Abstract
Objective
Bacterial endotoxin (lipopolysaccharide, LPS)-mediated sepsis involves dysregulated systemic inflammation, which injures the lung and other organs, often fatally. Vascular endothelial cells act as both targets and mediators of LPS-induced inflammatory responses. Dysfunction of endothelium results in increases of pro-inflammatory cytokine production and permeability leakage. Bone morphogenetic protein (BMP)-binding endothelial regulator (BMPER), an extracellular modulator of BMP signaling, has been identified as a vital component in chronic endothelial inflammatory responses and atherosclerosis. However, it is unclear whether BMPER also regulates inflammatory response in an acute setting such as sepsis. To address this question, we investigated the role of BMPER during LPS-induced acute lung injury.
Approach and results
Mice missing 1 allele of BMPER (BMPER+/− mice used in the place of BMPER−/− mice that die at birth) were used for LPS challenge. LPS-induced pulmonary inflammation and injury was reduced in BMPER+/− mice as shown by several measures, including survival rate, infiltration of inflammatory cells, edema and production of pro-inflammatory cytokines. Mechanistically, we have demonstrated that BMPER is required and sufficient for the activation of nuclear factor of activated T cells (NFAT) c1. This BMPER-induced NFAT activation is coordinated by multiple signaling pathways, including BMP-independent LRP1-ERK activation, calcineurin signaling and LRP1β-mediated nuclear factor-45 (NF45) nuclear export in response to BMPER treatment.
Conclusions
We conclude that BMPER plays a pivotal role in pulmonary inflammatory response, which provides new therapeutic options against sepsis shock. The new signaling pathway initiated by BMPER/LRP1 axis broadens our understanding about BMPER’s role in vascular homeostasis.
Keywords: Bone morphogenetic protein-binding endothelial regulator, Nuclear factor of activated T cells, Low density lipoprotein receptor-related protein 1, NF45, Endothelial inflammation
Subject codes: [138] Cell signaling/Signal transduction, [95] Endothelium/vascular type/nitric oxide
INTRODUCTION
Septic shock, induced in response to severe infection, remains a major cause of morbidity and mortality, despite of effective antimicrobial therapy. Severe sepsis is reported in 2% of patients admitted to the hospital. The number of cases exceeds 750,000 in the United States and 19 million worldwide per year1–3. Recent studies indicate that sepsis increases the risks of other complications such as impaired physical and neurocognitive function3. These clinical manifestations highlight a lack of understanding of pathways involved in sepsis and the demands for improved therapies. Impairment of pulmonary vascular integrity and endothelial dysfunction are key features observed in sepsis and other acute pulmonary dysfunctions including acute respiratory distress syndrome (ARDS), lung inflammation and ventilator-induced lung injuries4. In response to bacterial endotoxin lipopolysaccharide (LPS) in the blood, endothelial cells act as the first line of defense by recognizing invading pathogens through pattern recognition receptors and initiating inflammatory and coagulation cascades. Pro-inflammatory cytokines secreted from endothelial cells, which function in autocrine or paracrine loops, will further activate neutrophils, monocytes, tissue macrophages and/or endothelial cells. However, endothelial cell activation induced by LPS will eventually result in an increase in vascular permeability, allowing increased flux of proteins, fluid and immune cells across vessels into tissues. Therefore, maintenance of vascular endothelial integrity is crucial for vascular and tissue homeostasis. However, the underlying mechanisms mediating endothelial activation in response to LPS remain largely unknown.
Toll-like receptor 4 (TLR4) has been recognized as the major receptor for LPS. Ligation of LPS to TLR4 activates NFκB and nuclear factor of activated T cells (NFAT) pathways. In human umbilical vein endothelial cells (HUVECs), TLR4, MyD88 and Mal/TIRAP adaptor proteins are required for the activation of NFκB and production of interleukin-6 (IL-6)5. Animal studies with inactive NFκB signaling indicate that, in response to LPS-induced endotoxemia, NFκB activation acts as a quick adaptive response, which provides important survival signal and maintains a normal but dynamic endothelial barrier function6. On the other hand, LPS activates transcriptional factor NFAT in endothelial cells, which results in increased expression of pro-inflammatory cytokines such as IFN-γ and TNFα7. The activation of NFAT is mediated by reactive oxygen species (ROS)-driven Ca2+ signaling pathway and TLR4 may be required for ROS generation7. In activated immune cells such as dendritic cells, CD14 is another receptor of LPS for NFAT activation, likely through Src-family kinase and phospholipase Cγ2, and Ca2+/calcineurin signaling8. In endothelial cells, CD14 has also been suggested to function together with TLR4 for LPS-induced E-selectin and IL-6 production9. Although the exact roles of TLR4 and CD14 in LPS-mediated NFAT activation and endothelial inflammatory responses remain to be further clarified, NFAT activation is recognized as a crucial transcription factor controlling the expression of pro-inflammatory cytokines7.
Endothelial integrity and vascular homeostasis are tightly regulated by multiple signaling pathways such as bone morphogenetic protein (BMP) signaling10. BMP-binding endothelial regulator (BMPER), an extracellular regulator of BMPs11, 12, has been identified as an important regulator of vascular inflammation and atherosclerosis13–15. Knockdown of BMPER by its specific siRNA or its deficiency in BMPER+/− mice potentiates TNFα induced endothelial inflammatory responses14. This anti-inflammatory phenotype of BMPER is mediated by blocking BMP activity, which likely explains the atheroprotective function of BMPER15. However, it is not clear whether BMPER regulates inflammatory responses in endothelial cells in an acute setting, such as LPS challenge. We have recently demonstrated that LDLR-related protein (LRP) 1, a member of the LDL receptor family, is associated with BMPER and regulates angiogenesis during zebrafish vein development16. The mature form of LRP1 is a heterodimer composed of a 515-kDa α chain (LRP1α), possessing four extracellular ligand binding domains, and an 85-kDa membrane-anchored cytoplasmic β chain (LRP1β), which remains non-covalently associated with α chain. LRP1 is an endocytic receptor for multiple signaling pathways and mediates their signals through its β chain, which interacts with many scaffolding proteins17–23. In addition, processed forms of LRP1β can also translocate into nucleus and regulate the enzyme activity of PARP124 and expression of PPAR target genes by acting as a PPARγ co-activator25. Although LRP1 is required for the endocytosis of BMPER signaling complex in endothelial cells, it remains elusive whether the coupling of BMPER to LRP1 may also initiate their own signaling events.
BMPER null mice die at birth11. In this study, we used mice with BMPER haploinsufficiency to study the effect of reduced BMPER expression on LPS-induced endothelial inflammation. Surprisingly, we observed that BMPER+/− mice exhibit reduced vascular inflammatory responses upon LPS treatment as shown by several parameters, including wet/dry lung weight ratio, pulmonary edema, survival rate and production of pro-inflammatory cytokines. Interestingly, BMPER activates NFATc1 in a LRP1-dependent but BMP-independent manner. Furthermore, the coordinative actions of LRP1/NF45 nuclear export and NFATc1 nuclear import may play a crucial role for BMPER-induced NFATc1 activation. These results indicate that inhibition of BMPER signaling limits endotoxemia-induced pulmonary inflammatory responses and that BMPER/LRP1 signaling axis may provide potential therapeutic targets against sepsis-induced acute lung injury.
MATERIAL AND METHODS
Materials and Methods are available in the online-only supplement.
RESULTS
BMPER haploinsufficiency ameliorates pulmonary vascular leakage and lung injury in response to LPS treatment
Accumulated evidence suggests that BMPER protects endothelial cells from chronic inflammation and atherosclerosis by inhibiting BMP activity13–15. However, it is unknown how BMPER regulates acute inflammatory responses. Given that BMPER−/− mice die at birth11, we evaluated the inflammatory responses with BMPER+/− mice upon LPS challenge. First, we compared the lung injury-associated parameters, including pulmonary edema and capillary leakage, in BMPER+/− and BMPER+/+ mice where BMPER protein levels were decreased in serum and multiple tissues (Figure SIA, B). When BMPER+/+ control mice were challenged with a sub-lethal dose of LPS (10 mg/kg i.v.), they rapidly developed symptoms consistent with sepsis (e.g., lethargy, ocular discharge; data not shown). However, mice with BMPER haploinsufficiency (BMPER+/−) demonstrated less severe symptoms, compared to BMPER+/+ mice. Extravasation of Evans blue dye (EBD) from circulation into the lung tissue is often used as an indicator of elevated capillary permeability. We observed that extravasated EBD content in the lung tissue of BMPER+/− mice was significantly less than that from BMPER+/+ mice (Figure 1A, B). Similarly, the lung wet/dry weight ratio of BMPER+/− mice, following LPS injection, was significantly smaller than that of BMPER+/+ mice (Figure 1C). In addition, when challenged with a lethal dose of LPS (15 mg/kg), all BMPER+/+ mice died within 2 days while the majority of BMPER+/− mice recovered from this challenge with a 7-day survival rate at ~62.5% (Figure 1D). Then we evaluated histopathological changes of lung in mice following LPS-induced acute lung injury. Consistently, we observed a marked increase of inflammatory cell infiltration, interalveolar septal thickening, and interstitial edema in BMPER+/+ mice following LPS challenge (Figure 1E). However, BMPER+/− mice showed alleviated phenotypes of lung injury and a much reduced lung injury score (Figure 1E, F). These data suggest that BMPER haploinsufficiency protects lung from endotoxemia-induced injury.
Figure 1. BMPER haploinsufficiency ameliorates pulmonary vascular leakage and lung injury in response to LPS treatment.
A–C, Capillary leakage (A–B) and edema (C) are markedly improved in BMPER+/− mice versus BMPER+/+ mice. BMPER+/− or BMPER+/+ mice were injected i.v. with a sub-lethal dose (10 mg/kg) of LPS and 16 hours later with Evans Blue dye (EBD; 1% w/v). Mice were sacrificed 30 minutes later. Representative lung tissues with extravasated EBD were photographed and shown in (B). * P<0.05, compared with mice injected with saline; # P<0.05; n=5~6. D, Survival curves for BMPER+/− and BMPER+/+ mice injected i.v. with a lethal dose of LPS (15 mg/kg). P=0.003; n=7~8. E, representative photomicrographs of lung tissues stained with hematoxylin and eosin after LPS injection. LPS stimulated infiltration of inflammatory cells into lung interstitium and alveolar spaces as indicated by arrows, alveolar wall thickening, and intra-alveolar exudation. F, Lung tissues were screened for lung injury score. n=9 (BMPER+/+) and 4 (BMPER+/−). Analysis was two-way ANOVA followed by Fisher’s LSD multiple comparison test (for A, C), log-rank test (for D) and unpaired Student’s t-test (for F).
LPS-induced lung inflammation, apoptosis and permeability increase are attenuated in BMPER+/− mice
Endotoxemia-induced lung inflammation is associated with increases in the production of cytokines and leukocyte infiltration into lung tissue. Therefore, we evaluated the levels of LPS-induced pro-inflammatory cytokines in the serum of BMPER+/+ and BMPER+/− mice. IFNγ, IL-6, TNF-α, IL-2 and IL-5 were induced in BMPER+/+ mice by LPS treatment but their inductions were significantly decreased in BMPER+/− mice (Figure 2A–C and data not shown). To further access the influence of BMPER haploinsufficiency on pulmonary inflammation, we determined in bronchoalveolar lavage fluid (BALF) several parameters of lung inflammation, including cell infiltration, myeloperoxidase (MPO) activity, protein content and the levels of IFNγ, IL-6 and TNF-α. In response to LPS, both BMPER+/+ and BMPER+/− mice exhibited increased leukocyte infiltration into BALF, assessed by measuring the total recovered cell numbers of BALF. However, cell counts in BALF of BMPER+/− mice were significantly lower than those in BMPER+/+ mice (Figure 2D). In addition, myeloperoxidase (MPO) activity was also dramatically lower in BMPER+/− mice, compared to BMPER+/+ mice (Figure 2E). These results suggest that BMPER haploinsufficiency blocks leukocyte infiltration into alveolar space. Next, we measured total protein and cytokine levels in BALF and found that the protein level and individual levels of IFNγ, IL-6 and TNF-α were significantly decreased in BMPER+/− mice (Figure 2F–I). Taken together, BMPER+/− mice demonstrate alleviated pulmonary inflammatory response upon LPS challenge, exhibiting reductions in leukocyte infiltration and pro-inflammatory cytokine production.
Figure 2. LPS-induced pro-inflammatory responses are attenuated in BMPER+/− mice.
BMPER+/+ and BMPER+/− mice were treated with LPS (10 mg/kg i.v.) or PBS (for A–C) for 6 hours, indicated cytokine levels in serum (A–C) were measured. In BALF, total cell number (D), MPO activity (E), protein level (F), and indicated cytokine levels (G–I) were measured. * P<0.05, compared with same mice injected with PBS (A–C) or BMPER+/+ mice (D–I); # P<0.05; n=4~9. Analysis was two-way ANOVA followed by Fisher’s LSD multiple comparison test (for A–C) and unpaired Student’s t-test (for D–I).
Reports show that pulmonary microvascular endothelial cell apoptosis also contributes to pulmonary microvascular barrier dysfunction and edema during sepsis26. Therefore, we tested whether BMPER deficiency results in endothelial cell apoptosis in response to LPS. As expected, LPS injection in BMPER+/+ mice induced vascular cell apoptosis, as measured by TUNEL analysis (Figure SIIA, B). However, TUNEL positive cell number significantly decreased in BMPER+/− mice. This protective effect of BMPER haploinsufficiency was also observed in primary mouse lung microvascular endothelial cells (MLECs; Figure SIIC, D). We also assessed the effects of BMPER haploinsufficiency on endothelial permeability, we measured solute flux of FITC-Dextran across the endothelial monolayer. LPS treatment led to a 2.2-fold increase in paracellular permeability (Figure SIIE). However, this LPS-mediated permeability was partially inhibited in BMPER knockdown MLECs. Surprisingly, the protein level of claudin 5, a key determinant of endothelial permeability, was not affected by BMPER treatment (Figure SIIF), suggesting that BMPER-dependent permeability change is likely mediated through other mechanisms. Taken all together, these data suggest that BMPER deficiency reduces endothelial cell apoptosis and permeability in response to LPS.
BMPER is required for LPS-induced NFATc1 activation in endothelial cells
Host inflammatory response, rather than the nature of pathogen, is the primary determinant for the outcome of sepsis patients3. Endothelial cell is one of the major cornerstones of the innate immune responses by initiating a cascade of signaling responses to defend the pathogen3. Our group and other laboratories have demonstrated that BMPER is a key regulator of endothelial cell function12–16, 27–30. In particular, BMPER can be induced by inflammatory-regulatory stimuli, such as oscillatory shear stress and mevastatin, and inhibits endothelial inflammatory responses13, 15. BMPER haploinsufficiency inhibition of LPS-induced lung injury was unexpected because of the known anti-inflammatory role of BMPER in chronic inflammatory responses. Therefore, these data suggested that BMPER might regulate acute and chronic inflammatory responses through distinctive pathways. We tested whether BMPER plays a role in LPS signaling pathway in endothelial cells. By using MLECs, we investigated whether NFAT, an important transcription factor of LPS-induced acute inflammatory responses in endothelial cells7, can be regulated by BMPER. As expected, LPS increased NFAT activity as measured by NFAT reporter luciferase activity (Figure 3A). However, when BMPER expression in MLECs was knocked down with its specific siRNA (Figure SIIIA), we observed a ~two-fold decrease in NFAT activation, compared to control MLECs (Figure 3A). These data suggest that BMPER is required for NFAT activation in response to LPS.
Figure 3. BMPER is required and sufficient to activate NFATc1.
A, BMPER knockdown blocks NFAT activation in response to LPS (10 μg/ml). MLECs were transfected with a mixture of NFAT-responsive firefly luciferase and renilla constructs, and BMPER or control siRNA. One day later, cells were treated with LPS and the luciferase activity was measured after another day’s incubation. B, BMPER increases transcriptional activity of NFAT in MLECs. MLECs were transfected with a mixture of NFAT-responsive firefly luciferase and renilla constructs. After 24 hours, cells were treated with BMPER or control for another 24 hours and the luciferase activity was measured. C–D, BMPER increases nuclear translocation of NFATc1. MLECs were treated with 10 nM BMPER for indicated time periods. Nuclear and cytoplasmic enriched fractions of cell lysates were used to determine the translocation of NFATc1. Lamin B1 (nuclear marker) and HSP90 (cytosol marker) immunoblotting were used to verify the purity of the fractions. NFATc1 protein levels in nuclear and cytosol fractions were quantified and shown in D. E, NFATc1 is required for IFNγ induction upon BMPER treatment. MLECs were transfected with NFATc1 or control siRNA. After 24 hours, cells were treated with BMPER at 10 nM or control and incubated for another 8 hours. Cells were harvested for real time PCR with IFNγ-specific probe and primers. * P<0.05, compared to cells without LPS (A) or BMPER (B, D, E) treatment; # P<0.05; n=3~6. Analysis was two-way ANOVA followed by Fisher’s LSD multiple comparison test (for A, E) and one-way ANOVA (for B, D).
BMPER activates NFATc1 signaling
To understand the underlying molecular mechanisms responsible for BMPER’s action, we performed gene profiling analysis with BMPER treated endothelial cells and searched for changes in gene expression. Microarray analysis of endothelial cells treated with BMPER or control revealed that BMPER elicits either up-regulation or down-regulation of genes that are not associated with BMP transcriptional regulation (Figure SIIIB). To further examine these observations, we used TRANSFAC analysis to map transcription factor binding sites upstream of the identified BMP-independent BMPER-regulated genes. Interestingly, we found that a number of genes regulated by BMPER contain NFAT consensus binding sites including phospholipase Cβ1 and NFATc1, which itself is a member of NFAT transcription factors. There are mainly five members of NFAT transcriptional factors that are evolutionarily related to the REL- NF-κB family of transcription factors: NFAT1 (also known as NFATc2 or NFATp), NFAT2 (also known as NFATc1 or NFATc), NFAT3 (also known as NFATc4), NFAT4 (also known as NFATc3 or NFATx) and NFAT5 (also known as tonicity enhancer binding protein; TonEBP). To examine the relationship between BMPER and NFAT, we assessed the induction of NFATs in response to BMPER treatment. By performing real-time PCR analysis, we demonstrated that NFATc1 was the most induced NFAT in response to BMPER treatment, with the peak at one hour (Figure SIIIC). Next, we tested whether BMPER could activate NFAT by performing the NFAT reporter assay in endothelial cells in response to BMPER. When mouse lung endothelial cells were treated with BMPER, we observed an increase in NFAT transcriptional activity (Figure 3B). Since NFATc1 needs to be dephosphorylated and then translocated from cytoplasm to the nucleus for fully activation, we performed fractionation assays to determine whether subcellular localization of NFATc1 can be regulated by BMPER. Indeed, we observed an increase of NFATc1 accumulation in the nucleus after BMPER treatment for 15 to 60 minutes (Figure 3C, D). Immunostaining also confirmed the nuclear translocation of NFATc1 in response to BMPER in endothelial cells (Figure IIII). A large number of NFAT target genes including IFNγ are reported31, 32. Given that IFNγ levels in BALF and serum were decreased in BMPER+/− mice (Figure 2A, G), we tested whether NFATc1 is required for IFNγ induction in MLECs. Importantly, BMPER treatment dramatically increased the mRNA level of IFNγ in MLECs Figure 3E. However, knockdown of NFATc1 by its specific siRNA significantly blocked the induction of IFNγ Figure 3E. Using chromatin immunoprecipitation (ChIP) assays, we discovered that the association of NFATc1 with the promoter of IFNγ was increased in response to BMPER treatment (Figure SIIIE). Taken together, these data suggest that BMPER is not only necessary but also sufficient for NFATc1 activation and the induction of its target gene IFNγin endothelial cells.
LRP1 mediates NFAT activation induced by BMPER
To determine how BMPER activates NFATc1, inhibitors of different signaling pathways were used to dissect downstream signaling pathways. Importantly, we showed that cyclosporin A (CsA; calcineurin inhibitor) and U0126 (MEK1/ERK inhibitor) blocked the activation of NFAT upon BMPER treatment (Figure 4A). In addition, the inhibition of calcineurin activity by CsA improved the survival rate of BMPER+/+ mice upon LPS challenge at 15 mg/kg (Figure SIVA), which phenocopies the protective effect of BMPER haploinsufficiency (Figure 1D). However, Smad6, which is the inhibitor of the BMP’s downstream signaling mediators Smad1, 5 and 8, failed to inhibit NFAT activation (Figure 4A), suggesting that BMPER activates NFAT through BMP-independent pathways. We have recently demonstrated that LRP1 is associated with BMPER and is required for BMPER’s regulatory effects on angiogenesis16. Given that the intracellular domain of LRP1β contains multiple serine, threonine and tyrosine residues that can be phosphorylated by PKA or Src23, 33, we hypothesized that BMPER may initiate signaling cascades directly through LRP1. Treatment with BMPER at increased dosages promoted ERK activation. However, ERK activation induced by BMPER was not inhibited by BMP4 neutralizing antibody (Figure 4B, SIVB), suggesting that BMPER may induce ERK activation in a BMP4-independent manner. On the other hand, LRP1 knockdown dramatically decreased ERK activation upon BMPER treatment in MLECs that have been transiently transfected with LRP1 siRNA or mouse endothelial cells being stably transfected with LRP1 shRNA (EC50 is 51.20 nM; Figure 4C, SIVC), suggesting that LRP1 is required for BMPER-induced ERK activation. Given that ERK pathway is required for BMPER-induced NFAT activation, we then tested whether LRP1 is required for NFAT activation. Indeed, LRP1 knockdown significantly inhibited NFAT reporter activity in response to BMPER in MLECs (Figure 4D). More importantly, IFNγ mRNA levels were increased by BMPER, LPS or their combined treatments. These increases were inhibited by LRP1 knockdown in MLECs (Figure 4E). Taken for all, these results suggest that ERK activation mediated by LRP1 and calcium dependent calcineurin pathways are required for NFAT activation upon BMPER treatment. Many NFAT regulatory elements can also be regulated by NFκB. For example, CD28 response element of the IL-2 promoter contains a dimeric NFAT/NFκB site, which may bind both NFAT and NFκB and function cooperatively with AP-1 protein that is associated with the adjacent AP-1 site34, 35. Therefore, we tested whether BMPER can also activate NFκB transcriptional activity. In response to BMPER treatment, NFκB transcriptional activity was increased, determined by NFκB reporter assays (Figure SIVD). However, LRP1 knockdown by its specific siRNA decreased BMPER-induced NFκB activity. It suggests that BMPER/LRP1 axis can also activate NFκB transcriptional activity.
Figure 4. LRP1 is required for BMPER induced NFAT activation.
A, Inhibitors of calcineurin and ERK pathways block NFAT activation upon BMPER treatment. MLECs were transfected with constructs of NFAT-responsive luciferase, renilla and inhibitory Smad-Smad6. After 24 hours, cells were pre-treated with cyclosporine A (CsA), U0126 for half an hour and then treated with BMPER or control. Luciferase activity was measured after another day’s incubation. B, BMPER-induced ERK activation is not inhibited by BMP4 neutralizing antibody in MECs. Mouse cardiac-derived endothelial cells were treated with BMPER at indicated doses for 30 minutes. BMP4 neutralizing antibody (NAb; 40 ng/ml) was used to pretreat cells to block BMP4 activity (Figure SIVB). C, BMPER increases LRP1-dependent ERK activation. MLECs transiently transfected with LRP1 siRNA were treated with BMPER at 10 nM for 30 minutes, and then harvested for Western blotting. ERK activity was calculated by measuring the intensity of phospho-ERK and total ERK blots. D, LRP1 is required for NFAT activation induced by BMPER. MLECs were transfected with LRP1 or control siRNA, and NFAT-responsive luciferase and renilla constructs. After 24 hours, cells were treated with BMPER or control. Luciferase activity was measured after another day’s incubation. E, LRP1 is required for IFNγ induction upon LPS, BMPER, or their combined treatments. MLECs were transfected with LRP1 or control siRNA. After 24 hours, cells were treated with LPS at 10 μg/ml, BMPER at 10 nM, their co-treatment, or control, and then incubated for another 8 hours. Cells were harvested for real time PCR with IFNγ-specific probe and primers. * P<0.05, compared to control cells without BMPER or other indicated treatments; # P<0.05, compared as indicated (for A, C, D) or to control siRNA transfected cells upon same treatments (for E); n=3~4. NA, not significant. Analysis was two-way ANOVA followed by Fisher’s LSD multiple comparison test.
NF45 is associated with LRP1β and involved in NFAT activation
LRP1’s α chain (LRP1α) is responsible for extracellular ligand binding and β chain (LRP1β) for transducing signals. Additionally, LRP1β can be further processed36 and translocated to nucleus, where it interacts with nuclear proteins such as poly(ADP-ribose) polymerase-1 (PARP-1)24 and PPARγ25 to regulate cell cycle progression and gene transcription. Interestingly, we observed that LRP1β and its processed intracellular domain (~12 kDa) were localized in the nucleus at basal condition and their levels were decreased following the treatment of DAPT, a γ-secretase inhibitor (an inhibitor of presenillin-dependent γ-secretase activity, to generate the intracellular domain of LRP1 (~12 kDa) from the ~25 kDa processed form24, 36; VA-B). It is unclear why DAPT decreased LRP1βlevel in the nucleus. However, upon DAPT treatment, the level of the ~25 kDa fragment of LRP1 was increased in the cytosolic fraction, suggesting that LRP1β processing takes place at basal condition in a γ-secretase dependent manner. Because we have demonstrated that LRP1 is required for BMPER-induced ERK activation (Figure 4C), we wanted to determine if the nuclear LRP1β might play a role in BMPER-induced NFAT activation. To address this question, we started a search for LRP1β-interacting proteins as shown in our previous publication24. First, we performed liquid chromatography associated mass spectrometry (LC-MS/MS) analysis to identify LRP1β-associated proteins in HEK 293 cells (Figure 5A). Nuclear factor 45/ interleukin binding factor 2 (NF45/ILF2) was among proteins that co-immunoprecipitated with Flag-LRP1β (Figure 5A). NF45 is a nucleic acid-binding protein that regulates cellular gene expressions involved in DNA metabolism, transcription, translation, RNA export and microRNA biogenesis37–41. In addition, NF45 is considered as a transcriptional activator of IL-2 gene42. Previous reports show that NF45 and NFAT bind to the same promoter region of IL-2 promoter. However, they have different consensus binding sequences (TGTTTAC for NF45 and TGGAAAAT for NFAT)43. NF45, unlike NFAT, is associated with the IL-2 ARRE-2 site even at basal condition43. To date, it remains unknown whether this interchangeable binding of NF45 and NFAT transcriptional complex is important for IL-2 transcriptional activation. To study the role of NF45 in BMPER-induced gene expression, we first confirmed the interaction between overexpressed Flag-LRP1β and NF45 in HEK 293 cells (Figure 5B). Moreover, their endogenous association was detected in MLECs and blocked by the inhibitors of PKA- PKI, Src (PP2) or their combined treatments (Figure 5C, SVC), suggesting that the interaction of LRP1β with NF45 requires LRP1β phosphorylation by PKA and Src. Furthermore, both immunofluorescence imaging and subcellular fractionation analysis demonstrated that LRP1β and its processed intracellular domain, and NF45 were co-localized mainly in the nucleus of MLECs at basal condition (Figure 5D, E). Surprisingly, cytosolic levels of NF45, LRP1β and its intracellular domain, but not the processed form of LRP1β at 25 kDa, were increased in response to BMPER treatment (Figure 5E), suggesting that BMPER induces the cytosol translocation of NF45, LRP1β and its intracellular domain, but not the LRP1 processing. To further test whether NF45 translocation takes place in LPS-induced inflammatory setting, we performed immunofluorescent studies in MLECs and lung sections. In LPS-treated MLECs, NF45 positive signals in the cytosol fraction were significantly increased (Figure SVD, E). However, the increased signals were blocked in BMPER siRNA transfected MLECs. Additionally, 70.6% of NF45 signals were localized in cytosol of pulmonary vascular cells in BMPER+/+ mice upon LPS injection (Figure SVF, G). However, the cytosol-localized NF45 signals were decreased to 55.1% in BMPER+/− mice. All these results indicate that NF45 was translocated from the nucleus to cytoplasm in response to LPS and its translocation was inhibited by BMPER deficiency, which supports the hypothesis that NF45 nuclear export is regulated by LPS through a BMPER-dependent pathway.
Figure 5. NF45 is associated with LRP1β and involved in NFAT activation.
A, NF45 is a candidate protein associating with LRP1. Lysates of HEK 293 cells with stably transfected Flag-tagged LRP1 β chain (Flag-LRP1β) were immunoprecipitated with an anti-Flag antibody and stained with Coomassie blue. Positive bands are subjected to mass spectrometry analysis to identify interacting proteins. NF45 is one of these candidate proteins that are likely associated with LRP1. B, Flag-tagged LRP1β is associated with NF45 in HEK293 cells. Lysates of HEK 293 cells with stably expressing Flag-LRP1β were immunoprecipitated with an anti-flag resin and blotted with an anti-NF45 antibody. C, LRP1β is associated with NF45 in MLECs. Lysates of MLECs were immunoprecipitated with anti-LRP1β antibody or control IgG, and analyzed by Western blotting with an anti-NF45 antibody. D–E, LRP1β and NF45 are translocated from nucleus to cytoplasm upon BMPER treatment. MLECs were treated with BMPER at 10 nM for 15 minutes. After fixation, cells were stained with LRP1 (C-terminal) antibody (green), NF45 antibody (red) and DAPI (blue) for nucleus and representative pictures were shown in D. LRP1 (C-terminal) antibody recognizes both LRP1β and its processed fragments at 25 kDa and 12 kDa intracellular domain. The relative intensity of LRP1β and its processed fragments, and NF45 in nuclear and cytoplasmic fractions was quantified and the ratios of nuclear to cytoplasmic signals were presented in D. * P<0.05, compared to control cells; n=8~12. In addition, MLECs were treated with BMPER and fractionation assays was performed. Band intensity of LRP1β, its intracellular domain at 12 kDa (LRP1-ICD) and NF45 was calculated and normalized to the sample without BMPER treatment. The graph in E shows a decrease in nuclear NF45, LRP1β and LRP1-ICD protein levels but an increase in cytosolic NF45, LRP1β and LRP1-ICD. The protein level of the LRP1β processed form at 25 kDa was not changed in the cytosol in response to BMPER treatment. * P<0.05, compared to cells without BMPER treatment; n=3. F, NF45 knockdown promotes NFAT activity. MLECs were transfected with a mixture of NFAT-responsive firefly luciferase and renilla constructs, and NF45 or control siRNA. One day later, cells were treated with BMPER at 10 nM and the luciferase activity was measured after another day’s incubation. * P<0.05, compared to cells without BMPER treatment; # P<0.05; n=3. Analysis was Student’s t-test (for D), one-way ANOVA (for E) and two-way ANOVA followed by Fisher’s LSD multiple comparison test (for F).
In the meantime, we observed the translocation of NFATc1 from cytoplasm to nucleus (Figure 3C, D). Therefore, we hypothesize that NF45 may negatively regulate NFAT activity by competing to bind the promoter region of NFAT target genes. As expected, knockdown of NF45 by its specific siRNA significantly increased NFATc1 chromatin occupancy, and its promoter activity at basal condition and in response to BMPER treatment (Figure 5F, SVIA). In addition, when LRP1 was knocked down in MLECs, both BMPER-induced NF45 nuclear export and NFATc1 cytoplasmic-nuclear shuttling were inhibited (Figure SVIB-E). When LRP1 α chain was blocked by its specific antibody 8G1, BMPER-induced NF45 nuclear export was also blocked (Figure SVIF, G), suggesting that two signaling pathways, including the membrane LRP1-associated LRP1β signaling and nuclear LRP1β-NF45 signaling, are interdependent. However, exact mechanisms by which these two pathways are integrated remain unclear and will become one of our future research directions. Nevertheless, we conclude that NF45 acts as a repressor for NFAT activation and its nuclear export with LRP1β may contribute to BMPER-induced activation of NFATc1.
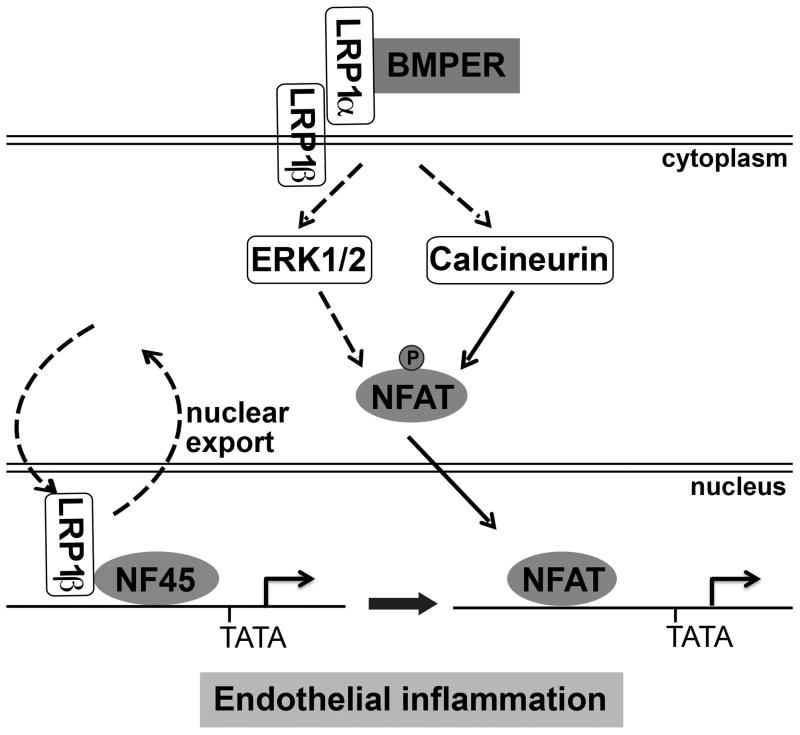
Taken all together, our results indicate that BMPER acts as a pro-inflammatory stimulus during acute lung injury. Its action requires coordinative actions of multiple signaling cascades, including the calcium-dependent calcineurin and LRP1-dependent ERK activation, the cytosolic translocation of NF45 and nuclear translocation of NFATc1, which eventually lead to the activation of NFATc1 and induction of its downstream inflammatory target genes (Figure 6). Our study provides BMPER signaling as a potential therapeutic target in preventing sepsis-induced pulmonary inflammation and injury.
Figure 6.
Schematic illustration to show how BMPER regulates NFATc1 activation via coordinative actions of LRP1, ERK, calcineurin and NF45.
DISCUSSION
The roles of BMPER in inflammation have been examined in several mouse models of inflammation including thioglycollate-induced peritonitis and high fat diet induced atherosclerosis14, 15. The studies suggest that BMPER plays an anti-inflammatory role through modulating BMP activity and thereby blocking TNF-α or oscillatory shear-mediated induction of adhesion molecules such as ICAM-1 and VCAM1. In this study, we have demonstrated for the first time a pro-inflammatory role of BMPER in LPS-induced acute lung injury. BMPER+/− mice exhibit alleviated responses upon LPS challenge. Our biochemical studies suggest that BMPER regulates inflammatory responses through NFAT pathway, which plays important roles in endothelial inflammation. In addition, we provide evidence suggesting that BMPER may initiate its own signaling pathways via LRP1, which expands our understanding in the roles of BMPER in vascular homeostasis.
How BMPER may play a pro-inflammatory role in LPS-induced acute lung injury? Our gene expression profiling analysis provided us a clue by suggesting that BMPER may regulate NFAT transcriptional activity. We then confirmed that BMPER is not only required but also sufficient for NFAT activation, which plays an essential role in pro-inflammatory cytokines production in endothelial cells during LPS-induced endotoxemia7. This BMPER-induced signaling event involves coordinative actions of multiple pathways. As we propose in the working model (Figure 6), BMPER activates ERK pathway in a LRP1-dependent manner, which is required for NFAT activation. Although it remains to be further determined how calcium/calcineurin signaling is activated by BMPER, the studies with calcineurin inhibitor cyclosporine suggest that calcium/calcineurin is also required for BMPER-induced NFAT activation. In addition, we discovered that LRP1β is associated with NF45 in the nucleus at basal condition. Upon BMPER stimulation, they are translocated to cytoplasm in a timely fashion when NFATc1 is translocated into nucleus. Given that NFATc1 and NF45 are associated with the same promoter regions of their genes, these observations indicate that NF45 may suppress NFAT transcriptional activity at basal condition. Upon the stimulation, the replacement of NF45 by NFAT transcriptional complex could be allowed in a fast fashion, which may explain the quick transcriptional events for pro-inflammatory cytokines. These timely reactions of endothelial cells, acting as the first line of defense, are essential for fighting against septic shock and the resolution of the acute inflammation.
We and others have identified both pro- and anti-BMP activities of BMPER11, 12, 44–51, resulting in some controversy as to whether BMPER is a BMP agonist or antagonist. To reconcile this issue, we have extensively analyzed the biochemical events resulting from BMPER’s interaction with BMP4 and identified a concentration-dependent switch of BMPER from pro- to anti -BMP signaling, which is modulated by the relative concentration of BMPER to BMP416. Specifically, when BMPER is at molar concentrations lower than BMP4 (sub-stoichiometric), BMP4 activity is enhanced, leading to increased phosphorylation of Smad1/5/8 in mouse endothelial cell. However, when BMPER is at molar concentrations higher than BMP4 (supra-stoichiometric), BMP4-mediated Smad1/5/8 phosphorylation is attenuated. Both the activation and inhibition of BMP4 signaling by BMPER require endocytosis of BMPER/BMP4 complex through a clathrin-dependent endocytosis pathway, suggesting that endocytosis of BMPER/BMP4 complex is involved in BMPER-mediated BMP4 signaling. This provides molecular mechanisms to support a role of BMPER in fine tuning BMP signaling at the single cell level. However, it may not necessarily explain all the pro- and anti- BMP phenotypes in different animal models. In this study, we provide strong evidence suggesting that BMPER, besides acting as an extracellular modulator of BMP, may also initiate its own signaling through LRP1 in endothelial cells. Even though this adds another layer of complexity to BMPER’s functional roles, it provides feasible explanations for BMPER’s different, sometimes even controversial, loss-of-function phenotypes in different animal models.
LPS-induced NFAT pathway plays a pivotal role in vascular endothelial activation. In endothelial cells, TLR4, and likely CD14 too, are main receptors of LPS. However, it is unclear whether TLR4 and/or CD14 are required for NFAT activation. In this study, we have discovered that BMPER is required and sufficient for NFAT activation. It will be interesting to determine how BMPER regulates LPS-induced NFAT activation. LRP1 has been reported to transactivate Trk receptors through a Src family kinase-dependent pathway52. It is likely that crosstalks may also exist between LRP1 and TLR4, CD14 and/ or their downstream mediators, which coordinately regulate NFAT activation. The detailed protein-protein interactions responsible for this crosstalk will become one of our further research directions.
It is worth to note that the decrease of BMPER expression in the BMPER+/− mouse is not limited in endothelial cells. Since BMPER is a secreted protein, it can act on many different cell types. Additionally, inflammation is a multi-cellular process that involves not only endothelial cells but also other cells such as circulating leukocytes and lung epithelial cells. Even though our investigation has only been focused on lung endothelial cells, the role of BMPER in sepsis or other lung inflammatory pathogenesis may be more complicated than what we observed within this endotoxemia model. Previous studies show that the decreased BMPER expression following bleomycin-induced lung injury impairs epithelial barrier function and epithelial morphology53. It is very likely that BMPER haploinsufficiency also regulates functions of epithelial cells, neutrophils or other cell types during LPS-induced acute lung injury, which remains to be further investigated. Nevertheless, given that BMPER is accessible as a secreted protein, our findings shed light that BMPER may become a great therapeutic target during endotoxemia, septic shock, and other pulmonary inflammatory conditions.
Supplementary Material
HIGHLIGHTS.
In this study, we have demonstrated for the first time a pro-inflammatory role of BMPER in LPS-induced acute lung injury. BMPER+/− mice exhibit alleviated responses upon LPS challenge.
Our biochemical studies suggest that BMPER regulates inflammatory responses through the activation of NFAT pathway, which plays important roles in endothelial inflammation.
In addition, we provide data suggesting that BMPER may initiate its own signaling pathways via LRP1, which expands our understanding in the roles of BMPER in vascular homeostasis.
Acknowledgments
We thank the Baylor College of Medicine Histology Core, Optical Imaging and Vital Microscopy Core and Mass Spectrometry Core Laboratories for their help.
SOURCES OF FUNDING
This work was supported by NIH R01s HL112890 and HL061656 (to X.P.) and HL122736 (to X.L.).
ABBREVIATIONS
- BMPER
bone morphogenetic protein- binding endothelial regulator
- BMP
bone morphogenetic protein
- NFAT
nuclear factor of activated T cells
- LRP1
low-density lipoprotein receptor related protein 1
- LRP1β
low-density lipoprotein receptor related protein 1 β chain
- MLEC
mouse lung microvascular endothelial cells
- LPS
lipopolysaccharide
- ARDS
acute respiratory distress syndrome
- TLR
toll-like receptor
- BALF
bronchoalveolar lavage fluid
- MPO
myeloperoxidase
- ROS
reactive oxygen species
- IFNγ
interferon gamma
- TNF-α
tumor necrosis factor alpha
- IL-6
interleukin 6
- NF45
nuclear factor 45
- ERK
extracellular signal-regulated kinase
Footnotes
DISCLOSURES
None.
References
- 1.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–61. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 2.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling, selection, and society. Crit Care. 2004;8:222–6. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 4.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–77. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 5.Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, Monaco C, Feldmann M, Foxwell BM. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103:2229–37. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 6.Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–63. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–8. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 9.Hijiya N, Miyake K, Akashi S, Matsuura K, Higuchi Y, Yamamoto S. Possible involvement of toll-like receptor 4 in endothelial cell activation of larger vessels in response to lipopolysaccharide. Pathobiology. 2002;70:18–25. doi: 10.1159/000066000. [DOI] [PubMed] [Google Scholar]
- 10.Dyer LA, Pi X, Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol Metab. 2014;25:472–80. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–79. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helbing T, Rothweiler R, Heinke J, Goetz L, Diehl P, Zirlik A, Patterson C, Bode C, Moser M. BMPER is upregulated by statins and modulates endothelial inflammation by intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2010;30:554–60. doi: 10.1161/ATVBAHA.109.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helbing T, Rothweiler R, Ketterer E, Goetz L, Heinke J, Grundmann S, Duerschmied D, Patterson C, Bode C, Moser M. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood. 2011;118:5040–9. doi: 10.1182/blood-2011-03-339762. [DOI] [PubMed] [Google Scholar]
- 15.Pi X, Lockyer P, Dyer LA, Schisler JC, Russell B, Carey S, Sweet DT, Chen Z, Tzima E, Willis MS, Homeister JW, Moser M, Patterson C. Bmper inhibits endothelial expression of inflammatory adhesion molecules and protects against atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2214–22. doi: 10.1161/ATVBAHA.112.252015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ, 3rd, Jin SW, Patterson C. LRP1-Dependent Endocytic Mechanism Governs the Signaling Output of the Bmp System in Endothelial Cells and in Angiogenesis. Circ Res. 2012;111:564–74. doi: 10.1161/CIRCRESAHA.112.274597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacskai BJ, Xia MQ, Strickland DK, Rebeck GW, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 2000;97:11551–6. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–41. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–7. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 20.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–82. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantuano E, Mukandala G, Li X, Campana WM, Gonias SL. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J Biol Chem. 2008;283:19904–11. doi: 10.1074/jbc.M801762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher P, Herz J. Signaling through LRP1: Protection from atherosclerosis and beyond. Biochem Pharmacol. 2011;81:1–5. doi: 10.1016/j.bcp.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Geer P. Phosphorylation of LRP1: regulation of transport and signal transduction. Trends Cardiovasc Med. 2002;12:160–5. doi: 10.1016/s1050-1738(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 24.Mao H, Lockyer P, Townley-Tilson D, Xie L, Pi X. LRP1 Regulates Retinal Angiogenesis by Inhibiting PARP-1 Activity and Endothelial Cell Proliferation. Arterioscler Thromb Vasc Biol. 2016;36:350–360. doi: 10.1161/ATVBAHA.115.306713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao H, Lockyer P, Li L, Ballantyne CM, Patterson C, Xie L, Pi X. Endothelial LRP1 regulates metabolic responses by acting as a co-activator of PPARgamma. Nat Commun. 2017;8:14960. doi: 10.1038/ncomms14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill SE, Taneja R, Rohan M, Wang L, Mehta S. Pulmonary microvascular albumin leak is associated with endothelial cell death in murine sepsis-induced lung injury in vivo. PLoS One. 2014;9:e88501. doi: 10.1371/journal.pone.0088501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–12. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 28.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–53. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyer L, Lockyer P, Wu Y, Saha A, Cyr C, Moser M, Pi X, Patterson C. BMPER Promotes Epithelial-Mesenchymal Transition in the Developing Cardiac Cushions. PLoS One. 2015;10:e0139209. doi: 10.1371/journal.pone.0139209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer L, Wu Y, Moser M, Patterson C. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev Biol. 2014;386:385–94. doi: 10.1016/j.ydbio.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–52. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 32.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–89. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, van Kerkhof P, Marzolo MP, Strous GJ, Bu G. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol Cell Biol. 2001;21:1185–95. doi: 10.1128/MCB.21.4.1185-1195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rooney JW, Sun YL, Glimcher LH, Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Mol Cell Biol. 1995;15:6299–310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himes SR, Coles LS, Reeves R, Shannon MF. High mobility group protein I(Y) is required for function and for c-Rel binding to CD28 response elements within the GM-CSF and IL-2 promoters. Immunity. 1996;5:479–89. doi: 10.1016/s1074-7613(00)80503-8. [DOI] [PubMed] [Google Scholar]
- 36.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 37.Shamanna RA, Hoque M, Lewis-Antes A, Azzam EI, Lagunoff D, Pe’ery T, Mathews MB. The NF90/NF45 complex participates in DNA break repair via nonhomologous end joining. Mol Cell Biol. 2011;31:4832–43. doi: 10.1128/MCB.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karmakar S, Mahajan MC, Schulz V, Boyapaty G, Weissman SM. A multiprotein complex necessary for both transcription and DNA replication at the beta-globin locus. EMBO J. 2010;29:3260–71. doi: 10.1038/emboj.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichman TW, Muniz LC, Mathews MB. The RNA binding protein nuclear factor 90 functions as both a positive and negative regulator of gene expression in mammalian cells. Mol Cell Biol. 2002;22:343–56. doi: 10.1128/MCB.22.1.343-356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakamoto S, Aoki K, Higuchi T, Todaka H, Morisawa K, Tamaki N, Hatano E, Fukushima A, Taniguchi T, Agata Y. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol Cell Biol. 2009;29:3754–69. doi: 10.1128/MCB.01836-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan D, Altan-Bonnet N, Parrott AM, Arrigo CJ, Li Q, Khaleduzzaman M, Li H, Lee CG, Pe’ery T, Mathews MB. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol Cell Biol. 2008;28:4629–41. doi: 10.1128/MCB.00120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao G, Shi L, Qiu D, Hu H, Kao PN. NF45/ILF2 tissue expression, promoter analysis, and interleukin-2 transactivating function. Exp Cell Res. 2005;305:312–23. doi: 10.1016/j.yexcr.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Nirula A, Moore DJ, Gaynor RB. Constitutive binding of the transcription factor interleukin-2 (IL-2) enhancer binding factor to the IL-2 promoter. J Biol Chem. 1997;272:7736–45. doi: 10.1074/jbc.272.12.7736. [DOI] [PubMed] [Google Scholar]
- 44.Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15:248–60. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coles E, Christiansen J, Economou A, Bronner-Fraser M, Wilkinson DG. A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development. 2004;131:5309–17. doi: 10.1242/dev.01419. [DOI] [PubMed] [Google Scholar]
- 46.Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–59. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- 47.Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, Kitamura T, Nosaka T, Sasai Y. Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev Biol. 2010;337:405–14. doi: 10.1016/j.ydbio.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133:4463–73. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- 49.Kamimura M, Matsumoto K, Koshiba-Takeuchi K, Ogura T. Vertebrate crossveinless 2 is secreted and acts as an extracellular modulator of the BMP signaling cascade. Dev Dyn. 2004;230:434–45. doi: 10.1002/dvdy.20069. [DOI] [PubMed] [Google Scholar]
- 50.Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–11. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- 51.Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–53. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helbing T, Herold EM, Hornstein A, Wintrich S, Heinke J, Grundmann S, Patterson C, Bode C, Moser M. Inhibition of BMP activity protects epithelial barrier function in lung injury. J Pathol. 2013;231:105–16. doi: 10.1002/path.4215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.