3282f5315f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi G, Manunta P, Hamlyn JM, Pavan E, De Toni R, Semplicini A, Pessina AC. Immunoreactive endogenous ouabain in primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J Hypertens. 1995;13:1181–91. doi: 10.1097/00004872-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens. 2002;20:1189–94. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Straub RH, Hall C, Kramer BK, Elbracht R, Palitzsch KD, Lang B, Scholmerich J. Atrial natriuretic factor and digoxin-like immunoreactive factor in diabetic patients: their interrelation and the influence of the autonomic nervous system. J Clin Endocrinol Metab. 1996;81:3385–9. doi: 10.1210/jcem.81.9.8784101. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216–24. doi: 10.1161/HYPERTENSIONAHA.112.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–6. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Zelenin S, Aperia A, Aizman O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-kappaB. J Am Soc Nephrol. 2006;17:1848–57. doi: 10.1681/ASN.2005080894. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Kennedy DJ, Ramakrishnan DP, Yang M, Huang W, Li Z, Xie Z, Chadwick AC, Sahoo D, Silverstein RL. Oxidized LDL-bound CD36 recruits an Na+/K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci Signal. 2015;8:ra91. doi: 10.1126/scisignal.aaa9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–8. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69:1288–95. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–73. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews WR, DuCharme DW, Hamlyn JM, Harris DW, Mandel F, Clark MA, Ludens JH. Mass spectral characterization of an endogenous digitalislike factor from human plasma. Hypertension. 1991;17:930–5. doi: 10.1161/01.hyp.17.6.930. [DOI] [PubMed] [Google Scholar]
- 28.Balzan S, Neglia D, Ghione S, D’Urso G, Baldacchino MC, Montali U, L’Abbate A. Increased circulating levels of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur J Heart Fail. 2001;3:165–71. doi: 10.1016/s1388-9842(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 29.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A. 2001;98:13420–4. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–45. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol. 2008;182:1153–69. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–26. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–8. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–42. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 35.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 36.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–7. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–40. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 38.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–21. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–45. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284:21066–76. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrari P. Rostafuroxin: an ouabain-inhibitor counteracting specific forms of hypertension. Biochim Biophys Acta. 2010;1802:1254–8. doi: 10.1016/j.bbadis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi W, O’Neal WT, Soliman EZ, Al-Mallah MH. Systematic review and meta-analysis of mortality and digoxin use in atrial fibrillation. Cardiol J. 2016;23:333–43. doi: 10.5603/CJ.a2016.0016. [DOI] [PubMed] [Google Scholar]
- 44.Al-Khateeb M, Qureshi WT, Odeh R, Ahmed AM, Sakr S, Elshawi R, Bdeir MB, Al-Mallah MH. The impact of digoxin on mortality in patients with chronic systolic heart failure: A propensity-matched cohort study. Int J Cardiol. 2016;228:214–218. doi: 10.1016/j.ijcard.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Erath JW, Vamos M, Hohnloser SH. Effects of digitalis on mortality in a large cohort of implantable cardioverter defibrillator recipients: results of a long-term follow-up study in 1020 patients. Eur Heart J Cardiovasc Pharmacother. 2016;2:168–74. doi: 10.1093/ehjcvp/pvw008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.