Abstract
Background
Population studies suggest that high body mass index (BMI) correlates with a reduced risk of death from lung cancer. The aim of our study was to evaluate definitively the influence of BMI on long term overall survival (OS) in surgical patients with non-small cell lung cancer (NSCLC).
Methods
Study population consisted of 1935 patients who underwent surgical resection for lung cancer at MD Anderson Cancer Center (2000-2014). Study variables included both patient and treatment related characteristics. Univariate and multivariate Cox regression analyses were performed to identify variables associated with overall survival.
Results
On univariate analysis, significant predictors of improved survival were higher BMI, pathologic tumor stage (stage I vs II, III, or IV), type of surgery (lobectomy/pneumonectomy versus wedge resection/segmentectomy), younger age, female gender, and adenocarcinoma histology (versus squamous) (all p<0.05, Table 2 and 3). Morbidly obese patients (BMI≥35) had a trend towards better outcomes than those classified as obese (BMI ≥30 and <35) (p=0.05), overweight (BMI ≥25 and <30) (p=0.13), or healthy weight (BMI <25) (p=0.37) (HR 0.727, 0.848, 0.926, and 1, respectively). On multivariate analysis, BMI remained an independent predictor of survival (p=0.02). Propensity matching analysis demonstrated significantly better OS (p=0.003) in patients with BMI≥30 as compared to BMI 25.
Conclusions
In this large, retrospective, single center series, after controlling for disease stage and other variables, higher BMI was associated with improved OS following surgical resection of NSCLC. Further studies are necessary to elucidate the precise relationship between BMI and treatment outcomes.
Introduction
Body mass index (BMI), a ratio of weight relative to height, measured in kilograms per meters squared of height has been correlated with various metabolic and cardiovascular diseases and cancer.1 While there are other measures of body composition, BMI has become a common tool in the estimation of health risk. Specifically, severe obesity, defined as BMI ≥ 40, has been associated with death rates from all cancers combined to be 52% higher for men and 62% higher for women, as compared to men and women of healthy weight.1 However, the relationship between high BMI and high risk of mortality from cancer is not consistent amongst different types of cancer. While obesity is associated with higher mortality rates from gastrointestinal malignancies (esophagus, stomach, gallbladder, pancreas, colon), prostate and breast cancer, obese individuals are less likely to die from renal cell cancer, diffuse large B cell lymphoma, and lung cancer.1 Especially in lung cancer, this paradoxically improved survival in patients with BMI 30-35 (relative risk of death RR 0.79, 0.73-0.86) and BMI >35 (RR 0.67, 0.54-0.84) remains largely unexplained.1 Whether this phenomenon represents simple protection from cancer related cachexia or if other molecular or metabolic mechanisms are engaged is yet to be determined.
In surgically resected lung cancer, two previous studies have focused on the analysis of short-term/peri-operative outcomes in patients with high BMI. In one study using National Surgical Quality Improvement Program database, high BMI did not negatively influence 30-day perioperative mortality, hospital length of stay, or perioperative complications after pulmonary resections.2 A similar study of 1369 patients treated surgically for lung cancer at a single institution also demonstrated similar perioperative outcomes despite varied range of BMIs.3
Long-term oncologic survival of lung cancer patients based on their BMI has been studied in patients with advanced lung cancer.4 In a study incorporating data from three cooperative group trials of first line chemotherapy in metastatic non-small cell lung cancer (NSCLC), obese patients were found to have longer overall survival than healthy or overweight patients in multivariate Cox models (HR=0.86, p=0.04; 95% CI: 0.75-0.99).4 One other study of 337 patients focused on long-term outcomes based on BMI in surgically resected lung cancer and found high BMI to have a protective effect.5 Recently, a meta-analysis of 25 surgical observational studies concluded that “obesity paradox” has a potential to exist in lung cancer surgery; this statement was based on the results, which demonstrated that obesity had favorable effect on both in-hospital outcomes as well as long-term survival of surgical patients with lung cancer
Our study aimed to contribute to prior work on this topic by evaluating the impact of BMI on overall survival (OS) in a large cohort of surgically resected NSCLC patients.).
Methods
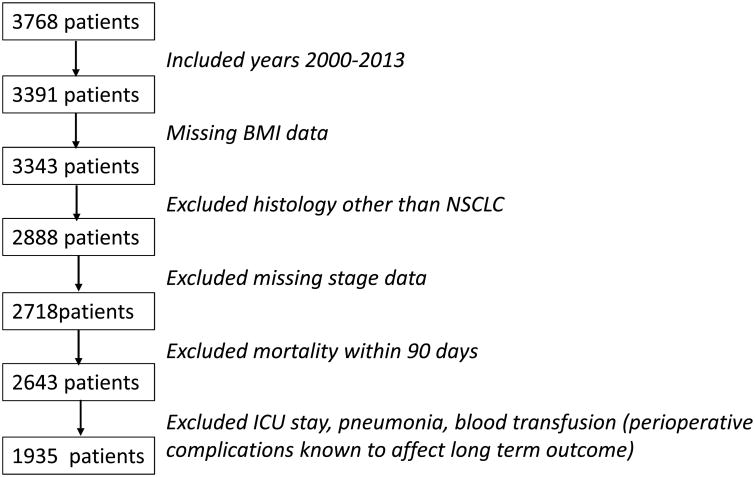
After obtaining the waiver of consent and approval for this retrospective study from the MD Anderson Cancer Center Institutional Review Board, a prospectively maintained thoracic surgical departmental database was queried for patients who underwent surgical resection for NSCLC between January 2000 and December 2013. Inclusion criteria consisted of patients with ages 18 through 85 who underwent wedge resection, segmentectomy, lobectomy, or pneumonectomy. Neoadjuvant or adjuvant chemotherapy or radiation therapy was allowed for the inclusion in the study. Only patients with non-small cell carcinoma histology (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, or NSCLC not otherwise specified) were included in the study. Considering the goal of studying long-term overall survival outcomes, we excluded patients whose long-term survival could be influenced by perioperative events, such as death within 90-days of operation, patients with intra-operative or post-operative blood transfusions, post-operative stay in the intensive care unit, or post-operative pneumonia. We also excluded patients with missing information about pathologic stage or BMI. The initial search identified 3768 patients from 1999-2013 in the database. After exclusions and limiting the series to years 2000-2013 the final study population consisted of 1935 patients (see CONSORT DIAGRAM, Figure 1). The reason for the above exclusion criteria was to minimize confounding variables, and to study the effect of BMI in a homogenous cohort of patients; patients who underwent uncomplicated surgical therapy for lung cancer.
Figure 1.
CONSORT Diagram of patient inclusion.
Collected study variables included patients' demographic characteristics, BMI, the type of operative procedure, the type of neoadjuvant and/or adjuvant therapy, tumor histology, pathologic stage, age, sex, smoking history, and the time from surgery to death or the last follow-up.
Statistics
The primary study outcome was OS of the entire patient population and patients' subgroups based on BMI. Four groups of patients based on their BMI were identified, BMI<25 (healthy), BMI 25-30 (overweight), BMI>30-35 (obese) and BMI>35 (morbid obesity). In the group with healthy BMI <25, we also included 20 patients with BMI <19 (underweight). In a separate preliminary analysis, these patients had similar outcomes to the group with BMI 20-25, and were therefore included together in the healthy BMI group. We initially performed univariate analysis to identify variables associated with survival; p value of <0.05 was considered statistically significant. Variables with p value <0.25 on univariate analysis were then tested in the multivariate Cox regression model. Survival analysis was performed using the method of Kaplan and Meier. Survival was calculated from the time of surgery to death or the last follow up. Patients who were alive were censored at the time of last follow up.
After we detected the difference in OS between the groups based on the BMI, we performed propensity score matching to learn whether the difference in survival between the groups persisted. The final Kaplan Meier analyses compared survival of patients with BMI<25 (healthy), BMI 25-30), BMI>30-35 and BMI>35 matched for variables significant on the multivariate analysis. Statistical analyses were performed using Version 23 SPSS program (IBM Corporation, 1 New Orchard Road Armonk, New York 10504-1722).
For an additional lung cancer survival analysis based on genes associated with obesity, we explored the obesity-related genes in The Cancer Genome Atlas (TCGA). Genetic and clinical data for lung adenocarcinoma and squamous cell carcinoma were downloaded from the TCGA database (http://www.cbioportal.org/). This was a separate dataset unrelated to the clinical data or tumor tissue for 1935 patients in this study.
Results
Demographic variables of the study population of 1935 patients are presented in Table 1. The median age was 66 years (range 18-88), and the study population was almost equally split based on sex (51% female, 49% male). In terms of the pathologic stage 59.4% (N=1149) of patients had stage I lung cancer, 22% (N=431) stage II lung cancer, 16% (N=299) stage III lung cancer and 2.9% (N=56) stage IV (oligometastatic) lung cancer. Stage I and II patients underwent surgical resection as the primary treatment modality. Adjuvant chemotherapy was indicated in stage II patients. Patients with clinically suspicious IIIA (N2) lung cancer underwent invasive mediastinal staging, and if N2 disease was confirmed in no more than single mediastinal station, patients received neoadjuvant chemotherapy followed by surgical resection. In terms of adjuvant chemotherapy, 11.2% (N=217) patients of the total 1935 patients had valid record of whether adjuvant chemotherapy was given and 70% (N=151) of these 217 patients received adjuvant chemotherapy. Due to the low ratio of patients with valid record of adjuvant chemotherapy, it is premature to analyze the effect of adjuvant chemotherapy on survival in this study. 11.2% Stage IV patients were treated with combination of therapies. In terms of multimodality, 81% (N=1568) of the total 1935 patients received preoperative treatment and 41% (N=23) of 56 stave IV patients received preoperative treatment. Lobectomy was performed in 1672 (86%) patients, segmentectomy in 103 (5%) patients, wedge resection in 95 (5%) patients and pneumonectomy in 65 (4%) patients. Most patients (65%, N=1252) had adenocarcinoma, with fewer squamous cell carcinoma (24%, N=472).
Table 1. Demographic and Clinical Variables.
| Age | Median 66 (18-88) | |
| BMI | <25 (Healthy) | 646 (33.4%) |
| ≥25 and <30 (Overweight) | 765 (39.5%) | |
| ≥30 and <35 (Obese) | 376 (19.4%) | |
| ≥35 (Moderately and Severely obese) | 148 (7.6%) | |
|
| ||
| Sex | Female | 984 (50.9%) |
| Male | 951 (49.1%) | |
|
| ||
| Stage | I | 1149 (59.4%) |
| II | 431 (22.3%) | |
| III | 299 (15.5%) | |
| IV | 56 (2.9%) | |
|
| ||
| Surgery type | Lobectomy | 1672 (86.4%) |
| Pneumonectomy | 65 (3.3%) | |
| Wedge resection | 95 (4.9%) | |
| Segmentectomy | 103 (5.3%) | |
|
| ||
| Histology | Adenocarcinoma | 1252 (64.7%) |
| Squamous Cell Carcinoma | 472 (24.4%) | |
| Other | 211 (10.9%) | |
| Smoking | Recent or former smokers | 1551 (80%) |
Patients were divided into four groups based on their BMI: healthy BMI <25 (33%, N=646), overweight BMI ≥25 and <30 (40%, N=765), obese BMI ≥ 30 and <35 (19%, N=376), and severely obese BMI ≥35 (8%, N=148).
On univariate analysis, only obesity (BMI>35) was associated with improved OS (p=0.05, OR 0.727 0.526-1.005). Variables associated with poor OS included increasing disease stage, pneumonectomy, wedge resection, squamous cell carcinoma histology, smoking, and male sex (Table 2). When BMI was tested as a continuous variable, a significant (p<0.01) association was observed with survival with OR 0.98, implying that for every one unit increase in BMI, there was 2% improvement in overall survival (Table 2). In a multivariate Cox regression model, increasing BMI remained as a significant independent predictor of survival (p=0.03, OR 0.977) (Table 3). Other factors associated with survival in the multivariate model included age, male sex, wedge resection, segmentectomy, and smoking (Table 3).
Table 2. Univariate Cox Model (hazard ratios represent the risk of death).
| Overall Survival HR (95% CI) | P-Value | ||
|---|---|---|---|
| Age | Continuous variable | 1.019 (1.011-1.027) | <0.01 |
|
| |||
| Sex | Female (Reference) | 1.000 | <0.01 |
| Male | 1.304 (1.125-1.511) | ||
| Smoking | No (Reference) | 1.00 | <0.01 |
| History | Yes | 1.471 (1.192-1.814) | |
| Diabetes | No (Reference) | 1.00 | 0.162 |
| Yes | 1.179 (0.936-1.484) | ||
|
| |||
| Stage | I (Reference) | 1.000 | <0.01 |
| II | 1.843 (1.538-2.206) | <0.01 | |
| III | 2.452 (2.026-2.967) | <0.01 | |
| IV | 3.258 (2.319-4.576) | <0.01 | |
|
| |||
| Surgery type | Lobectomy | 1.000 | <0.01 |
| Pneumonectomy | 2.168 (1.533-3.066) | <0.01 | |
| Segmentectomy | 1.152 (0.833-1.594) | 0.392 | |
| Wedge resection | 2.061 (1.56-2.718) | <0.01 | |
|
| |||
| Histology | Adenocarcinoma (Reference) | 1.000 | |
| Squamous Cell Carcinoma | 1.314 (1.114-1.551) | <0.01 | |
| Other | 0.928 (0.726-1.187) | 0.55 | |
|
| |||
| BMI | Continuous variable (per 1 unit) | 0.980 | <0.01 |
|
| |||
| BMI | <25 (Reference) | 1.000 | |
| ≥25 and <30 | 0.926 (0.782-1.095) | 0.37 | |
| ≥30 and <35 | 0.848 (0.684-1.052) | 0.13 | |
| >35 | 0.727 (0.526-1.005) | 0.05 | |
Table 3. Multivariate Cox Model (hazard ratios represent the risk of death).
| Overall Survival HR (95% CI) | P-Value | ||
|---|---|---|---|
| Age | Continuous variable | 1.024 (1.015-1.032) | <0.01 |
|
| |||
| Sex | Female (Reference) | 1.000 | |
| Male | 1.236 (1.061-1.441) | <0.01 | |
|
| |||
| Stage | I (Reference) | 1.000 | |
| II | 1.888 (1.571-2.269) | ||
| III | 2.743 (1.942-3.875) | ||
| IV | 2.662 (1.855-3.821) | ||
|
| |||
| Surgery type | Lobectomy (Reference) | 1.000 | <0.01 |
| Segmentectomy | 1.474 (1.061-2.047) | <0.02 | |
| Wedge resection | 1.848 (1.376-2.481) | <0.01 | |
| Pneumonectomy | 1.478 (1.033-2.115) | 0.03 | |
|
| |||
| Pre-op therapy | No (Reference) | 1.000 | <0.01 |
| Yes | 1.403 (1.164-1.692) | ||
|
| |||
| Histology | Adenocarcinoma (Reference) | 1.000 | |
| Squamous Cell Carcinoma | 1.225 (1.035-1.451) | 0.02 | |
| Other | 0.959 (0.747-1.231) | 0.74 | |
|
| |||
| BMI | Continuous (per 1 unit) | 0.977 (0.962-0.992) | 0.03 |
| BMI | <25 Reference | 1.00 | |
| >=25 | 0.841 (0.719-0.984) | 0.03 | |
| Smoking | No (Reference) | 1.00 | |
| Yes | 1.358 (1.095-1.684) | <0.01 | |
The initial Kaplan Meier analyses demonstrated significant differences in survival in patients with BMI <25 compared with BMI ≥ 30 and BMI ≥35. Based on these findings, we performed propensity-score matching analysis of patients with BMI <25 and patients with BMI ≥30, and BMI ≥35 (Figure 2). A total of 464 patients in each group were matched on the following variables: age, sex, type of surgery, histology, pathologic stage, and smoking. Kaplan Meier analyses of propensity-score matched patients demonstrated significantly better OS in patients with BMI ≥30 compared to patients with BMI <25 and even better OS in patients with BMI ≥35 (p=0.003, Figure 2).
Figure 2.
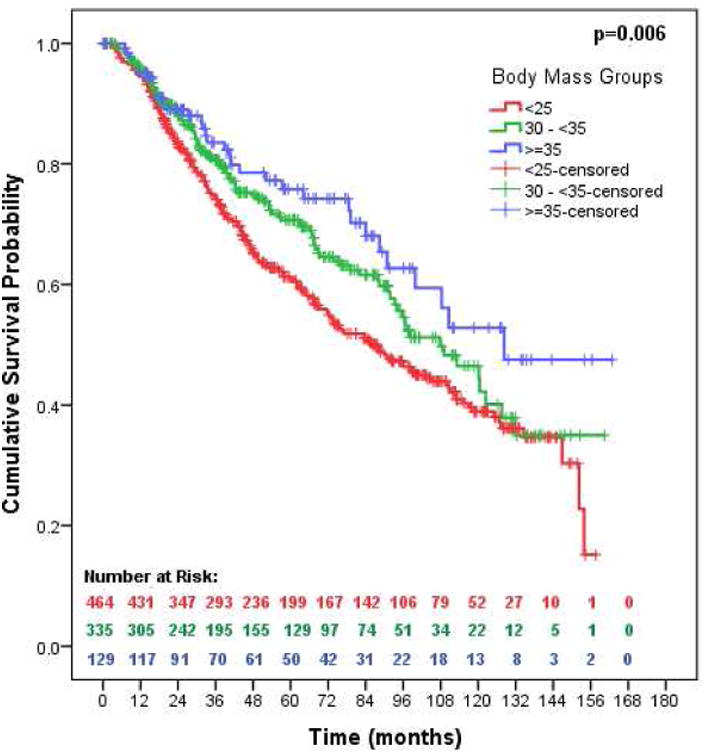
Overall survival based on propensity matching. 464 patients with BMI <25, 335 patients with BMI 30-35, and 129 patients with BMI >35 were matched on age, sex, type of surgery, histology, smoking, and pathologic stage. Alive patients were censored at the time of the last follow up.
For the exploratory analysis of genes associated with obesity and lung cancer survival we further analyzed the association between obesity genes (LEP, LEPR, PCSK1, POMC, MC4R, BMIQ1, BMIQ2, BMIQ3, UCP2, BMIQ5, BMIQ6, INSIG2, FTO, TMEM18, GNPDA2, NEGR1, BDNF, KCTD15, SH2B1, MTCH2 and NPC1)9 and lung cancer survival from a separate Cancer Genome Atlas data set (TCGA). The TCGA is an independent and centralized database with genomic and genetic profiles of various cancer patients, including lung cancer. The only significant difference in OS we detected on exploratory Kaplan Meier analyses in this TCGA cohort was in the group high Uncoupling Protein 2 (UCP2) expression.
Discussion
Our retrospective analysis of 1935 lung cancer patients from a single center demonstrates that patients with BMI ≥30, classified as obese, experience superior OS to patients with healthy (BMI <25).
These results are consistent with the previous reports evaluating the role of BMI in OS in advanced NSCLC4 as well as in surgically resected lung cancer.5,6
In the study by Dahlberg et al, authors combined data from three phase III clinical trials of first-line systemic chemotherapy for stage IIIB, stage IV or recurrent NSCLC. They analyzed OS for 2585 patients based on their BMI and found that within the first 16 months patients with obese range BMI experienced superior survival to non-obese patients (HR 0.86). This trend, however, was reversed at 16 months (HR 1.54).4 In our study, the observation of improved outcome in the high BMI group persisted throughout the study follow up period (mean follow up time 45.16 months). Although authors did not provide an explanation for the early survival benefit in obese patients, they postulated that the synergy between perixome proliferator-activated receptor ligands such as fatty acids, anti-diabetic drugs, and platinum chemotherapy could provide a biological basis for reasoning behind their observation.4 A group from Liverpool (UK) reported on outcomes of 337 propensity matched patients with BMI >30 and BMI <30 who underwent surgical resection for lung cancer. Similar to our study, authors observed significantly improved OS in patients with BMI ≥30, as compared to patients with BMI<30.5
Recently, Li et al conducted a review and meta-analysis of the prognostic role of BMI for patient undergoing lung cancer surgery.6 In the review they identified 25 observational studies with 78,143 patients who were included in the analysis. With respect to long-term survival obesity was associated with favorable long-term prognosis with OR 0.78 (0.63-0.98, p=0.031). Authors concluded that “obesity paradox” does have the potential to exist in the lung cancer surgery and offered some interesting hypotheses for possible mechanisms underlying obesity paradox.6 They suggested that obesity may be more prevalent in younger patients, these patients are more likely to withstand surgical trauma, and may recover better. Another hypothesis suggests that obese patients have more medical problems and therefore seek medical attention sooner, and are therefore better medically managed than patients with healthy BMI, who may avoid medical care. They also postulate that obese patient have better nutritional status, which leads to their better longevity post-surgical lung cancer therapy. Lastly, they suggest that it is the undernourished patients who do poorly rather than obese patients who do better.6 While all these hypotheses may have some merit, they remain speculative.
Another study combining large datasets of lung cancer patients focused on the relationship between survival and obesity or weight loss in 76,086 patients of which 14,751 presented with obesity and/or weight loss.7 In this large population study, obese patients at the time of lung cancer diagnosis demonstrated longer median survival relative to non-obese patients (13.0 versus 8.6 months, p<0.001). This observation was significant across all stages and histologic subtypes. Authors hypothesized that the protective effect of obesity in lung cancer survival might relate to greater physiologic reserve, and life prolongation due to avoidance of cancer related cachexia.6 While the death from cancer cachexia may sound logical, in reality there is no clear evidence that cancer related cachexia is the major mode of mortality in patients had lung cancer. Conversely a study by Ross et al found that weight loss as a symptom of lung cancer predicted chemotherapy treatment toxicity and was associated with shortened survival.8 Patients who experienced weight loss were less likely to finish three cycles of chemotherapy, and developed significantly more anemia.8 A small number of studies investigate possible cellular mechanisms for lung cancer-related cachexia. A small study by Murton et al found lung cancer cachexia patients with high mRNA levels of interleukin 6 and low intramyocellular lipid content in slow twitch fibers.9 Fukawa et al studied fatty acid oxidation and muscle atrophy in cancer related cachexia in vitro and in vivo.10 They found cachectic cancer cells secret inflammatory factors and rapidly induce excessive fatty acid oxidation in myotubules, which causes oxidative stress and muscle atrophy.10 Could the protective survival effect of higher BMI be due to larger fatty acid storage? This hypothesis would require further exploration.
With the available data from TCGA database, we sought to explore the genetic connection behind the correlation of high BMI and better lung cancer survival. Most genetic abnormalities in lung cancer tissue are somatic change while the genetic predisposition to obesity is germline, which makes the association analysis of obesity and lung cancer more complex. In addition, the genetic abnormalities related to lung cancer survival are usually studied in cancer tissue while those related with obesity are usually studied in other tissues.
We examined the association between genes associated with obesity and survival in the TCGA dataset (11). The chief genes of interest from the TCGA were (LEP, LEPR, PCSK1, POMC, MC4R, BMIQ1, BMIQ2, BMIQ3, UCP2, BMIQ5, BMIQ6, INSIG2, FTO, TMEM18, GNPDA2, NEGR1, BDNF, KCTD15, SH2B1, MTCH2 and NPC1)11 The TCGA dataset showed significantly better OS in patients with high Uncoupling Protein 2 (UCP2) expression. UCP2 belongs to the mitochondrial uncoupling protein family and was reported to suppress mitochondrial reactive oxygen species (ROS) production. However, recent studies showed that UCP2 promotes fatty acid oxidation and limits glycolysisderived pyruvate utilization. As a consequence, upregulation of UCP2 inhibits proliferation of cancer cells.12,13
We recognize that our study has limitations, mainly related to its retrospective nature. Considering the fact that our cohort consists mainly of surgical patients, selection bias may play a role in our results. However, other confounding variables could not be controlled and might have influenced our results. For example, patients' metabolic profiles (lipid level, glucose level and all other metabolic parameters that were measured in clinic) were not well studied, which could potentially provide hypothesis for future study. Another limitation of our study is the lack of valid information of adjuvant chemotherapy (only 11.2% patients had valid record of adjuvant chemotherapy), which limit our ability to analyze how adjuvant therapy affect patients survival. This should be addressed in future study.
Our results independently confirmed the study by Attaran and Li,5,6 and strengthened the argument that the obesity paradox in surgically resected lung cancer patients is a consistent clinical feature of human lung cancer. In our study, we elected to exclude patients with peri-operative mortality or major complications to avoid confounding factors known to influence long term survival. We also excluded patients with perioperative blood transfusions, since the meta-analysis by Luan et al demonstrated worse oncologic outcomes in lung cancer patients following peri-operative blood transfusion.14 In addition to the factors discussed, we also compared the follow up time in each BMI group. In our study, the median follow-up time is 45.16 months and there was no statistic significant difference between all BMI groups (p=0.5).
Interestingly, we found that BMI is an independent variable in prediction of lung cancer patients' survival after surgery. We also analyzed recent or former smokers in our cohort (80% patients) and found the same conclusion. This observation is important, since in the large study by Calle et al the inverse association between BMI and mortality seemed to have disappeared in female non-smokers,1 while in our study, BMI is independent of patients' gender and smoking history. Future study in non-smokers patients is needed to understand this.
In summary, in this large, single center retrospective series of lung cancer patients, after controlling for disease stage and other variables, BMI >30 was associated with improved OS following surgical resection of NSCLC. Further studies are necessary to confirm these findings and to define the molecular or metabolic mechanisms linking BMI and treatment outcomes.
Acknowledgments
Financial Support: This study was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) grants R01-CA087546 (ED) and R01-CA190722 (ED), a Samuel Waxman Cancer Research Foundation Award (ED), a UT-STARs award (ED), an American Cancer Society Clinical Research Professorship (ED).
Dr. Gold reports grants from Pharmacyclics, personal fees from Ariad Pharmaceuticals, personal fees from Genentech, non-financial support from Astra Zeneca, outside the submitted work.
Footnotes
The study was presented at World Lung Cancer Conference, Denver, CO, September, 2015
Disclosure: All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Mungo B, Zogg CK, Hooker CM, Yang SC, Battafarano RJ, Brock MV, Molena D. Does obesity affect the outcomes of pulmonary resections for lung cancer? A National Surgical Quality Improvement Program analysis. Surgery. 2015 Apr;157(4):792–800. doi: 10.1016/j.surg.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson MK, Im HK, Watson S, Johnson E, Wigfield CH, Vigneswaran WT. Association of body mass index and outcomes after major lung resection. Eur J Cardiothorac Surg. 2014 Apr;45(4):e94–9. doi: 10.1093/ejcts/ezu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, Johnson DH. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013 Sep;8(9):1121–7. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attaran S, McShane J, Whittle I, Poullis M, Shackcloth M. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. 2012 Oct;42(4):653–8. doi: 10.1093/ejcts/ezs135. Epub 2012 Apr 19. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Wang Z, Huang J, Fan J, Du H, Liu L, Che G. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg. 2016 Dec 30; doi: 10.1093/ejcts/ezw386. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011 Sep;170(1):e75–83. doi: 10.1016/j.jss.2011.04.061. Epub 2011 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O'Brien ME. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004 May 17;90(10):1905–11. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murton AJ, Maddocks M, Stephens FB, Marimuthu K, England R, Wilcock A. Consequences of Late-Stage Non-Small-Cell Lung Cancer Cachexia on Muscle Metabolic Processes. Clin Lung Cancer. 2016 Jun 25; doi: 10.1016/j.cllc.2016.06.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama HO, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan KK, Ho YS, Tan IB, Teh BT, Shyh-Chang N. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med. 2016 Jun;22(6):666–71. doi: 10.1038/nm.4093. [DOI] [PubMed] [Google Scholar]
- 11.Xia Q, Grant SFA. The genetics of human obesity. Ann NY Acad Sci. 2013 Apr;1281(1):178–190. doi: 10.1111/nyas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, RicquierD, Miroux B, Thompson CB. Uncoupling protein-2 controls proliferation bypromoting fatty acid oxidation and limiting glycolysis-derived pyruvateutilization. FASEB J. 2008 Jan;22(1):9–18. doi: 10.1096/fj.07-8945com. Epub 2007 Sep 13. [DOI] [PubMed] [Google Scholar]
- 13.Esteves P, Pecqueur C, Alves-Guerra MC. UCP2 induces metabolic reprogramming to inhibit proliferation of cancer cells. Mol Cell Oncol. 2014 Dec 1;2(1):e975024. doi: 10.4161/23723556.2014.975024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luan H, Ye F, Wu L, Zhou Y, Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014 May 23;14:34. doi: 10.1186/1471-2482-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]