Abstract
Immunological identity is traditionally defined by genetically encoded antigens, with equal maternal and paternal contributions as a result of Mendelian inheritance. However, vertically transferred maternal cells also persist in individuals at very low levels throughout postnatal development. Reciprocally, mothers are seeded during pregnancy with genetically foreign fetal cells that persist long after parturition. Recent findings suggest that these microchimeric cells expressing noninherited familially relevant antigenic traits are not accidental souvenirs of pregnancy, but are purposefully retained within mothers and their offspring to promote genetic fitness by improving the outcome of future pregnancies. Here, we discuss the immunological implications, benefits and potential consequences of individuals being constitutively chimeric with a biologically active ‘microchiome’ of genetically foreign cells.
The unique composition of genetically encoded antigenic traits in each individual has traditionally been used to define their immunological identity [G]. This binary classification of antigens as either ‘self’ or ‘non-self’ has established a conceptual framework for evaluating how the adaptive immune system responds to microbial infection, or to antigen stimulation in other contexts such as immunization or transplantation1,2. Importantly, however, the classical immunological tenets of self-tolerance, which have been based primarily on the analysis of highly inbred animal strains, do not properly reflect the genetic diversity in outbred populations, where each individual contains a distinct immunological signature defined by unique MHC haplotypes and other minor alloantigens. This limitation of investigating tolerance exclusively using genetically identical inbred animals is magnified when addressing the immunological shifts that occur during pregnancy, when expanded tolerance to genetically discordant fetal tissue is likely to be essential for successful reproduction.
The physiological exposure of individuals to foreign antigens during pregnancy and early life development has been used to establish working models of immunological identity and tolerance. In the 1940s, Ray Owen recognized the plasticity of immune tolerance, which naturally extends beyond genetically encoded self-antigens, based on experiments showing expanded blood group compatibility among dizygotic twin cattle with mixed circulatory systems during in utero development3. In the 1950s, Sir Peter Medawar articulated the immunological conundrum that is associated with viviparity [G], by describing the contrast between rapid rejection of allogeneic skin grafts compared with the persistence of fetal tissues in mothers during pregnancy4. Thus, pregnancy activates unique adaptations in mothers for maintaining fetal tolerance [G]. Given the dominant role of reproductive fitness in trait selection, adaptations that reinforce fetal tolerance and promote maternal well-being are likely to be engrained within the reproductive process through refining positive selection. Accordingly, we propose that further dissecting how maternal–fetal conflict is averted has exciting potential to reveal not only new strategies for improving pregnancy outcomes, but also fundamental insights into how immune tolerance works in other biological contexts.
Many of the known mechanisms that maintain fetal tolerance function at the maternal–fetal interface, including the production of immunosuppressive molecules, exclusion of immune cells through chemokine gene silencing, reduced complement deposition and entrapment of professional antigen-presenting cells, have been recently summarized and are not discussed further here (see REFS 5–7). However, given the limited macroscopic anatomical distribution of fetal tissues in women during pregnancy, it remains unclear why systemic immunological changes are also involved. In this context, a remarkable, but somewhat underappreciated, aspect of mammalian pregnancy is the bidirectional transfer and systemic seeding of small numbers of genetically foreign cells, termed microchimeric cells [G], between mother and offspring.
Beginning early in pregnancy, fetal cells are found in the maternal blood and tissues, with the number of these cells progressively increasing until term8,9. Reciprocally, maternal cells are found in human fetal tissues beginning in the second trimester of pregnancy10,11. Perhaps more remarkable is the long-term persistence of these genetically discordant fetal cells in mothers many years after pregnancy, and the retention of maternal cells in offspring throughout postnatal development into adulthood12,13. Despite near uniform agreement that all individuals contain these microchimeric cells, surprisingly little is known regarding their biological function and molecular properties. These knowledge gaps primarily stem from the lack of tools for experimental manipulation and consistent identification of these exceptionally rare cells (Table 1). Nonetheless, fetal microchimerism (FMC) and maternal microchimerism (MMC) have been increasingly shown to occur for various hematopoietic, undifferentiated and tissue restricted cell types (Supplementary information S1 (Table)). Interestingly, recent findings suggest that these microchimeric cells are not accidental souvenirs of pregnancy, but instead are purposefully retained to help promote the success of future pregnancies14. Thus, further investigating the fundamental biology of microchimeric cells, including their origins and the mechanisms by which they evade immunological rejection, has the potential to redefine immunological identity to also include genetically foreign, but familially relevant antigenic traits. Here, we discuss accumulating evidence regarding the persistence, cellular identity and molecular phenotype of microchimeric cells, their potential biological benefits and harmful consequences, and the broader immunological implications of considering individuals as being constitutively chimeric.
Table 1.
Current methods for identifying genetically foreign microchimeric cells
| Technique | Target | Advantages | Disadvantages |
|---|---|---|---|
| DNA amplification by polymerase chain reaction (PCR) |
|
|
|
| Fluorescence in situ hybridization (FISH) |
|
|
|
| Flow cytometry |
|
|
|
Expanded immune tolerance during pregnancy
For placental mammals, prolonged in utero maturation allowing more devoted and purposeful investment to each concepti promotes the survival of offspring, while minimizing wasted allocation of resources to non-surviving offspring. However, the close physical approximation of maternal and fetal tissues during in utero maturation also highlights the immunological conundrum for why maternal immune cells do not reject genetically foreign fetal tissues, and how fetal immune components with compulsory exposure to maternal tissues adapt to genetically foreign noninherited maternal antigens (NIMAs) [G]. Here, we discuss expanded immune tolerance that averts maternal-fetal conflict during pregnancy from the perspective of microchimeric cells.
Maternal tolerance to paternal–fetal antigens
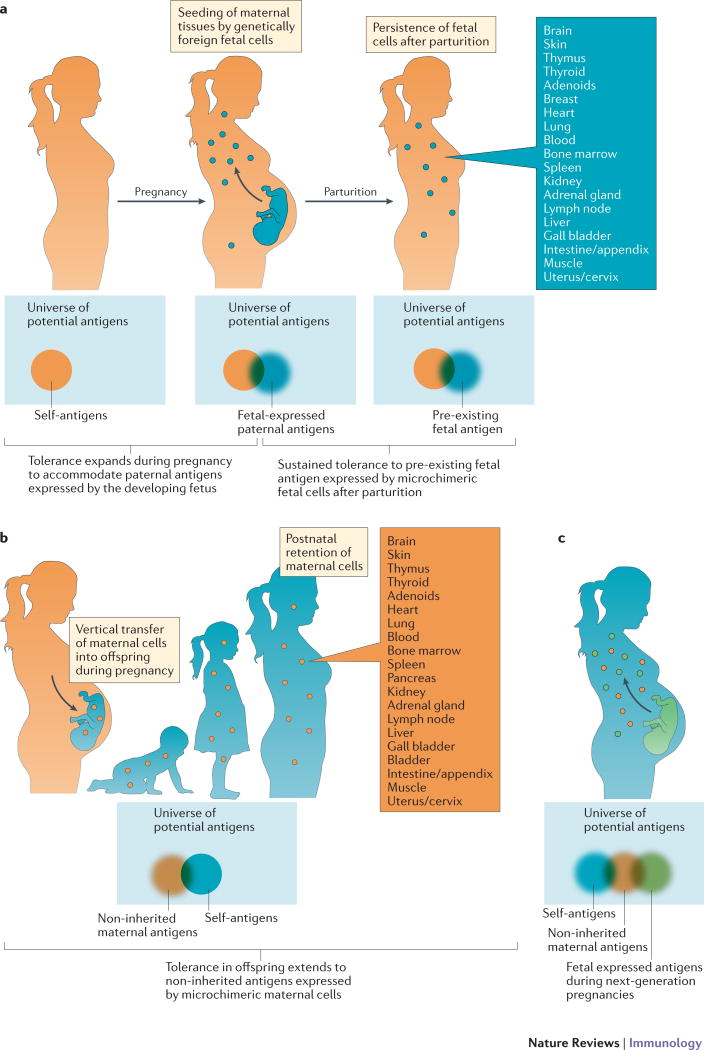
Despite the many localized mechanisms at the maternal–fetal interface for averting immune rejection of the high density of fetal cells that are contained within the pregnant uterus5–7, potent immunological changes also occur systemically in women during pregnancy. For example, the severity of autoimmune disorders that affect non-reproductive tissues, such as rheumatoid arthritis and multiple sclerosis, markedly improves for women during pregnancy15,16. Similarly, the serological response to inactivated influenza vaccine is blunted during human pregnancy17,18. These systemic shifts in immune reactivity that occur during pregnancy probably extend to maternal immune components with specificity for fetal-expressed antigens given the FMC that results from widespread seeding and retention of genetically foreign fetal cells in the peripheral tissues of mothers (Fig. 1a).
Fig. 1. Pregnancy imprints expanded immune tolerance in mothers and offspring.
(a) Among the universe of all possible antigens to which the immune system can theoretically respond, immune tolerance expands in mothers during pregnancy beyond her own genetically encoded self-antigens (orange), to encompass genetically foreign paternal antigens expressed by the developing fetus (blue). This coincides with the widespread seeding of maternal tissues by genetically foreign fetal cells during pregnancy. Long-term persistence of these fetal cells after parturition suggests that expanded tolerance is maintained in mothers to include fetal antigens from prior pregnancy. (b) Immune tolerance expands in offspring beyond genetically encoded self-antigens (blue), to also include genetically foreign non-inherited maternal antigens (NIMAs) (orange). This begins with the vertical transfer of maternal cells into offspring during in utero development. The long-term postnatal retention of these genetically foreign maternal cells in the tissue of offspring is indicative of persistently expanded tolerance to NIMAs. (c) Immune tolerance further expands during next-generation pregnancies in female offspring beyond her own genetically encoded self-antigens (blue) and noninherited antigens from her own mother (orange), to include genetically foreign paternal antigens expressed by the developing fetus (green).
Early studies using various methods (Table 1) to identify fetal microchimeric cells that express unique paternally derived markers showed that fetal cells are present amongst maternal peripheral blood mononuclear cells (PBMCs) at a frequency of 1 in 103 cells by 14–15 weeks of gestation19, and that fetal cells can be detected in maternal blood as early as 7 weeks gestation before complete establishment of the fetal placental vasculature20. The number of fetal cells in maternal blood progressively increases throughout pregnancy, reaching peak levels of more than 100 fetal cells per mL of maternal blood at parturition20,21. Similarly, fetal DNA-containing cells have been found in the cadaveric lung, spleen, liver, kidney and heart tissues of women during pregnancy8. Importantly, fetal microchimeric cells are intact cells and represent a completely distinct source of fetal DNA from the placenta-derived, cell-free fetal DNA in the serum of mothers during pregnancy that is increasingly being used for prenatal analysis.
The transfer of fetal cells to mothers during pregnancy is highly conserved, and is thought to occur in all placental mammalian species22. For rhesus macaque non-human primates, fetal cells are found among maternal PBMCs during the second and third trimesters of pregnancy, and in various maternal tissues including the heart, liver and spleen at the time of term delivery23. Similarly in mice, fetal cells of many unique cell types are found amongst multiple maternal tissues (Supplementary information S1 (Table)), beginning shortly after implantation and reaching a peak level of 1 fetal cell per 104–106 maternal cells by late gestation9,24.
The progressively increasing mass and number of fetal cells across maternal tissues during pregnancy correlates with the systemic expansion of immunosuppressive, CD4+ regulatory T (Treg) cells25. Circulating maternal Treg cells begin to accumulate in mice the day after conception and their numbers increase by approximately two-fold by midgestation during healthy pregnancies25–27. By contrast, fetal wastage is associated with defective maternal Treg cell expansion in abortion-prone matings between defined strains of inbred mice, or a reduction in the suppressive capacity of Treg cells on a per cell basis triggered by prenatal infection28–30. Allogeneic pregnancies [G] promote a greater expansion of maternal Treg cell populations than syngeneic pregnancies, which highlights the importance of fetal–maternal MHC mismatch in driving maternal Treg cell accumulation26,27. The modest expansion of maternal Treg cell populations that does occur during syngeneic pregnancies is thought to reflect maternal mismatch to Y-chromosome-expressed fetal alloantigens31, or the response to self-antigen stimulation32, and may coincide with the increased need for tissue repair and homeostasis during pregnancy33,34. By investigating pregnancy outcomes in mice after partial depletion of maternal Treg cells to pre-pregnancy levels, it was confirmed that a sustained increase in maternal Treg cell numbers is necessary to avert fetal wastage in allogeneic but not syngeneic pregnancies27,35. Interestingly, blunted systemic expansion and decidual accumulation of maternal Treg cell populations are also associated with complications in human pregnancy such as preeclampsia and spontaneous abortion25, although there is no clear-cut evidence that this is not simply correlative. Thus, the expanded immune tolerance during pregnancy in mothers that is required to accommodate foreign paternal–fetal antigens, both locally in the uterus and systemically seeded fetal microchimeric cells, parallels the systemic accumulation of maternal Treg cells, although further work is required to establish the necessity of expanded maternal Treg cells in human pregnancy.
Antigen-specific tools that track how mothers uniquely respond to stimulation with fetal-expressed antigens show that pregnancy-induced immunological changes are most pronounced for maternal immune components with paternal–fetal specificity27,29,35,36. For example, during pregnancies sired by transgenic male mice that express defined model antigens, most fetal-specific CD8+ T cells in the mother undergo clonal deletion and do not acquire cytotoxic effector properties despite extensive proliferation36. The remaining fetal-specific CD8+ T cells express low levels of the chemokine receptor CXCR3, and are restricted from accessing the maternal–fetal interface through epigenetically silenced expression of the chemokine ligands CXCL9 and CXCL10 by decidual stromal cells37,38.
By contrast, maternal CD4+ T cells with specificity for fetal antigens proliferate and preferentially differentiate into Treg cells, as demonstrated by their increased FOXP3 expression35. The necessity for fetal-specific Treg cells for successful pregnancy in mice is demonstrated by the fetal loss that occurs after blocking Treg cell differentiation selectively amongst maternal CD4+ T cells with fetal specificity39. The accumulation of fetal-specific FOXP3+CD4+ T cells is primarily driven by peripherally induced Treg cells [G], rather than by the proliferation of thymus-derived Treg cells, as FOXP3− donor CD4+ T cells transferred to female mice prior to pregnancy readily proliferate and upregulate FOXP3 expression with fetal antigen stimulation35. Similarly, fetal wastage occurs in mice with targeted deletion of the CNS1 enhancer, which is required for induced FOXP3 expression40. Interestingly, CNS1 is highly conserved across placental mammals, but is absent in marsupials and egg-laying mammals, which suggests that viviparity may have co-evolved with the capacity for peripherally induced Treg cell differentiation40. Thus, considering that humans share with other placental mammals such as mice the need to avert maternal–fetal immunological conflict during pregnancy, animal pregnancy models allowing for experimental manipulation are invaluable tools for investigating how immune tolerance functions during the reproductive process, although species-specific differences limit the direct translation of these findings to human pregnancy.
Fetal tolerance to noninherited maternal antigens
In addition to maternal exposure to fetal antigens, the fetus is reciprocally exposed to an equally vast array of genetically foreign NIMAs during in utero maturation. Nascent T cells begin seeding human fetal lymphoid tissue by gestational week 10, and they progressively accumulate throughout in utero development41. However, immunological attack of and damage to genetically foreign maternal tissues does not occur even though fetal T cells are capable of alloantigen-induced proliferation11. Instead, fetal CD4+ T cells preferentially undergo Treg cell differentiation in response to NIMA stimulation, and this expanded fetal Treg cell pool is thought to avert fetal–maternal conflict by suppressing NIMA-specific effector T cells11.
Although the factors during in utero development that are responsible for this shift from T cell sensitization to tolerance remain largely undefined, an interesting parallel occurrence is the vertical transfer and persistence of genetically discordant microchimeric maternal cells in offspring (Fig. 1b). For example, cells with two X chromosomes, which are therefore presumed to be of maternal origin, are consistently found in the cord blood of human male offspring42. Furthermore, maternal cells have been identified in various human fetal tissues (REFS 10,11,43). Interestingly in humans, the kinetics of maternal cell seeding in the fetus parallels maturation of the fetal thymus and peripheral lymphoid tissues41,44, which suggests that vertically transferred maternal cells might have a role in delivering NIMAs to developing fetal immune cells for priming tolerogenic responses.
The vertical transfer of maternal cells to offspring is widely conserved across placental mammalian species, despite marked differences in the maturation state of fetal adaptive immune cells at the time of birth41,45,46. In rodent offspring, where peripheral B and T cells are not detected until after birth, maternal cells are nonetheless present in the fetus by midgestation and progressively accumulate in various fetal tissues47,48. Accordingly, although vertically transferred maternal cells are an important source of NIMAs that have been implicated in averting fetal–maternal conflict in humans and other species that have functional fetal adaptive immune cells at birth11,41, this explanation is probably incomplete since maternal cells are similarly transferred to offspring in species where adaptive immune cells do not develop until after birth. Thus, the highly conserved nature of pregnancy-induced microchimerism across mammalian species suggests that additional biological advantages aside from supporting fetal–maternal tolerance during pregnancy promote the maintenance of these phenomena during reproduction.
Persistent immune tolerance after parturition
Despite brisk anatomical separation of fetal and maternal tissues at the time of birth, expanded immune tolerance primed by pregnancy persists in both mothers and offspring. This may explain why vertically transferred maternal cells are retained in offspring throughout postnatal development, and why fetal cells persist in mothers decades after giving birth, even though the rarity of these cells may preclude their consistent identification in more limited tissue samplings from some individuals. Here, we discuss how pregnancy imprints expanded bidirectional tolerance in mothers and their offspring in relation to the persistence of microchimeric cells.
Postnatal tolerance to noninherited maternal antigens
Postnatal NIMA-specific tolerance in humans gives rise to some remarkable immunological phenotypes, including reduced sensitization to erythrocyte Rhesus factor (Rh) antigens among Rh-negative women born to Rh-positive mothers49, and suppressed serological sensitization to noninherited maternal HLA antigens in transfusion-dependent individuals who develop antibodies against almost all other HLA alloantigens50. In a groundbreaking analysis of the outcomes of kidney allografts, long-term graft survival was shown to be significantly improved in NIMA-matched sibling donor–recipient pairings51. The severity of graft-versus-host disease (GVHD) after bone marrow transplantation is also reduced in recipients of NIMA-matched donor stem cells52,53. Postnatal persistence of NIMA-specific tolerance is similarly recapitulated in animals. For example, long-term survival of cardiac H-2d allografts occurs in recipient mice exposed to H-2d as an NIMA, compared with the rapid rejection of these grafts in recipient mice without developmental H-2d exposure45. Likewise, the severity of GVHD is reduced in mice after engraftment of allogeneic stem cells from donor mice with developmentally matched NIMA exposure54.
Animal cross-fostering studies and parental breastfeeding surveys of human transplant recipients show the improved survival of NIMA-matched allografts is overturned when postnatal NIMA exposure through breastfeeding is eliminated, which indicates that breastfeeding is essential for the maintenance of NIMA-specific tolerance in offspring45,55. However, postnatal ingestion of maternal cells or soluble maternal MHC molecules alone is insufficient to prime NIMA-specific tolerance, as the survival of NIMA-matched donor allografts does not improve in mice without in utero NIMA exposure45,56–58. Thus, NIMA-specific tolerance requires exposure to maternal cells both prenatally and postnatally through breastfeeding.
The highly conserved postnatal persistence of MMC across species suggests that important teleological benefits may promote the maintenance of NIMA-specific tolerance in offspring11,45,46. In this regard, although the vertical transfer of maternal cells probably protects against fetal–maternal conflict during in utero development by conditioning fetal immune cells for NIMA-specific tolerance11,41, a perplexing question is why MMC persists postnatally in offspring after birth (Fig. 1b). In adult humans and mice, maternal microchimeric cells are distributed across a range of tissues (Fig. 1b)13,22,58,59, which parallels the systemic accumulation of NIMA-specific Treg cells11,14,60. Reciprocally in mice, a rapid decline in the number of FOXP3+CD4+ T cells with NIMA specificity occurs following the depletion of maternal microchimeric cells, which suggests that ongoing postnatal NIMA exposure maintains the expanded accumulation of NIMA-specific Treg cells in offspring14.
Interestingly, by investigating the outcomes of next generation pregnancies in mice, it has been observed that susceptibility to fetal wastage triggered by disruptions in fetal tolerance, induced by either infection with the prenatal pathogen Listeria monocytogenes or partial depletion of maternal Foxp3+CD4+ T cells, is markedly reduced during pregnancies sired by males expressing NIMA-matched MHC haplotypes compared with pregnancies sired by mismatched males14. Protection against fetal wastage was associated with robust expansion of NIMA-specific maternal Treg cell populations with overlapping paternal–fetal specificity compared with fetal-specific Treg cells without overlapping NIMA specificity. Reciprocally, the depletion of maternal microchimeric cells from female mice overturned this protection against fetal wastage during subsequent pregnancy and eliminated the expanded accumulation of NIMA-specific Treg cells14. These cross-generational reproductive benefits of NIMA-specific tolerance suggest that, together with the transfer of maternal genetic traits encoded by homologous chromosomes through Mendelian inheritance, the vertical transfer and postnatal retention of maternal cells in offspring promotes the genetic survival of non-inherited maternal traits by reinforcing fetal tolerance to these during next-generation pregnancies14. However, as protection against fetal wastage was shown when complete overlap in MHC haplotype alleles was present between NIMAs in mothers and fetal expressed paternal antigens using defined strains of inbred mice for mating14 — it will be necessary to further investigate the degree of protection achieved when antigenic overlap is restricted to individual MHC loci and/or other alloantigens that would more likely occur amongst humans and individuals in outbred populations.
Maternal memory to fetal antigen persists postpartum
Similar to the postnatal persistence of maternal cells in offspring, fetal cells are also retained in various maternal tissues after parturition (Fig. 1a). For example, Y-chromosome DNA can be amplified in maternal PBMCs up to 27 years after giving birth to a son12. Fetal cells identified by Y-chromosome or HLA-haplotype DNA persist in many lymphoid and non-lymphoid tissues, and amongst multiple cell types, in mothers after parturition (reviewed in REFS 61,62) (Supplementary information S1 (Table)). The retention of fetal cells in maternal tissues is also highly conserved across mammalian species, which suggests that there are likely to be biological benefits that drive the maintenance of tolerance to fetal antigens in mothers after pregnancy9,23,63.
Analogous to how the persistence of maternal microchimeric cells in female offspring promotes the genetic survival of noninherited maternal traits by enforcing fetal tolerance to these during next-generation pregnancies14, a provocative hypothesis is that fetal microchimeric cells retained in mothers after pregnancy may similarly reinforce fetal tolerance during future pregnancies. This notion is supported in humans by partner-specific protective benefits of a prior pregnancy against complications in future pregnancies. For example, the incidence of preeclampsia is reduced during second pregnancies sired by the same father, whereas these protective benefits are eliminated when the second pregnancy is sired by a new male partner64,65. These human partner-specific benefits of prior pregnancy correspond in animals to the persistence of fetal-specific maternal Treg cells after parturition, their more rapid secondary expansion after re-stimulation with fetal antigen, and protection against fetal wastage induced by the partial depletion of maternal FOXP3+CD4+ T cells in secondary compared with primary pregnancy35. Thus, the persistence of fetal microchimeric cells expressing pre-existing fetal antigens may represent an altruistic act of first children to promote tolerance in their mothers to genetically similar future siblings66, and conflict with genetically discordant siblings67.
The protective benefits of prior pregnancy in humans can wane with progressively increased inter-pregnancy intervals68,69, which suggests that the durability of expanded T cell tolerance primed by fetal microchimeric cells might be reduced compared with that primed by maternal microchimeric cells. By contrast, serological sensitization to fetal-expressed HLA antigens primed by pregnancy in mothers is considerably more durable, but surprisingly is not known to be associated with any positive or negative effect on the outcomes of subsequent pregnancies70,71. Whether this reflects the balanced effects of antibodies, which can promote the rejection of genetically foreign tissues after transplantation but can also suppress rejection through accommodation72 or enhancement73, remains to be established. Nonetheless, considering that females can become pregnant multiple times, it will be important to establish how memory maternal adaptive immune components and fetal microchimeric cells retained from prior pregnancies each respond to newly seeded fetal cells during subsequent pregnancies.
Redefining immunological identity
With a growing appreciation of the active cross-talk that occurs between host adaptive immune components and commensal microorganisms, the paradigm that immunological identity is exclusively defined by genetically encoded ‘self’ antigens has already undergone revision to include the expanded repertoire of ‘extended-self’ antigens encoded by the microbiome74. Given the postnatal persistence of MMC in adult individuals, and additional seeding of adult females with fetal microchimeric cells during pregnancy, these genetically discordant microchimeric cells can analogously be viewed as a biologically active ‘microchiome’ containing an expanded repertoire of familially relevant ‘extended-self’ antigens (Fig. 2). In turn, changes to the number, phenotype or distribution of microchimeric cells can be envisioned to modulate health and disease in a similar manner to how commensal microorganisms are now recognized to control susceptibility to a wide variety of immunological and non-immunological disorders74,75.
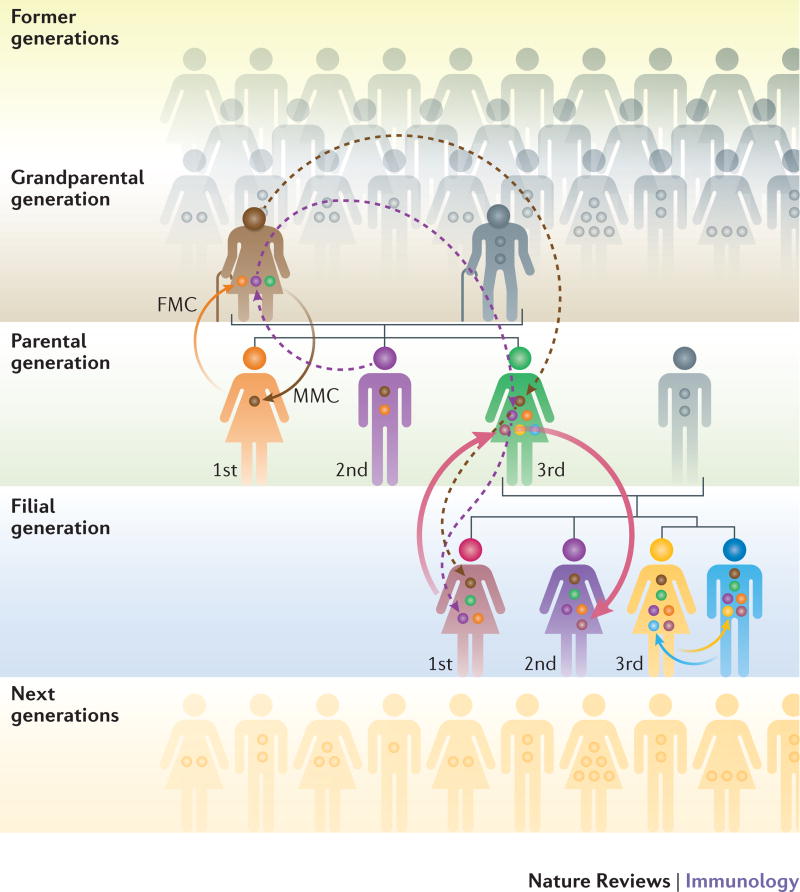
Fig. 2. Familial sources of microchimeric cells that establish the ‘microchiome’.
The bidirectional transfer of genetically foreign cells between a mother and her fetus results in fetal microchimerism (FMC) in the mother (orange arrow) and maternal microchimerism (MMC) in the offspring (brown arrow). In addition to vertical transfer of maternal cells to next-generation (filial) offspring, it is also possible that cross-generational transfer of microchimeric cells from the maternal grandmother may occur (dashed brown and dashed dark blue arrows). As mothers receive genetically distinct fetal microchimeric cell populations in each successive pregnancy, microchimeric cells from older siblings may also be transferred into younger siblings (pink arrow). This exchange of genetically foreign cells may also occur in utero between dizygotic twins (yellow and light blue arrows). Thus, females are the primary reservoirs for transferring microchimeric cells between past and future generations, and the diversity of microchimeric cells in each individual, or their ‘microchiome’, is likely further influenced by birth order and parity.
Considering that the reservoir of microchimeric cells is more transferable and potentially unstable in females than in males (Fig. 2), the effects of microchimerism would be predicted to be more pronounced in females62. For example, a cross-sectional analysis of the prevalence of maternal and fetal microchimeric cells in parous women found that MMC decreased with increasing parity76, which indicates the probable replacement of maternal microchimeric cells by fetal microchimeric cells during each successive pregnancy. Accordingly, the wider diversity and potential dynamic interplay between microchimeric cells of maternal and fetal origins, or genetically discordant fetal cells retained after each successive pregnancy, may contribute to the increased susceptibility of women during their reproductive years to autoimmune disorders and others diseases caused by aberrantly activated immune cells77.
Extending the complexity of the ‘microchiome’ is the possibility that pre-existing subsets of microchimeric cells retained in women from either previous pregnancies or their own siblings can be vertically transferred to offspring (Fig. 2). For example, Y chromosome DNA is frequently identified in the cord blood of younger female siblings with older brothers, and amongst the tissues of adult women who have never given birth to a son78. Further highlighting the importance of sibling birth order, and hence unidirectional tolerance to sibling-derived microchimeric cells, is the lower incidence of GVHD among individuals with hematologic malignancy following stem cell transplants involving HLA-matched younger-sibling donors compared with older-sibling donors79,80. However, these protective benefits of microchimerism were not associated with improved survival after stem cell transplantation for individuals with aplastic anemia81, which may reflect the contribution of other immunological mechanisms in this form of rejection, such as natural killer cell expression of discordant killer cell immunoglobulin receptors82.
There is also the intriguing potential for maternal microchimeric cells to be transferred across generations from a maternal grandmother to her grandchild (Fig. 2), which — similar to other hereditary sources of genetically foreign cells — may instigate or protect against autoimmune disorders (reviewed in REF. 62) (Table 3). However, direct evidence is still lacking to confirm the existence of grandmaternal microchimeric cells in second-generation offspring, mainly as a result of the lack of tools for precise identification of these likely very rare cells. In this regard, date-marking based on Carbon-14 (14C) integration83, which identifies when DNA synthesis occurred, could be used to analyze the age, and hence potential multigenerational heritage, of microchimeric cells. Nonetheless, the accumulation of and dynamic interplay between this ‘microchiome’ of genetically discordant microchimeric cells within the same individual creates an exciting new framework to view immunological identity beyond the traditional tenets of ‘self’ versus ‘non-self’ antigen distinction1,2.
Beneficial and harmful effects of microchimeric cells
The highly conserved nature of microchimerism across placental mammalian species suggests enhanced genetic fitness associated with the persistence of microchimeric cells is likely to be shared by all mothers and their female offspring. However, the purposeful retention of microchimeric cells that reinforces fetal tolerance during future pregnancies has the potential to provoke aberrant alloimmune activation, and new ways to investigate the pathogenesis of autoimmunity or idiopathic auto-inflammatory disorders. In turn, a more comprehensive analysis of the tissue distribution, phenotype and dynamic interplay between microchimeric cells and host cells may show other non-reproductive benefits and harmful consequences associated with individuals being constitutively chimeric.
FMC in maternal autoimmunity and tissue homeostasis
There is a provocative association between increased FMC and greater susceptibility of women during their reproductive years to various autoimmune disorders (reviewed in REFS 62,66). Most of these studies show increased numbers of fetal microchimeric cells in diseased tissue or the circulation of women with autoimmunity, which suggests that alloreactivity to fetal microchimeric cells or tissue seeding by fetal adaptive immune cells may aggravate or initiate autoimmunity. For example, FMC is significantly increased in the blood of women with scleroderma compared with healthy controls84. Interestingly, increased levels of FMC coincides with greater fetal–maternal HLA class II compatibility amongst women with scleroderma84,85, which suggests that this MHC matching may promote the retention of fetal microchimeric cells by protecting them from rejection. However, because these fetal microchimeric cells express other alloantigens that are foreign to mothers, their presence can also cause low-grade inflammation and maternal susceptibility to immune-mediated tissue injury. Similarly, enforced fetal tolerance that occurs in allogeneic pregnancies sired by males expressing NIMA-matched MHC haplotypes14 may promote increased levels of FMC, and greater maternal susceptibility to autoimmunity. Together, these findings suggest fetal cells expressing foreign MHC alleles or other alloantigens that seed maternal tissues may be the target of maternal alloimmunity, which is likely to be clinically indistinguishable from autoimmunity.
Given the lack of representative disease models allowing for experimental manipulation of microchimeric cells, the causative relationship between increased FMC and maternal autoimmunity remains speculative. This uncertainty is compounded by a potential protective role for fetal microchimeric cells in tissue homeostasis and replacement of injured cells in diseased tissues (Table 2). Another interesting consideration is that emerging data show a reduced risk for autoimmune disorders such as scleroderma, rheumatoid arthritis and multiple sclerosis in women with increasing number of prior pregnancies86–91. Although the number and diversity of fetal microchimeric cells were not tested in these studies, the benefits of prior pregnancy, and potentially increasing number of prior pregnancies, in reducing the risk for autoimmunity may reflect the greater accumulation and heterogeneity of fetal microchimeric cells retained in mothers after each pregnancy.
Table 2.
Overview of the potential beneficial and harmful effects of fetal and maternal microchimeric cells
| Potential beneficial roles | Potential harmful effects | |
|---|---|---|
| Fetal microchimeric cells in mothers |
|
|
| Maternal microchimeric cells in offspring |
NIMA, non-inherited maternal antigen; Treg cell, regulatory T cell.
The severity and relapse rate of preexisting autoimmune disorders, such as multiple sclerosis and rheumatoid arthritis, are also reduced during pregnancy15,16. These beneficial effects of pregnancy are most apparent during the last trimester when levels of FMC are highest15,20,92,93. In turn, the remission of multiple sclerosis and rheumatoid arthritis is often reversed shortly after parturition, which parallels the sharp numerical decline in FMC. Female reproductive hormones, levels of which increase during pregnancy, probably contribute to these clinical phenotypes given the reduced relapse rate of multiple sclerosis in non-pregnant women administered exogenous oestriol94. Pregnancy-induced expansion of maternal Treg cells may mediate this cross-talk between female reproductive hormones and protection against relapse of autoimmunity since glucocorticoid receptor stimulation of maternal T cells is necessary for the peripheral induction of Treg cell differentiation and protection against paralysis in animal models of multiple sclerosis95. Thus, these associations highlight the need for additional studies that definitively establish the role of FMC in driving the expansion of maternal Treg cells during pregnancy.
Beyond the context of immunological tolerance, tissue seeding of fetal microchimeric cells with multi-lineage potential can also ameliorate disease by simply replacing injured maternal cell subsets. For example, fetal-derived pancreatic acinar cells have been detected in mouse models of diabetes, suggesting fetal origin cells with multi-lineage potential can replace defective islet cells in maternal type 1 diabetes96. Likewise, beneficial properties of FMC have also been described after non-immune tissue injury, including models of myocardial infarction or Parkinson’s disease, where pluripotent fetal microchimeric cells are thought to differentiate into cell types that infiltrate and replace injured cells in diseased tissues97–102. Protective benefits associated with FMC may also be mediated by neoangiogenesis during wound healing103,104. Interestingly, deliberate seeding of fetal microchimeric cells into defined maternal tissues has also been proposed to promote postpartum care of offspring (reviewed in REF 66). For example, the accumulation of fetal microchimeric cells in maternal breast tissue may promote lactation, and in the brain may enhance maternal attention66. Thus, fetal microchimeric cells, and their likely greater accumulation and wider heterogeneity with increasing parity, could influence the health of mothers during and after pregnancy through both immune and non-immune pathways, which warrants further study using animal disease models that incorporate the manipulation of microchimeric cells.
MMC in the immunological development of offspring
Increased levels of MMC have been observed in a wide variety of human autoimmune disorders including type 1 diabetes, juvenile dermatomyositis, myopathies, scleroderma, biliary atresia and neonatal lupus congenital heart block22,62. This suggests that maternal microchimeric cells — similar to the effects of fetal microchimeric cells in the mother — may be targets of an alloimmune response by the offspring, or trigger an alloimmune reaction against genetically foreign antigens expressed by the offspring (Table 2). Although alloimmunity mediated by maternal microchimeric cells has not yet been conclusively demonstrated in humans, animal disease models suggest that alloreactive maternal T cells promote autoimmune diabetes105 and intestinal inflammation106. Importantly, however, these potential harmful effects of maternal microchimeric cells are likely to be counterbalanced by the evolutionary advantages conferred by these cells in terms of the health of offspring. Together with the aforementioned cross-generational reproductive benefits of NIMA-specific tolerance14, these health benefits include a regenerative function for maternal microchimeric cells with multi-lineage potential, as female insulin-producing cells have been detected in the pancreatic islets of men with type 1 diabetes107,108. Another example of maternal microchimeric cell-mediated protection against tissue injury occurs in pityriasis lichenoides, where maternal cells can adopt keratinocytic phenotypes109, and in neonatal lupus congenital heart block, where maternal cells have the phenotype of cardiomyocytes110. However, despite these associations, the lack of tools for selectively manipulating microchimeric maternal cells within individual tissues precludes definitively establishing their beneficial properties, and excluding their potential role in instigating inflammation as a result of loss of tolerance to these cells.
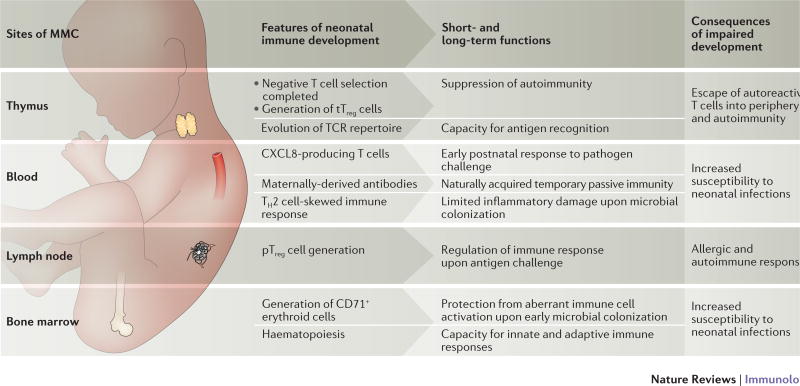
Despite similarities in the distribution and persistence of maternal and fetal microchimeric cells, an inherent distinction between them lies in the immunological maturity and responsiveness of the recipient host that these cell types initially encounter. Whereas fetal microchimeric cells seed an immunologically mature mother, maternal cells are transferred to the fetus prior to or during key milestones in immunological development10,11,41,44,111,112 (Fig. 3). Potential protective effects of maternal microchimeric cells are indicated by their presence in primary and secondary lymphoid organs before the fetal immune system is fully developed in healthy and immune-deficient offspring10,11,48,113. In human infants with severe combined immunodeficiency, the presence of expanded populations of circulating maternal T cells improves the health of offspring by augmenting host defense against microorganisms that cause opportunistic infection such as Epstein–Barr virus114. Maternal microchimeric cells have also been shown to produce IgG in murine offspring that are genetically deficient for B cells115, and to produce interleukin (IL-2) in the thymus and spleen of IL-2-deficient offspring116. Although these observations show that maternal cells can replace missing fetal immune components in immune-deficient offspring, insights as to the beneficial effects of maternal microchimeric cells on the physiological course of immune ontogeny remain sparse. Nonetheless, considering that a major threat to offspring survival in the neonatal period is caused by microbial infection, it is tempting to speculate that maternal microchimeric cells could accelerate the maturation of fetal immune cells by delivering essential growth and differentiation factors. Alternatively, the expanded accumulation of Treg cells with specificity for NIMAs may also promote the health of offspring by dampening inflammation induced by microbial colonization during the perinatal period, and by suppressing aberrant immune responses that ultimately lead to allergy and autoimmunity117–119. This window of expanded tolerance in early development can also be exploited by microorganisms, such as hepatitis B virus, which is transmitted to offspring from the mother during the perinatal period to establish persistence120.
Fig. 3. Potential pathways by which maternal microchimeric cells seeded in fetal tissues may influence immune system development in offspring.
Several primary and secondary lymphoid tissues (thymus, blood, lymph node and bone marrow) are seeded by genetically foreign maternal microchimeric cells during gestation59,62. This seeding coincides with important milestones in the development of fetal and neonatal haematopoietic cells including the development of central tolerance, and differentiation of regulatory T (Treg) cells in the thymus (tTreg cells) and periphery (pTreg cells)41,126,127. Evolutionary adaptations including the emergence of immunosuppressive CD71+ erythroid cells and diminished responsiveness of neonatal immune cells favour the development of immune tolerance in early postnatal development to avert pathological inflammation driven by commensal microbial colonization. Given the near ubiquitous presence of maternal cells in these developing fetal tissues, and the profound short and long-term implications of impaired or delayed fetal–neonatal immune ontogeny, important areas for future investigation include how maternal microchimeric cells may affect functional hematopoiesis in offspring.
The transfer and persistence of microchimeric maternal cells can be reciprocally influenced by genetic and external factors. For example, the extent of maternal–fetal histocompatibility [G] has been shown to increase MMC in offspring48,121,122. Similarly, pertussis toxin-induced inflammation in mothers during pregnancy causes increased cellular trafficking across the placenta in offspring123. Increased levels of maternal DNA are also found in offspring after intrauterine fetal trauma induced by intrahepatic saline injection in mice124, and after in utero fetal surgical repair of spina bifida in humans125. Furthermore, given the known influence of maternal stress, infection and malnutrition during pregnancy on the immunological development of the offspring117, understanding how these factors affect the number and phenotype of maternal cells that are transferred and retained in offspring may uncover innovative new strategies for improving the health of infants and children.
Outlook and future directions
Important discoveries regarding the existence of immunological tolerance have historically been made by investigating nature’s allograft, embodied by the genetically foreign fetus5–7. Interestingly, in an extension to these early studies, we now know that expanded tolerance during fetal development is not limited to antigens expressed by fraternal twin siblings3, but also efficiently extends to NIMAs49–51. Similarly, partner-specific protective benefits of prior pregnancy on reduced complications in future pregnancies suggest that tolerance retained in mothers also expands to encompass pre-existing fetal antigens64,65. Thus, reproduction and pregnancy highlight the remarkable plasticity of immune tolerance that extends classical definitions of ‘self’ versus ‘non-self’ antigen distinction1,2.
But how and why is this expanded tolerance achieved? Persistent bidirectional tolerance in mothers and offspring after pregnancy is demonstrated by the long-term retention of microchimeric cells62. Interestingly, recent findings suggest that these genetically foreign cells are probably not the beneficiaries, but rather the instigators of expanded immune tolerance14. Thus, the intentional transfer and retention of genetically foreign microchimeric cells forces reconsideration of how we define immunological identity in individuals to include familially relevant antigens encoded by microchimeric cells. In turn, establishing how microchimeric cells prime expanded tolerance to evade rejection may reveal exciting new ways for us to re-conceptualize how immunological tolerance naturally works.
With improved tools for the identification and experimental manipulation of rare microchimeric cells, important questions for future study will include investigating whether microchimeric cells randomly seed recipient tissues or are actively recruited to specific fetal and maternal organs; which chemoattractants instruct tissue-specific homing; and whether microchimeric cells require differentiation cues via costimulation, inflammatory mediators and/or growth factors to proliferate and acquire tolerogenic properties. Moreover, considering the dominant role that reproductive benefits have in driving trait selection, the protective effects of microchimeric cells in promoting success of future pregnancies may outweigh their potential harmful roles in causing aberrant alloreactivity to genetically foreign cells. Thus, considering individuals as being constitutively chimeric, with a biologically active ‘microchiome’ of genetically foreign cells, has exciting potential with further study to not only reveal new approaches for improving the outcomes of pregnancy, but also for developing innovative therapeutic solutions for other immunological problems such as autoimmunity and transplantation.
Supplementary Material
Key points.
The benefits of viviparity in placental mammals require dedicated immunologicaladaptations in mothers and offspring to avert maternal–fetal conflict during pregnancy. Given the dominant role that reproductive fitness has in driving positive refining selection, adaptions that enforce fetal tolerance and promote maternal wellbeing are likely engrained in mammalian reproduction.
Expanded systemic immune tolerance occurs in mothers that allows the widespread seeding and persistence of genetically foreign fetal microchimeric cells in maternal tissues during pregnancy and after parturition.
Genetically foreign maternal cells, that express non-inherited maternal antigens, are vertically transferred into offspring during pregnancy. These maternal microchimeric cells persist throughout postnatal development into adulthood, and sustain in the offspring a persistent immunological tolerance to non-inherited maternal antigens.
The bi-directional transfer of genetically foreign cells between mothers and their offspring during pregnancy is probably not accidental. Instead, microchimeric cells that express familially relevant traits are purposefully retained to promote genetic fitness by improving the outcome of future pregnancies.
Expanded immune tolerance to genetically foreign antigens expressed by microchimeric cells (the ‘microchiome’) extends how the immunological identity of individuals is defined beyond classical models of binary ‘self’ versus ‘non-self’ antigen discrimination to include an expanded repertoire of familially relevant ‘extended-self’ antigens.
Despite uniform agreement on the existence of microchimeric cells, little is currently known about their cellular identity, molecular phenotype and interactions with the immune system. Further studying the effects of microchimeric cells may not only reveal new approaches for improving the outcomes of pregnancy, but also for developing innovative therapeutic solutions to other immunological problems such as autoimmunity and transplantation.
Acknowledgments
The writing of this Review and reference to our own work was made possible through funding by Cusanuswerk-Studienförderung (to I.A.S.), Deutsche Forschungsgemeinschaft (AR232/106 in KFO296, AR232/107 to P.C.A.), the US National Institutes of Health, Office of the Director (DP1AI131080 to S.S.W.), US National Institute of Allergy and Infectious Disease (R01AI100934, R01AI120202 to S.S.W.) and March of Dimes Foundation (FY15-254 to S.S.W.). S.S.W. is a Burroughs Wellcome Fund Investigator in the pathogenesis of infectious disease and Howard Hughes Medical Institute Faculty Scholar.
Glossary terms
- Immunological identity
The signature of distinct protein antigens encoded by the unique DNA of each individual that includes MHC haplotype alleles and other alloantigens.
- Viviparity
Development of offspring inside the body of the parent that results in the birth of live offspring capable of independent existence.
- Fetal tolerance
The processes that allow fetal cells and tissues that express genetically foreign paternal antigens to avoid immune rejection and co-exist in harmony inside expecting mothers during pregnancy.
- Microchimeric cells
Rare cells found in one individual that originate from another individual and are genetically distinct from the host individual.
- Non-inherited maternal antigen (NIMA)
The half of genetically encoded maternal antigens that are not transmitted to offspring by classical Mendelian inheritance.
- Allogeneic pregnancy
The result of mating between individuals that are genetically distinct. For genetically identical inbred animal strains, this refers to matings between unique male and female strains with discordant MHC haplotypes, which recapitulates the natural diversity of MHC alleles among individuals in outbred populations.
- Peripherally induced Treg cells
CD4+ T cells that are induced to express FOXP3 and acquire immunosuppressive properties by cognate antigen stimulation in extra-thymic peripheral tissues.
- Maternal–fetal histocompatibility
The degree of similarity between genetically encoded MHC alleles in each mother–child pair.
Biographies
Jeremy Kinder is a postdoctoral fellow in the Division of Infectious Disease at Cincinnati Children’s Hospital, Ohio, USA. His studies explore the reproductive benefits conferred by genetically foreign microchimeric cells that persist after parturition in mothers and offspring.
Ina Stelzer is a postdoctoral fellow in the Department of Obstetrics and Prenatal Medicine at University Medical Center Hamburg-Eppendorf, Germany. She received her Ph.D. for her studies aiming to identify the phenotype and function of microchimeric cells in the tissues of offspring.
Petra Clara Arck is a Professor for Feto-Maternal Medicine at the University Medical Center Hamburg-Eppendorf in Germany. Her laboratory works on maternal immune-endocrine adaptation to pregnancy and related consequences for maternal and offspring's health and disease.
Sing Sing Way is an infectious disease pediatrician at Cincinnati Children’s Hospital and University of Cincinnati College of Medicine, Ohio, USA. He provides clinical care for infants and children with communicable infection, and his laboratory investigates the pathogenesis of fetal wastage that occurs with prenatal infection and maternal–fetal immune tolerance.
Footnotes
Competing interests statement The authors declare no competing interests.
References
- 1.Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin. Immunol. 2000;12:185–188. doi: 10.1006/smim.2000.0230. discussion 257-344. [DOI] [PubMed] [Google Scholar]
- 2.Paul WE. Self/Nonself-Immune Recognition and Signaling: A new journal tackles a problem at the center of immunological science. Self Nonself. 2010;1:2–3. doi: 10.4161/self.1.1.10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. Pioneering description of expanded immune tolerance primed by early developmental exposure to genetically foreign antigens. [DOI] [PubMed] [Google Scholar]
- 4.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953;7:320–338. [Google Scholar]
- 5.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat. Rev. Immunol. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 6.Erlebacher A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 7.Robertson SA, Petroff MG, Hunt J. In: Physiology of Reproduction. Plant TM, Zeleznik AJ, editors. Academic Press; 2015. pp. 1835–1874. Ch. 41. [Google Scholar]
- 8.Rijnink EC, et al. Tissue microchimerism is increased during pregnancy: a human autopsy study. Mol. Hum. Reprod. 2015;21:857–864. doi: 10.1093/molehr/gav047. [DOI] [PubMed] [Google Scholar]
- 9.Khosrotehrani K, Johnson KL, Guegan S, Stroh H, Bianchi DW. Natural history of fetal cell microchimerism during and following murine pregnancy. J. Reprod. Immunol. 2005;66:1–12. doi: 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson AM, Uzunel M, Gotherstrom C, Papadogiannakis N, Westgren M. Maternal microchimerism in human fetal tissues. Am. J. Obstet. Gynecol. 2008;198:325, e321–326. doi: 10.1016/j.ajog.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 11.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. Evidence that fetal effector T cells are capable of allo-reactivity, but are actively suppressed by fetal immune suppressive regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi D, Zickwolf G, Weil G, Sylvester S, DeMaria M. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. PNAS. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. Definitive demonstration that genetically foreign male cells of presumed fetal origin can persist in mothers long-term (27 years) after parturition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloney S, et al. Microchimerism of maternal origin persists into adult life. J. Clin. Invest. 1999;104:41–47. doi: 10.1172/JCI6611. Definitive demonstration that maternal cells persist in healthy offspring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinder JM, et al. Cross-generational reproductive fitness enforced by microchimeric maternal cells. Cell. 2015;162:505–515. doi: 10.1016/j.cell.2015.07.006. Established cross-generational reproductive benefits of microchimeric maternal cells retained in offspring using tools for the selective in vivo depletion of these cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 16.Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin. Immunopathol. 2007;29:185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff AL, et al. Altered response to A(H1N1)pnd09 vaccination in pregnant women: a single blinded randomized controlled trial. PLoS One. 2013;8:e56700. doi: 10.1371/journal.pone.0056700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaudecker EP, McNeal MM, Dodd CN, Ranz JB, Steinhoff MC. Pregnancy modifies the antibody response to trivalent influenza immunization. J. Infect. Dis. 2012;206:1670–1673. doi: 10.1093/infdis/jis592. [DOI] [PubMed] [Google Scholar]
- 19.Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. PNAS. 1979;76:1453–1455. doi: 10.1073/pnas.76.3.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ariga H, et al. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion (Paris) 2001;41:1524–1530. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 21.Krabchi K, et al. Quantification of all fetal nucleated cells in maternal blood between the 18th and 22nd weeks of pregnancy using molecular cytogenetic techniques. Clin. Genet. 2001;60:145–150. doi: 10.1034/j.1399-0004.2001.600209.x. [DOI] [PubMed] [Google Scholar]
- 22.Gammill H, Nelson J. Naturally acquired microchimerism. Int. J. Dev. Biol. 2010;54:531–543. doi: 10.1387/ijdb.082767hg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez DF, Leapley AC, Lee CI, Ultsch MN, Tarantal AF. Fetal CD34+ cells in the maternal circulation and long-term microchimerism in rhesus monkeys (Macaca mulatta) Transplantation. 2005;79:142–146. doi: 10.1097/01.tp.0000144468.71962.aa. [DOI] [PubMed] [Google Scholar]
- 24.Fujiki Y, Johnson KL, Tighiouart H, Peter I, Bianchi DW. Fetomaternal trafficking in the mouse increases as delivery approaches and is highest in the maternal lung. Biol. Reprod. 2008;79:841–848. doi: 10.1095/biolreprod.108.068973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang TT, et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J. Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aluvihare V, Kallikourdis M, Betz A. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 27.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonney EA, Brown SA. To drive or be driven: the path of a mouse model of recurrent pregnancy loss. Reproduction. 2014;147:R153–167. doi: 10.1530/REP-13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe JH, Ertelt JM, Xin L, Way SS. Listeria monocytogenes cytoplasmic entry induces fetal wastage by disrupting maternal FoxP3+ regulatory cell-sustained fetal tolerance. PLoS Pathog. 2012;8:e1002873. doi: 10.1371/journal.ppat.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zenclussen AC, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahn D, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. PNAS. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J. Immunol. 2013;191:2273–2281. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuerer M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. PNAS. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuswanto W, et al. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlebacher A, Vencato D, Price K, Zhang D, Glimcher L. Constraints in antigen presentation severely restric T cell recognition of allogeneic fetus. The Journal of Clinical Investigation. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaturvedi V, et al. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. The Journal of Clinical Investigation. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nancy P, et al. Chemokine gene silencing in decidual stromal cells limits T cell access to maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin L, et al. Cutting Edge: Committed Th1 CD4+ T Cell Differentiation Blocks Pregnancy-Induced Foxp3 Expression with Antigen-Specific Fetal Loss. J. Immunol. 2014;192:2970–2974. doi: 10.4049/jimmunol.1302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv. Immunol. 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5. [DOI] [PubMed] [Google Scholar]
- 42.Hall JM, et al. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–2832. [PubMed] [Google Scholar]
- 43.Stevens AM, Hermes HM, Kiefer MM, Rutledge JC, Nelson JL. Chimeric maternal cells with tissue-specific antigen expression and morphology are common in infant tissues. Pediatr. Dev. Pathol. 2009;12:337–346. doi: 10.2350/08-07-0499.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haynes BF. Phenotypic characterization and ontogeny of components of the human thymic microenvironment. Clin. Res. 1984;32:500–507. [PubMed] [Google Scholar]
- 45.Andrassy J, et al. Tolerance to noninherited maternal MHC antigens in mice. J. Immunol. 2003;171:5554–5561. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 46.Bakkour S, et al. Analysis of maternal microchimerism in rhesus monkeys (Macaca mulatta) using real-time quantitative PCR amplification of MHC polymorphisms. Chimerism. 2014;5:6–15. doi: 10.4161/chim.27778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marleau AM, Greenwood JD, Wei Q, Singh B, Croy BA. Chimerism of murine fetal bone marrow by maternal cells occurs in late gestation and persists into adulthood. Lab Invest. 2003;83:673–681. doi: 10.1097/01.lab.0000067500.85003.32. [DOI] [PubMed] [Google Scholar]
- 48.Piotrowski P, Croy BA. Maternal cells are widely distributed in murine fetuses in utero. Biol. Reprod. 1996;54:1103–1110. doi: 10.1095/biolreprod54.5.1103. Pioneering immunohistochemical analysis showing the presence and widespread distribution of microchimeric maternal cells in fetal tissues. [DOI] [PubMed] [Google Scholar]
- 49.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. PNAS. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. Classical study showing developmental exposure to genetically foreign maternal antigens confers long lasting tolerance by reduced sensitization to the erythrocyte Rh antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. Developmental exposure to genetically foreign non-inherited maternal HLA confers long lasting functional tolerance in humans shown by diminished priming of anti-HLA antibodies. [DOI] [PubMed] [Google Scholar]
- 51.Burlingham WJ, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. The New England Journal of Medicine. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. Developmental exposure to genetically foreign non-inherited maternal HLA confers long lasting functional tolerance in humans shown by prolonged renal allograft survival. [DOI] [PubMed] [Google Scholar]
- 52.Ichinohe T, et al. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family members linked with long-term fetomaternal microchimerism. Blood. 2004;104:3821–3828. doi: 10.1182/blood-2004-03-1212. [DOI] [PubMed] [Google Scholar]
- 53.van Rood JJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. Developmental exposure to genetically foreign non-inherited maternal HLA confers long lasting functional tolerance in humans shown by diminished rates of severe graft-versus-host disease. [DOI] [PubMed] [Google Scholar]
- 54.Matsuoka K, et al. Fetal tolerance to maternal antigens improves the outcome of allogeneic bone marrow transplantation by a CD4+ CD25+ T-cell-dependent mechanism. Blood. 2006;107:404–409. doi: 10.1182/blood-2005-07-3045. [DOI] [PubMed] [Google Scholar]
- 55.Campbell DA, Jr, et al. Breast feeding and maternal-donor renal allografts. Possibly the original donor-specific transfusion. Transplantation. 1984;37:340–344. doi: 10.1097/00007890-198404000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Molitor ML, Haynes LD, Jankowska-Gan E, Mulder A, Burlingham WJ. HLA class I noninherited maternal antigens in cord blood and breast milk. Hum. Immunol. 2004;65:231–239. doi: 10.1016/j.humimm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Zhou L, et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–580. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutta P, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–3587. doi: 10.1182/blood-2009-03-213561. Microchimeric maternal cells are widely distributed in the tissues of adult offspring, while exposure to non-inherited maternal antigens during lactation is essential for persisting tolerance to maternal alloantigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stelzer IA, Thiele K, Solano ME. Maternal microchimerism: lessons learned from murine models. J. Reprod. Immunol. 2015;108:12–25. doi: 10.1016/j.jri.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Molitor-Dart ML, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J. Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 61.Eikmans M, et al. Naturally acquired microchimerism: implications for transplantation outcome and novel methodologies for detection. Chimerism. 2014;5:24–39. doi: 10.4161/chim.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson JL. The otherness of self: microchimerism in health and disease. Trends Immunol. 2012;33:421–427. doi: 10.1016/j.it.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Axiak-Bechtel SM, Kumar SR, Hansen SA, Bryan JN. Y-chromosome DNA is present in the blood of female dogs suggesting the presence of fetal microchimerism. PLoS One. 2013;8:e68114. doi: 10.1371/journal.pone.0068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell D, MacGillivray I, Carr-Hill R. Pre-eclampsia in second pregnancy. Br. J. Obstet. Gynaecol. 1985;92:131–140. doi: 10.1111/j.1471-0528.1985.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 65.Li DK, Wi S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am. J. Epidemiol. 2000;151:57–62. doi: 10.1093/oxfordjournals.aje.a010122. [DOI] [PubMed] [Google Scholar]
- 66.Boddy AM, Fortunato A, Wilson Sayres M, Aktipis A. Fetal microchimerism and maternal health: a review and evolutionary analysis of cooperation and conflict beyond the womb. Bioessays. 2015;37:1106–1118. doi: 10.1002/bies.201500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haig D. Does microchimerism mediate kin conflicts? Chimerism. 2014;5:53–55. doi: 10.4161/chim.29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N. Engl. J. Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 69.Tandberg A, Klungsoyr K, Romundstad LB, Skjaerven R. Pre-eclampsia and assisted reproductive technologies: consequences of advanced maternal age, interbirth intervals, new partner and smoking habits. BJOG. 2015;122:915–922. doi: 10.1111/1471-0528.13051. [DOI] [PubMed] [Google Scholar]
- 70.Masson E, et al. Incidence and risk factors of anti-HLA immunization after pregnancy. Hum. Immunol. 2013;74:946–951. doi: 10.1016/j.humimm.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 71.Vilches M, Nieto A. Analysis of Pregnancy-Induced Anti-HLA Antibodies Using Luminex Platform. Transplant. Proc. 2015;47:2608–2610. doi: 10.1016/j.transproceed.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 72.Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008;13:165–170. doi: 10.1097/MOT.0b013e3282f6391e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris PJ. Suppression of rejection of organ allografts by alloantibody. Immunol. Rev. 1980;49:93–125. doi: 10.1111/j.1600-065x.1980.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 74.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gammill HS, Guthrie KA, Aydelotte TM, Adams Waldorf KM, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood. 2010;116:2706–2712. doi: 10.1182/blood-2010-02-270942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 78.Muller AC, et al. Microchimerism of male origin in a cohort of Danish girls. Chimerism. 2015;6:65–71. doi: 10.1080/19381956.2016.1218583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bucher C, et al. Role of primacy of birth in HLA-identical sibling transplantation. Blood. 2007;110:468–469. doi: 10.1182/blood-2007-02-076257. [DOI] [PubMed] [Google Scholar]
- 80.Dobbelstein C, et al. Birth order and transplantation outcome in HLA-identical sibling stem cell transplantation: an analysis on behalf of the Center for International Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2013;19:741–745. doi: 10.1016/j.bbmt.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gratwohl A, et al. Birth order and outcome after HLA-identical sibling donor transplantation. Blood. 2009;114:5569–5570. doi: 10.1182/blood-2009-10-249060. [DOI] [PubMed] [Google Scholar]
- 82.Mancusi A, et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces nonrelapse mortality. Blood. 2015;125:3173–3182. doi: 10.1182/blood-2014-09-599993. [DOI] [PubMed] [Google Scholar]
- 83.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 84.Nelson JL, et al. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351:559–562. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 85.Lambert NC, et al. Cutting edge: persistent fetal microchimerism in T lymphocytes is associated with HLA-DQA1*0501: implications in autoimmunity. J. Immunol. 2000;164:5545–5548. doi: 10.4049/jimmunol.164.11.5545. [DOI] [PubMed] [Google Scholar]
- 86.Ponsonby AL, et al. Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology. 2012;78:867–874. doi: 10.1212/WNL.0b013e31824c4648. [DOI] [PubMed] [Google Scholar]
- 87.Guthrie KA, Dugowson CE, Voigt LF, Koepsell TD, Nelson JL. Does pregnancy provide vaccine-like protection against rheumatoid arthritis? Arthritis Rheum. 2010;62:1842–1848. doi: 10.1002/art.27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Pregnancy and the risk of developing rheumatoid arthritis. Arthritis Rheum. 1990;33:1770–1775. doi: 10.1002/art.1780331203. [DOI] [PubMed] [Google Scholar]
- 89.Lambe M, Bjornadal L, Neregard P, Nyren O, Cooper GS. Childbearing and the risk of scleroderma: a population-based study in Sweden. Am. J. Epidemiol. 2004;159:162–166. doi: 10.1093/aje/kwh027. [DOI] [PubMed] [Google Scholar]
- 90.Masera S, et al. Parity is associated with a longer time to reach irreversible disability milestones in women with multiple sclerosis. Mult. Scler. 2015;21:1291–1297. doi: 10.1177/1352458514561907. [DOI] [PubMed] [Google Scholar]
- 91.Pisa FE, et al. Reproductive factors and the risk of scleroderma: an Italian case-control study. Arthritis Rheum. 2002;46:451–456. doi: 10.1002/art.10178. [DOI] [PubMed] [Google Scholar]
- 92.Patas K, Engler JB, Friese MA, Gold SM. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J. Reprod. Immunol. 2013;97:140–146. doi: 10.1016/j.jri.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Straub RH, Buttgereit F, Cutolo M. Benefit of pregnancy in inflammatory arthritis. Ann. Rheum. Dis. 2005;64:801–803. doi: 10.1136/ard.2005.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voskuhl RR, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:35–46. doi: 10.1016/S1474-4422(15)00322-1. [DOI] [PubMed] [Google Scholar]
- 95.Engler JB, et al. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E181–E190. doi: 10.1073/pnas.1617115114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sunami R, Komuro M, Yuminamochi T, Hoshi K, Hirata S. Fetal cell microchimerism develops through the migration of fetus-derived cells to the maternal organs early after implantation. J. Reprod. Immunol. 2010;84:117–123. doi: 10.1016/j.jri.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Mahmood U, O'Donoghue K. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism. 2014;5:40–52. doi: 10.4161/chim.28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kara RJ, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ. Res. 2012;110:82–93. doi: 10.1161/CIRCRESAHA.111.249037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roy E, et al. Biphasic recruitment of microchimeric fetal mesenchymal cells in fibrosis following acute kidney injury. Kidney Int. 2014;85:600–610. doi: 10.1038/ki.2013.459. [DOI] [PubMed] [Google Scholar]
- 100.Santos MA, O'Donoghue K, Wyatt-Ashmead J, Fisk NM. Fetal cells in the maternal appendix: a marker of inflammation or fetal tissue repair? Hum. Reprod. 2008;23:2319–2325. doi: 10.1093/humrep/den261. [DOI] [PubMed] [Google Scholar]
- 101.Seppanen E, Fisk NM, Khosrotehrani K. Pregnancy-acquired fetal progenitor cells. J. Reprod. Immunol. 2013;97:27–35. doi: 10.1016/j.jri.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Zeng XX, et al. Pregnancy-associated progenitor cells differentiate and mature into neurons in the maternal brain. Stem Cells Dev. 2010;19:1819–1830. doi: 10.1089/scd.2010.0046. [DOI] [PubMed] [Google Scholar]
- 103.Nassar D, et al. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–157. doi: 10.1096/fj.11-180695. [DOI] [PubMed] [Google Scholar]
- 104.Nguyen Huu S, et al. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1871–1876. doi: 10.1073/pnas.0606490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy E, et al. Specific maternal microchimeric T cells targeting fetal antigens in beta cells predispose to auto-immune diabetes in the child. J. Autoimmun. 2011;36:253–262. doi: 10.1016/j.jaut.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Leveque L, et al. Selective organ specific inflammation in offspring harbouring microchimerism from strongly alloreactive mothers. J. Autoimmun. 2014;50:51–58. doi: 10.1016/j.jaut.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Nelson JL, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. PNAS. 2007;104:1637–1642. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye J, Vives-Pi M, Gillespie KM. Maternal microchimerism: increased in the insulin positive compartment of type 1 diabetes pancreas but not in infiltrating immune cells or replicating islet cells. PLoS One. 2014;9:e86985. doi: 10.1371/journal.pone.0086985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khosrotehrani K, et al. Presence of chimeric maternally derived keratinocytes in cutaneous inflammatory diseases of children: the example of pityriasis lichenoides. J. Invest. Dermatol. 2006;126:345–348. doi: 10.1038/sj.jid.5700060. [DOI] [PubMed] [Google Scholar]
- 110.Stevens AM, Hermes HM, Rutledge JC, Buyon JP, Nelson JL. Myocardial-tissue-specific phenotype of maternal microchimerism in neonatal lupus congenital heart block. Lancet. 2003;362:1617–1623. doi: 10.1016/S0140-6736(03)14795-2. [DOI] [PubMed] [Google Scholar]
- 111.von Hoegen P, Sarin S, Krowka JF. Deficiency in T cell responses of human fetal lymph node cells: a lack of accessory cells. Immunol. Cell Biol. 1995;73:353–361. doi: 10.1038/icb.1995.54. [DOI] [PubMed] [Google Scholar]
- 112.Petit T, et al. Detection of maternal cells in human fetal blood during the third trimester of pregnancy using allele-specific PCR amplification. Br. J. Haematol. 1997;98:767–771. doi: 10.1046/j.1365-2141.1997.2603076.x. [DOI] [PubMed] [Google Scholar]
- 113.Srivatsa B, Srivatsa S, Johnson KL, Bianchi DW. Maternal cell microchimerism in newborn tissues. J. Pediatr. 2003;142:31–35. doi: 10.1067/mpd.2003.mpd0327. [DOI] [PubMed] [Google Scholar]
- 114.Touzot F, et al. Massive expansion of maternal T cells in response to EBV infection in a patient with SCID-Xl. Blood. 2012;120:1957–1959. doi: 10.1182/blood-2012-04-426833. [DOI] [PubMed] [Google Scholar]
- 115.Arvola M, et al. Immunoglobulin-secreting cells of maternal origin can be detected in B cell-deficient mice. Biol. Reprod. 2000;63:1817–1824. doi: 10.1095/biolreprod63.6.1817. [DOI] [PubMed] [Google Scholar]
- 116.Wrenshall LE, Stevens ET, Smith DR, Miller JD. Maternal microchimerism leads to the presence of interleukin-2 in interleukin-2 knock out mice: implications for the role of interleukin-2 in thymic function. Cell. Immunol. 2007;245:80–90. doi: 10.1016/j.cellimm.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 118.von Mutius E. The microbial environment and its influence on asthma prevention in early life. J. Allergy Clin. Immunol. 2016;137:680–689. doi: 10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- 119.Elahi S, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tian Y, Kuo CF, Akbari O, Ou JH. Hepatitis B virus persistence in offspring after vertical transmission is driven by macrophages that are altered by viral e antigen in mother. Immunity. 2016 doi: 10.1016/j.immuni.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berry SM, et al. Association of maternal histocompatibility at class II HLA loci with maternal microchimerism in the fetus. Pediatr. Res. 2004;56:73–78. doi: 10.1203/01.PDR.0000129656.10005.A6. [DOI] [PubMed] [Google Scholar]
- 122.Kaplan J, Land S. Influence of maternal-fetal histocompatibility and MHC zygosity on maternal microchimerism. J. Immunol. 2005;174:7123–7128. doi: 10.4049/jimmunol.174.11.7123. [DOI] [PubMed] [Google Scholar]
- 123.Wienecke J, et al. Pro-inflammatory effector Th cells transmigrate through anti-inflammatory environments into the murine fetus. Placenta. 2012;33:39–46. doi: 10.1016/j.placenta.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Nijagal A, et al. Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice. J. Clin. Invest. 2011;121:582–592. doi: 10.1172/JCI44907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saadai P, MacKenzie TC. Increased maternal microchimerism after open fetal surgery. Chimerism. 2012;3:1–3. doi: 10.4161/chim.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4:97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- 127.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 128.Kinder JM, et al. Tolerance to noninherited maternal antigens, reproductive microchimerism and regulatory T cell memory 60 years after 'Evidence for actively acquired tolerance to Rh antigens'. Chimerism. 2015;6:8–20. doi: 10.1080/19381956.2015.1107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stevens AM. Maternal microchimerism in health and disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2016;31:121–130. doi: 10.1016/j.bpobgyn.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Leveque L, Khosrotehrani K. Feto-maternal allo-immunity, regulatory T cells and predisposition to auto-immunity: Does it all start in utero? Chimerism. 2014;5 doi: 10.4161/chim.29844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.