Abstract
Background: Mindfulness-based interventions (MBIs) to address self-regulation and lifestyle behaviors (diet, physical activity) may benefit endometrial cancer survivors (ECS), who are at increased risk for morbidity and mortality associated with obesity. However, the acceptability of mindfulness training and whether it can augment behavior change in ECS is unknown. We aimed to examine; 1) the feasibility of the Mindfulness in Motion + Diet (MIM+D) intervention and 2) the preliminary efficacy of MIM+D for improving mindfulness, diet, PA and health-related quality of life (HRQL). Methods: ECS (Mage=62.4, ±5yrs from diagnosis) completed assessments at baseline, 8 and 14 weeks. Feasibility was determined by intervention completion surveys, attendance and adherence data. We used repeated measures ANOVA’s (SPSS 22.0) and effect size estimates (Cohen’s d) to examine changes in mindfulness, diet, PA, and HRQL over time. Results: Thirteen ECS (76%) completed the MIM+D program and attendance (≥6/8 sessions) was 90%. Women reported favorably on the overall quality (mean of 4.75/5) and benefits of the MIM+D program; however, would have preferred receiving MIM+D closer to diagnosis. Intention to treat analyses found MIM+D did not significantly improve any outcomes. However, an intervention completers analysis showed significant change in mindfulness (p=.0039) and small to moderate estimates for change in fruits and vegetable intake (d=.23), MVPA (d=.45), RAND SF-36: MCS (d=.46), and sleep quality (d=.68). Conclusions: Integrating mindfulness training into behavioral interventions is feasible and ECS that adhere to these lifestyle programs may benefit. However, to future research should examine the-long term effects of mindfulness-based behavioral lifestyle interventions.
Keywords: endometrial cancer, survivorship, mindfulness, lifestyle behaviors, physical activity, diet
Introduction
Endometrial cancer (EC) is the most prevalent form of gynecologic cancer affecting women in the United States, with an estimated 60 050 new cases in 2016.1 Risk for developing EC is strongly linked with obesity,2,3 which also affects disease-specific and all-cause mortality.4 The relative risk of death as a result of the disease is 6.25 (95% confidence interval [CI] = 3.75-10.42) for body mass index (BMI) >40 kg/m2 versus normal weight (BMI = 18.5-24.9 kg/m2).5 However, the majority of EC survivors diagnosed with early-stage disease actually die from cardiovascular disease (CVD) and associated comorbidity.6 It is therefore critical to reduce the excess burden of morbidity and mortality associated with being obese as an EC survivor.4,7,8 However, obesity is a complex and multifactorial condition that some consider a disease itself,9 and while weight loss is challenging for most people, it may be especially so for women who are concurrently dealing with a diagnosis of cancer. Novel behavioral interventions, such as those that include a mindfulness-based approach, may offer one option for changing dietary behaviors and healthy lifestyle behaviors that may help with sustainable weight management.
Mindfulness is a Western conceptualization of a traditionally Eastern practice of meditation and concentration that was popularized by Jon Kabat-Zinn, who describes mindfulness as “paying attention in a particular way: on purpose, in the present moment, and non-judgmentally.”10(p 4) This focus on developing nonjudgmental awareness has significant implications for the ways in which people react to stressful life events and self-regulate their behavior. Mindfulness can be thought of both as a practice and as a state of awareness and has previously been the focus of a number of interventions in cancer care. One review reports that mindfulness-based interventions (MBIs) are associated with significant improvements in anxiety, depression, stress, sexual difficulties, physiological arousal, and immune functioning, as well as self-reported quality of life.11 More recently, there has been a growing interest in the potential of mindfulness-based approaches to support weight loss efforts12,13 and as a way of controlling eating habits.14 One hypothesis is that unregulated emotional responses are often integrated into eating behaviors (emotional eating); however, evidence is mixed.15 Broadly, mindfulness posits that people need to slow down and disengage from habitual automatic responses to environmental or emotional cues, thereby providing space to cognitively make better choices related to their lifestyle behaviors.
In terms of lifestyle modification, dietary adjustment alone can be effective, but it is well established that diet in combination with physical activity (PA) is the most beneficial behavioral strategy, especially with regard to maintaining healthy, metabolically active lean tissue.16 Therefore, modifying both diet and PA to address obesity-related outcomes in these survivors is critical. Not surprisingly, evidence suggests that only a small number of survivors currently meet the dietary (15% to 19%) and PA (12% to 29%) guidelines.7,17 However, to date, only a limited number of studies have examined lifestyle interventions that aim to address this,18,19 none of which has incorporated mindfulness into their intervention.
A recent review that included 8 studies—4 cross-sectional, 1 retrospective, 1 prospective, and 2 randomized trials—examined the relationships between obesity, diet, PA, and health-related quality of life (HRQL) in EC survivors and reported that meeting PA and dietary recommendations was positively associated with overall HRQL. Obesity was also negatively associated with HRQL, physical well-being, and fatigue.20 While EC survivors do seem to have the capacity to improve their lifestyle behaviors, especially in the context of structured interventions, there is much evidence to suggest that as a population, EC survivors do not see their condition as especially threatening and therefore are not motivated to change their behaviors. Furthermore, interventions that aim to increase moderate to vigorous physical activity (MVPA) to the level of the guidelines (≥150 minutes)21 may be limited by the functional capacity of morbidly obese women with no prior experience of exercise; it is not yet clear if behavioral changes can occur with less intensive contact or whether alternative methods of supporting lifestyle change may be more or less efficacious.22 Because lifestyle behaviors are often complex, utilizing comprehensive approaches to behavior change may hold potential, especially in a hard-to-reach population, such as EC survivors. In addition to mindfulness, another technique that is increasingly adopted as part of behavioral interventions and in health coaching is motivational interviewing (MI). Miller and Rollnick describe MI in lay terms as “a collaborative conversation style for strengthening a person’s own motivation and commitment to change.”23(p12) A meta-analysis showed that MI has previously been effective in addressing BMI, total blood cholesterol, systolic blood pressure, and alcohol use.22 Furthermore, mindfulness that leads to less emotional reactivity and nonjudgmental awareness may actually support the use of MI techniques. For EC survivors, who are known to have poor diets, low levels of PA, and diminished HRQL, mindfulness-based approaches could potentially be incorporated into traditional lifestyle interventions to enhance adoption of these healthy behaviors. We were therefore interested in whether an alternative behavioral lifestyle intervention that incorporated a mindfulness-based approach could feasibly improve dietary and PA behaviors by enhancing behavioral self-regulation.
As far as we know, this single-group, pre-post study is the first to test the feasibility and preliminary efficacy of delivering a mindfulness-based dietary counselling intervention (Mindfulness In Motion + Diet [MIM+D]) to a group of overweight and obese, early-stage, type I EC survivors. Our primary hypothesis was that EC survivors would be (a) interested in participating and (b) find the MIM+D program feasible and acceptable. Secondary exploratory aims of this study were to examine the effects of the intervention on levels of mindfulness, diet, PA participation, physical function, and HRQL. Our secondary hypotheses were that, for those women who adhered to the program, we would see improvements in mindfulness, lifestyle behaviors, physical functioning, and HRQL. We did not assess weight loss as a primary outcome given the short time frame of the study, and while the intervention was not focused on PA per se, it did incorporate gentle yoga and a dietary counselling program that espoused the benefits of PA. These data may inform the design and delivery of subsequent lifestyle intervention trials targeting EC survivors.
Methods
This study was approved by the Institutional Review Board (Cancer) of the Ohio State University. This pre-post intervention study was designed to examine the feasibility and preliminary efficacy of delivering a mindfulness-based dietary counseling intervention to EC survivors. While the primary focus was on diet, we were still interested in whether participants would begin to incorporate PA participation, based on its inclusion in conversations related to diet and health. Over a period of 24 months, there were approximately 500 EC patients treated through our OB-GYN clinics, and we estimated that a minimum recruitment rate of 15% would allow us to estimate effect sizes for the design of a larger trial, while also allowing us to reach our recruitment targets within the available timeframe. We aimed to assess mindfulness, dietary behavior, PA, physical function, and select HRQL outcomes in a group of surgically treated, early-stage (I and II) type I EC survivors. Participants attended weekly sessions of the MIM+D intervention for 8 weeks at the cancer center and transitioned to home-based practice during weeks 9 to 14. Assessments were completed at baseline, follow-up 1 (8 weeks), and follow-up 2 (14 weeks). The person completing assessments also delivered the intervention. To assess the feasibility of our approach, on completion of the study, we asked participants to evaluate separately the mindfulness and dietary counseling components of the study. Adherence was tracked through attendance to study sessions and with self-report logs.
Participants
The focal point for recruitment was a large metropolitan university’s gynecological (GYN) oncology clinic, but also included patients of other local GYN oncology clinicians. Patient records and in-clinic visits were the primary means for identifying potential participants. After patients were identified, the study staff conducted telephone-screening interviews to assess interest and confirm that participants satisfied the eligibility criteria (Table 1). They were then scheduled baseline assessment visits. All possible subjects must have been surgically staged and treated for type 1, early-stage (I and II) EC. We targeted these women because they were most at risk for disease recurrence, future comorbidity (CVD and type 2 diabetes), and experiencing compromised HRQL due to sedentary lifestyles and poor dietary practices. The recruitment procedures for this study included a combination of mail-out invitations and in-clinic offers to participate in the study.
Table 1.
Eligibility Criteria.
| Inclusion criteria |
| • Female |
| • English speaking |
| • Previous diagnosis of grade 1 or 2, stage I or II, endometrioid endometrial cancers (“Type I cancers”)—confirmed during surgical intervention for treatment |
| • Overweight or obese (BMI > 25 kg/m2) |
| • Anytime from treatment |
| • Ambulatory or able to engage in walking for at least 45 minutes |
| • Sedentary lifestyle, as engaging in less than 100 minutes structured aerobic walking, cycling, or swimming per week |
| Exclusion criteria |
| • No prior type I endometrial cancer diagnosis |
| • Prior diagnosis of other cancer |
| • Currently (previous 6 months) engaged in structured exercise either aerobic or yoga based |
| • Severe heart or systemic disease: evidence of documented myocardial infarction, chronic unstable angina, symptomatic congestive heart failure, uncontrolled hypertension |
| • Severe musculoskeletal disease: Severe muscle or joint disorders due to disease or trauma, amputations, or any condition that significantly impair physical capabilities, as defined by the physician |
| • Nonambulatory |
| • Concurrent diagnosis of organic brain syndrome, dementia, mental retardation, or significant sensory deficit |
| • Major mental illness (eg, schizophrenia, major depressive disorder) |
| • Unwilling to give consent |
Mindfulness in Motion + Diet Intervention
Mindfulness in Motion (MIM)24 is an 8-week MBI that emphasizes mindful yoga as a way to develop a healthy relationship with the body and provides a practical strategy for stress management. It includes music, yoga, and mindfulness instruction and practice, and has been described in detail elsewhere.25 The movements and breathing exercises are simple and can be practiced daily in the worksite or home. Participants were provided with a CD/DVD and guidelines for home-based mindfulness and yoga, as has been done in prior studies, and is an integral part of the protocol.25-28 The components of each week’s MIM and Dietary sessions are shown in Table 2. At 14 weeks, participants completed a second follow-up to determine the adherence to the training postintervention and the efficacy of the approach.
Table 2.
Intervention Components.
| Mindfulness in Motion | Dietary Counselling | |
|---|---|---|
| Week 1 | Willingness toward daily practice | Introduction and overview |
| Week 2 | Cultivating mindful sleep | Principles of energy balance, portion sizes |
| Week 3 | Supported by the breath | Macronutrients and SMART goal setting |
| Week 4 | Mindful eating | Micronutrients and barrier problem solving |
| Week 5 | Movement through balance | Meal planning for balance, variety, and moderation |
| Week 6 | Centering through sensation | Myths and misconceptions |
| Week 7 | Clarity and release | Resources for self-monitoring (apps/software) |
| Week 8 | Strength of the mountain | Staying motivated and dealing with lapses |
Abbreviation: SMART, Specific, Measureable, Attainable, Realistic, Time-related.
Dietary Counselling
The 30-minute dietary counselling sessions were conducted by a registered dietitian. These sessions immediately followed the MIM session during weeks 1 to 8. The specific dietary objectives were consistent with the Therapeutic Lifestyle Changes recommended in the Adult Treatment Panel III Report of the National Cholesterol Education Program29 and the American Institute of Cancer Research.30 The nutrition intervention encourages reductions in portion size and caloric and fat consumption together with a gradual transition from an animal-based diet to a more plant-rich diet while still incorporating animal foods, including milk and meat, with an emphasis on monitoring food proportion and portion size. Specific goals of the dietary component included: (a) reduction in energy intake by 500 to 1000 kcal per day; (b) reduction in total fats to 25% to 30%, saturated fats to 7%, and protein to 15% of total calories; (c) increase in fruit and vegetable consumption to 5 servings per day; and (d) intake of 3 or more servings of whole grains and a gradual increase to at least 25 grams of dietary fiber per day. The nutrition counseling used an MI approach that has been demonstrated to be an effective approach to promote behavior change in cancer patients.28,31 The nutrition counselling also builds on cognitive-behavioral theory and self-management strategies32 as well as aspects of mindfulness utilized in the MIM intervention. Participants were taught how to set realistic goals for changing their dietary behavior, while learning to self-monitor through the completion of dietary logs. They were also taught the importance of anticipating and overcoming barriers to dietary behavior change. These skills were intended to support and improve participant’s self-efficacy for dietary behavior change. Participants did not explicitly set goals of weight loss or for PA; however, they could have done this on their own using the techniques taught. We specifically focused goal-setting on dietary behavior itself, with the intention being that this would foster weight loss in the long term. Broadly, this theory-based intervention was designed to facilitate the development of behavioral self-regulatory skills needed to successfully adopt and maintain a change in dietary behavior over the long term.
Measures
Demographics, medical history, mindfulness, dietary quality, and measures of psychosocial and HRQL/QOL (quality of life) variables were conducted using surveys. We used both self-report and accelerometry for PA and a standard battery of functional tests for assessing physical function. Finally, we assessed weight status using the IDXA lunar (GE) for body composition.
Mindfulness was assessed with the 5-Facet Mindfulness Questionnaire (FFMQ)33 and the Mindfulness Attention Awareness Scale (MAAS).34 The FFMQ measure was created using a factor analysis approach to identify the underlying independent facets tapped by multiple mindfulness questionnaires and has demonstrated acceptable psychometric properties when comparing experienced meditators with nonmeditators with α coefficients for all facets ranging from .67 to .92.33,35 The 5 underlying constructs targeted are Observing, Acting with awareness, Nonreacting, Nonjudging, and Describing—all of which have shown strong expected relationships with psychological metrics. The MAAS is a measure proposed to tap a unitary operationalization of mindfulness and has been previously validated in a cancer population.36
To measure the dietary intakes of EC survivors, we used the Food Habits Questionnaire (FHQ).37 This measure was developed as a screening tool for dietary quality related to the prevention of cancer and CVD, with a focus on the assessment of dietary fats and whole grains. It typically includes 49 questions related to frequency of food intake over 6 categories. In the RENO diet heart study, the test-retest reliability of this measure was (Pearson r = .92) and a Cronbach’s α level of .85. It is recognized that there are more in-depth analyses of food intakes available; however, for the purposes of this study, we chose this measure for its brevity as well as ability to capture meaningful data in conjunction with other measures as part of a larger survey.
PA was assessed using both objective accelerometers and self-reported data via the modified Paffenbarger Physical Activity Questionnaire (PAQ)38 to measure weekly MVPA levels in EC survivors. PA participation was assessed objectively with the uniaxial, Kenz LIFECORDER (Suzuken Kenz Inc Limited, Tokyo, Japan) accelerometer. The LIFECORDER (LC) has previously established validity and reliability.39-41 The PAQ measures the amount of time typically spent by an individual in various types of PA, including stair climbing, walking, and structured exercise. Comparison of the level of accumulated weekly PA can be made using the current American College of Sports Medicine/US Department of Health and Human Services guidelines of 150 minutes of MVPA per week. Prior research has demonstrated the PAQ has acceptable validity and reliability.42 Physical function was assessed using 4 valid and reliable tests. The Short Physical Performance Battery (SPPB) is composed of 3 subcomponents that test balance, gait speed, and leg strength and endurance.43 We also conducted 3 timed performance-related mobility tasks: the 400-m walk, stair-climb, and lift and carry task.44-47 For the 400-m walk, participants completed 10 clockwise laps around a 40-m indoor course as quickly as possible. For the stair-climb task, participants were timed while they ascended a set of 10 stairs turning around at the top of the platform, and then descending the stairs. In the lift and carry task, participants picked up a container—weighing 10 lbs—with both hands, turned and walked around a cone placed 5 m away. They then returned to the shelf to place the container down. Performance on each test was measured in the total time (in minutes and/or seconds) to complete the task.
Quality of life was assessed from both a global perspective as well as within a more disease specific context (HRQL). Globally we used the Satisfaction with Life Scale (SWLS), a 10-item, Likert-type questionnaire with reported validity and reliability,48 and for the specific aspects of HRQL we used the RAND SF-36, which is well-known and has acceptable validity and reliability.49 The Pittsburg Sleep Quality Index (PSQI) was used to sample typical sleep patterns over the previous month, which is determined to be a time frame within which transient issues with this aspect of normal functioning may vary. The measure has been shown to be valid and reliable in prior research.50
To assess the feasibility of our approach, on completion of the study, we asked participants to evaluate separately the mindfulness and dietary counseling components of the study (Figure 2). Adherence was tracked through attendance to study sessions and with self-report logs. The preliminary efficacy analyses were conducted with the Statistical Package for Social Sciences (SPSS, version 22). Data were cleaned and examined for outliers and missing values. SPSS by default conducts analyses by dropping cases for which there are missing data. We therefore carried data forward from the last visit for which there was an assessment to conservatively evaluate the effect of the intervention. Following an examination of the demographics of the study population, we conducted bivariate correlations to determine the relationships between the primary variables of interest. Alpha was set a priori at a level of P = .05. Repeated-measures ANOVAs were then used to determine the effects of the MIM+D intervention on changes in all outcomes of interest. Analyses were conducted using the intention-to-treat principle with the last-value-carried-forward approach used to account for missing data. Where assumptions of sphericity were violated as determined by Mauchley’s test, we used the Greenhouse-Geisser adjustment to interpret main effects. Given this was a feasibility and preliminary efficacy study involving a small sample size, effect sizes (Cohen’s d) were calculated by taking the mean difference and dividing by the pooled standard deviation to better estimate the meaningfulness of change for each observed outcome following the intervention. By convention, the values and meanings for effect size estimates are a small effect size (d = .2), a moderate effect size (d = .5), and a large effect size (d = .8). Finally, we also conducted an exploratory analysis for intervention completers (adherence) following the same steps as above where appropriate.
Figure 2.
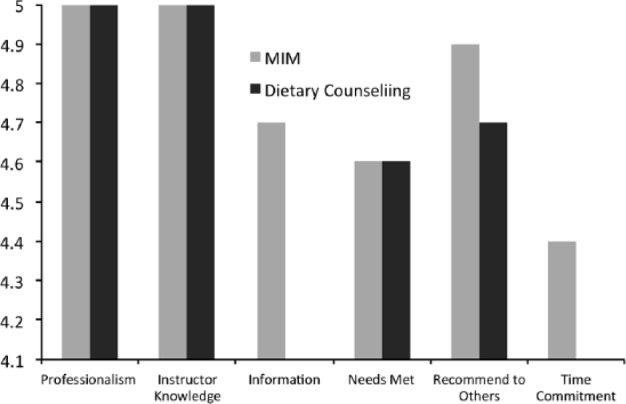
Completion surveys of the MIM+Diet intervention.
Results
Recruitment processes and flow are detailed in the CONSORT diagram (Figure 1). Briefly, 438 patients were screened and contacted about the study, leading to 43 (9.8%) that showed an interest. Of these, we excluded 26 patients who were (a) too active (5), (b) had recently participated in yoga (3), (c) were underweight (4), (d) who withdrew interest after receiving more details (9), and (e) for miscellaneous reasons (5). There were a total of 17 participants that completed baseline measures on all outcomes of interest (40%). Two subjects did not start the intervention due to scheduling conflicts, and a further 2 dropped out by follow-up 1 and another 2 by follow-up 2, leaving a total of 13/15 that completed the program (86%) but 11/17 who completed the entire study (65%). While we did have some dropout over the course of the intervention, those who completed the study reported a high level of satisfaction with the intervention (Figure 2). In particular, it was notable that the majority of the participants reported that they felt they would have benefitted most from this intervention had it been offered to them when they were first diagnosed and early in the course of their treatment.
Figure 1.
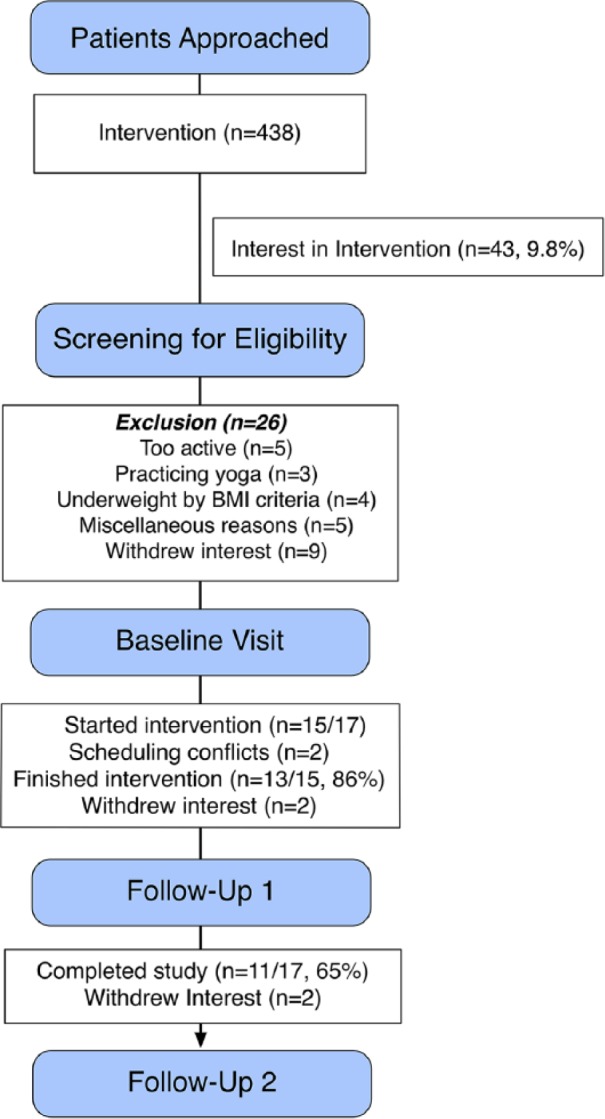
CONSORT diagram for participant flow.
Women in the study were predominantly white (88%), well-educated, with a mean age of 61.1 ± 7 years, considered class I obese (BMI = 33.8 ± 6.5; body fat % = 46.8 ± 6.6), with multiple comorbidities, including 30% who were type 2 diabetic, 65% who had hypertension, 24% with arthritis, and 12% with depressive symptoms (see Table 3). Interestingly, in the 2 women that were only slightly overweight by BMI criteria, body fat % (IDXA) measures showed they were actually obese. Given the short time frame of the study, we did not expect to see significant weight loss. However, we did examine body composition changes for those women who completed the intervention. These results are reported with the rest of the intervention completion data and are shown in Table 7. The 95% CIs for all analyses are available by request from the corresponding author.
Table 3.
Demographics (N = 17).
| % or Mean (SD) | Range | |
|---|---|---|
| Age | 61.1 (7.0) | 45-70 |
| BMI | 33.8 (6.5) | 23.0-49.5 |
| Body fat % (IDXA) | 46.8 (6.6) | 30.3-50.3 |
| Type 2 diabetes mellitus | 29.4% | |
| Hypertension | 64.7% | |
| Arthritis | 23.5% | |
| Depression | 11.8% | |
| Race | ||
| White | 88% | |
| African American | 12% | |
| Education | ||
| High school | 18% | |
| College | 60% | |
| Graduate school or higher | 23.8% | |
| Employment | ||
| Currently working (full-time) | 35% | |
| Currently working (part-time) | 12% | |
| Retired | 53% | |
| Household income (US$) | ||
| 75 000 or more (highest) | 53% | |
| 50 000 to 74 999 | 12% | |
| <50 000 | 35% | |
Abbreviation: BMI, body mass index.
Table 7.
Body Composition for Intervention Completers.
| IDXA Body Composition Measures | Baseline, Mean (SD) | 14 Weeks, Mean (SD) | ES, d | P |
|---|---|---|---|---|
| Body fat % | 45.6 (6.9) | 45.0 (8.3) | −.07 | NS |
| Lean body mass | 105.3 (11.8) | 104.5 (12.2) | −.07 | NS |
| Visceral adipose tissue | 3.5 (2.4) | 3.8 (2.4) | .12 | .05 |
| Weight | 199.3 (29.6) | 197.3 (33.9) | −.06 | NS |
Abbreviation: NS, nonsignificant.
Mindfulness
Table 4 shows the results of the intervention on mindfulness scores over time. For the 5 different factors of the FFMQ, there were no statistically significant or meaningful effect size changes over time. In terms of the MAAS, we again did not see a statistically significant change (p = .15; 95% CI = −0.58 to 0.09), but effect size estimates (Cohen’s d = .22) pointed toward a small improvement.
Table 4.
Mindfulness.
| Mindfulness Measures | Baseline, Mean (SD) | 8 Weeks, Mean (SD) | ES, d | 14 Weeks, Mean (SD) | ES, d | P |
|---|---|---|---|---|---|---|
| FFMQ | ||||||
| Observe | 30.2 (5.1) | 31.1 (5.3) | .17a | 30.7 (5.7) | .09 | NS |
| Nonjudge | 30.8 (6.3) | 30.2 (6.6) | −.09 | 30.6 (5.7) | −.03 | NS |
| Nonreact | 23.9 (4.8) | 23.2 (5.5) | −.14 | 24.4 (5.6) | .09 | NS |
| Describe | 30.1 (5.5) | 29.9 (5.9) | −.03 | 30.1 (4.8) | .00 | NS |
| Act with Awareness | 28.9 (3.9) | 28.8 (3.4) | −.02 | 29.2 (3.7) | .08 | NS |
| MAAS | 4.44 (0.79) | 4.53 (0.59) | .13 | 4.6 (0.66) | .22a | .15 |
Abbreviations: ES, effect size; FFMQ, Five-Factor Mindfulness Questionnaire; MAAS, Mindfulness Attention Awareness Scale; NS, nonsignificant.
Small to moderate effect size (.2-.7).
Dietary Quality
Food habits data are reported in Table 5. Scores in each category represent the relative quality of eating habits, ranging from poor to excellent. At baseline, only 2 women showed poor choices in the grains category, with no scores below 8, which indicates a high risk for cancer.37 There were no poor choices in the fruits and vegetables category, 3 (18%) in the fairly good range, and the rest (82%) in the excellent range. However, 24% of women had poor dairy choices, with the majority (59%) in the fair category. Meat scores are a measure of protein intake and include items such as beans and fish. No subjects were in the poor category, 29% in the fair category, 65% fairly good, and one in the excellent category. The fats and oils category indicates the general way a person cooks their meals or the type of meals they typically choose if eating out. Eighty-eight percent of women were making poor choices. Given the sample, this is not surprising. Seventy-seven percent of participants were making poor choices in the “other” category, which referred to fast food, sugary foods, and items such as alcoholic beverages. There were no statistically significant changes in the dietary quality of participants following the intervention. Effect size estimates did not reveal any meaningful change either, except for the Fats and Oils category where we actually saw a moderate decline (d = −.44), though not significant (p = .06; 95% CI = 0.31-2.5).
Table 5.
Food Habits Scores.
| Food Categorya | Baseline, Mean (SD) | 8 Weeks, Mean (SD) | ES, d | 14 Weeks, Mean (SD) | ES, d | P |
|---|---|---|---|---|---|---|
| Grains | 15.9 (3.1) | 16.0 (3.5) | .03 | 15.5 (3.4) | −12 | NS |
| Fruits and vegetables | 27.3 (4.2) | 28.1 (4.0) | .20b | 27.6 (4.4) | .07 | NS |
| Dairy | 26.0 (5.2) | 27.0 (6.5) | .17 | 26.4 (6.5) | .07 | NS |
| Meat | 25.2 (3.5) | 25.2 (3.6) | 0 | 24.9 (3.7) | −.08 | NS |
| Fats and oils | 21.8 (2.7) | 21.0 (3.5) | −.25 | 20.4 (3.6) | −.44b | .06 |
| Other | 17.0 (4.6) | 16.9 (3.5) | −.03 | 16.5 (4.0) | −.12 | NS |
| Total FHQ score | 136.4 (19.3) | 134.3 (15.3) | −.12 | 131.6 (17.2) | −.26b | NS |
Abbreviations: ES, effect size; NS, nonsignificant; FHQ, Food Habits Questionnaire.
Grains: <8 = risk for cancer, 8-11 = needs improvement, 12-15 = fairly good, ≥16 = excellent. Fruits and vegetables: <11 = high risk, 11-16 = needs improvement, 17-22 = fairly good, ≥23 = excellent. Dairy: <22 = high fat/risk, 22-31 needs improvement, 32-42 = good, ≥43 = excellent. Meat: <16 = poor (high fat choices), 16-23 = fair, 24-31 = good, ≥32 = excellent. Fats and oils: <25 = poor, 25-29 = good, 30-33 = fair, ≥33 = excellent. Other: <21 = poor, 21-27 = good, ≥28 = excellent.
Small to moderate effect size (.2-.7).
Physical Activity and Physical Function
Physical activity, physical function, and QOL are reported in Table 6. At baseline, 6 (31%) participants self-reported no daily PA; the objective PA assessment indicated that 80% of participants did not accrue even half of the recommended weekly MVPA (M = 65.7, ±81.1). There was a wide range of activity levels, but on average women were taking about 5000 steps per day and accruing roughly 65 minutes of MVPA and 330 minutes of light activity per week. The intervention did not lead to any statistically significant changes in activity levels; however, effect size estimates revealed there were small changes in MVPA (d = .18) and self-reported walking minutes (p = .18; 95% CI = −161.8 to 29.9; d = .41).
Table 6.
Physical Activity, Physical Function, and Quality of Life.
| Physical Activity/Physical Function and QOL | Baseline, Mean (SD) | 8 Weeks, Mean (SD) | ES, d | 14 Weeks, Mean (SD) | ES, d | P |
|---|---|---|---|---|---|---|
| Steps/day (Lifecorder) | 5334 (2686) | 5272 (3820) | .02 | 5714 (4069) | .11 | NS |
| LPA, min/week (Lifecorder) | 330 (129) | 324 (150) | .04 | 349 (169) | .12 | NS |
| MVPA, min/week (Lifecorder) | 65.7 (81.1) | 82.6 (162.4) | .13 | 88.0 (161.0) | .18 | NS |
| Walking, min/week (PAQ) | 57.5 (68.7) | 72.8 (101.1) | .18 | 123.4 (214.5) | .41a | .18 |
| Stair flights/day (PAQ) | 4.0 (5.4) | 4.3 (5.3) | .05 | 3.8 (3.5) | −.04 | NS |
| SPPB | 10.4 (1.0) | 10.8 (1.1) | .38 | 11.0 (1.0) | .60a | .01 |
| Gait speed (m/s) | 1.11 (.22) | 1.14 (.20) | .14 | 1.14 (.20) | .14 | NS |
| Chair stand (s) | 14.6 (5.0) | 13.0 (3.4) | .37 | 12.6 (4.6) | .42a | <.01 |
| 400-m Walk Time (s) | 342.5 (64.8) | 342.9 (64.8) | 0 | 338.5 (69.0) | .06 | NS |
| Stair climb time | 10.7 (3.4) | 10.7 (3.1) | 0 | 10.7 (3.2) | 0 | NS |
| Lift and carry | 11.5 (2.4) | 11.3 (2.0) | .09 | 11.1 (2.2) | .17a | .18 |
| SF-36 MCS (RAND) | 75.9 (17.2) | 80.1 (12.6) | .27a | 80.6 (9.6) | .34a | NS |
| SF-36 PCS (RAND) | 81.7 (10.0) | 83.3 (9.9) | .16 | 83.0 (9.5) | .13 | NS |
| PSQI | 11.8 (3.2) | 10.7 (2.8) | .36a | 10.5 (2.5) | .45a | .06 |
| SWLS | 25.6 (8.1) | 27.5 (6.4) | .26a | 27.1 (7.2) | .20a | .06 |
Abbreviations: QOL, quality of life; ES, effect size; LPA, light physical activity; MVPA, moderate to vigorous physical activity; PAQ, Physical Activity Questionnaire; SPPB, Short Physical Performance Battery; SF-36 MCS, Short Form-36 Mental Component Scores; SF-36 PCS, Short Form-36 Physical Component Scores; PSQI, Pittsburgh Sleep Quality Index; SWLS, Satisfaction With Life Scale.
Small to moderate effect size (.2-.7).
Physical functioning measures indicated that the majority of the participants were high functioning. The overall SPPB, gait speed, and balance were indicative of good functioning; however, of the 3 tasks, women experienced the most challenge completing the timed chair stands (M = 14.6, ±5.0), with 3 women taking more than 20 seconds to complete this task. For all tests of PF, there was a larger range in scores with some participants scoring on the low end, indicating some deficits in functioning. Repeated-measures ANOVAs showed that there was a significant main effect of time on SPPB, F(1.38, 22.01) = 6.55, p = .011; 95% CI = −0.38 to 0.03. Effect size estimates revealed a moderate effect size (d = .60). For the chair stand aspect of the SPPB, results showed a significant main effect of time, F(2, 32) = 6.38, p = <.01; 95% CI = 0.67 to 3.8, and effect size estimates indicated a moderate effect (d = .42). There was no significant main effect of time on stair climb time, F(2, 32) = 0.02, p = .98; however, effect size estimates indicated that there was a small change (d = .17) and no other significant effects of the intervention on aspects of physical function, including the 400-m walk test or the lift and carry test.
For generic HRQL, the RAND SF-36 component summary scales showed that, in general, women in the study reported high mental and physical HRQL; however, at baseline the physical component scale was slightly higher than the mental component subscale, 75.9 ± 17.2 versus 81.7 ± 10.0. Despite a high baseline level of HRQL, following the intervention, effect size estimates indicated that there was a slight improvement in the mental component summary scale (d = .17); however, this was not statistically significant. The PSQI, a self-report measure of sleep quality with 7 distinct component scores, indicated the mean score at baseline was M = 11.8 ± 3.2, and while effect size estimates indicate there was a moderate improvement (d = .45) over time, this was neither statistically significant (p = .06; 95% CI = −0.19 to 2.9) nor clinically meaningful, as the mean score was still above 5 following the study, indicating on average a poor quality of sleep. Globally, QOL was measured with the SWLS. Findings yielded a mean score of M = 25.6 ± 8.1 (p = .06; 95% CI = −3.1 to −0.01), which, according to the norms provided by Diener et al,48 would be considered high. This is again an indication that there was an overall high quality of life reported within this sample. However, it should again be noted that there were certain individuals who had a relatively poor score on this measure, with a low score of 5 reported.
Intervention Completers’ Analysis
In an exploratory sense, we ran analyses for the EC survivors that completed the entire study to get a sense of the potential benefits of the intervention for those women who adhered (not shown in tables). While there were no significant changes in body composition we did explore these outcomes further (Table 7). On average, women lost 0.6% body fat and roughly 2 lbs of body weight over the course of the intervention. Interestingly, we saw that visceral adipose tissue increased slightly, which was deemed a significant change despite being a small effect. For the rest of the analyses, we specifically focused on measures that effect size estimates indicated had potentially changed. Mindfulness measured with the MAAS was significantly improved, F(2, 18) = 3.924, p = .039, with an effect size estimate of (d = .63). In terms of dietary intake, for the Fats and Oils category, while not significant, the effect size estimate increased (d = 1.86), as it did for fruits and vegetables (d = .23). There were no significant changes in PA; however, the effect size estimate for steps per day (d = .53) showed a moderate effect, LPA (d = .28) and MVPA (d = .45). It should be noted that this effect is most likely due to a few individuals who increased their exercise markedly over the course of the intervention. In terms of PAQ self-report, for walking, the pattern was the same with an effect size change to (d = .53). For physical functioning, repeated-measure ANOVAs only indicated a significant main effect of time on chair stand performance, F(1, 9) = 16.958, p = .003, a component of the SPPB. For quality of life, the mental component scale (MCS) of the RAND SF-36 did not significantly change, but the effect size estimate did slightly increase (d = .46). Sleep quality did not significantly change for intervention completers, F(2, 20) = 3.271, p = .059, but the effect size estimates did indicate a moderate improvement (d = .68).
Discussion
This single-group, pre-post study examined the feasibility and preliminary efficacy of a novel mindfulness-based lifestyle intervention in EC survivors. Despite recruiting challenges, women who were eligible and completed the study reported favorably on the intervention. Seventeen women participated in baseline screening, 13 of whom completed the intervention (76%). We did not see changes in dietary behavior; however, there was some evidence that outcomes important to cancer survivors may have improved. From a preliminary efficacy standpoint, there may be some potential for incorporating a MBI into more conventional behavioral lifestyle approaches for EC survivors; however, the small sample size and study design mean further investigation is warranted.
From the demographics of the study participants, it is apparent that obesity was prevalent and that a high burden of comorbidity existed, further reinforcing the need for behavioral modification in this group of survivors.4 Particularly interesting was the fact that 2 participants who were only marginally overweight by BMI criteria were found to have a high degree of body fatness when assessed on the IDXA, a more accurate measurement of body fat. The implications of this are that while BMI is useful for determining risk in a large population, individually women who have poor dietary habits and who do not engage in PA may still have high levels of adipose tissue. This is a specific risk factor for developing type I EC and further places these women at high risk for future comorbidity.51 Lifestyle modification is therefore an important goal for overweight and obese EC patients and survivors who (a) may be at risk for disease recurrence and (b) have a high burden of comorbidity and reduced quality of life.17,20,52-54 Despite the well-known characteristics of this patient population (sedentary, poor diet, and high comorbidity), there are few systematic, supportive care approaches focused on healthy lifestyle behaviors during survivorship.
One study—a randomized controlled trial designed to test the feasibility of a lifestyle intervention for weight loss and PA in 55 EC survivors—did find that women in the lifestyle arm lost weight and increased their PA relative to the control group at 1 year.55 The authors also examined HRQL and mediators of change in the feasibility intervention and reported that self-efficacy was much lower in morbidly obese survivors and that the intervention did not improve HRQL.56 In a larger follow-up trial, these findings were confirmed, suggesting that lifestyle interventions can be effective when survivors adhere to exercise and dietary guidance.57 More recently, Basen-Engquist and colleagues examined the response of obese versus nonobese EC survivors to an exercise intervention. In the STEPS to Health study, 100 posttreatment, stage I-IIIa survivors participated in a single-arm 6-month study of home-based exercise. At baseline, obese survivors had worse cardiorespiratory fitness and self-reported measures of HRQL.58 While nonobese survivors improved their fitness and exercise behavior to a greater extent than obese survivors did, both groups improved their HRQL and reduced stress to the same degree. Obese survivors also improved their exercise from their baseline levels.
From a feasibility standpoint, there were some difficulties in recruiting patients to this study. One example of the kind of difficulties met was the response from one particular EC patient when asked about her interest in a lifestyle intervention study for healthy EC survivorship. She replied that she “had been treated for her cancer and was now better.” This perhaps is an indication of the fact that many patients are not acutely aware of the importance of lifestyle behaviors on their risk for developing EC in the first place or on their continued health following diagnosis.59 While clinicians do recognize the importance of weight loss and increasing PA for these patients, they do not always have the time or the specialized training to meaningfully impact this aspect of their patient’s lives.60,61 Furthermore, women with type I EC may be unique as cancer patients and survivors in part due to the fact that the majority of them are surgically treated without adjuvant therapies and are survivors at 5 years postdiagnosis.62 Despite these challenges, women who did show an interest in the study reported favorably on both the mindfulness training and the dietary counselling components. The once-weekly meeting schedule was also an achievable demand on time. It was reported by one woman that she had become uncomfortable because she had begun to feel responsible for causing her health problems; while unfortunate, there is always a concern that a program to increase awareness will cause some psychological discomfort and therefore this was specifically addressed as a risk in the informed consent document. Overall, we do feel this type of intervention is safe and feasible in this population of cancer survivors.
Due to the small sample size, we were not statistically powered to detect significant differences in primary or secondary outcomes. However, we still felt it was valuable to explore the preliminary efficacy of our intervention. Specifically, we did not see statistically significant improvements in mindfulness measured with the FFMQ, dietary quality, PA, or HRQL utilizing an intention-to-treat approach. Effect size estimates and an exploratory completers’ analysis did indicate the potential of our intervention to improve some outcomes. Results for the FFMQ at baseline showed that EC survivors had similar scores compared with a population of regular meditators, meaning it may be that we saw a ceiling effect with the FFMQ as these scores were already high at baseline. Scores for the MAAS at baseline were, mean = 4.44, SD = 0.79), which were also similar to those previously found in a mixed group of cancer patients (mean = 4.2, SD = 0.64) and a large US adult sample (mean = 4.26, SD = 0.63) but slightly higher than another sample of cancer patients (mean = 4.08, SD = 0.74).36 We did see a significant improvement measured with the MAAS, and it is feasible that this unitary operationalization of mindfulness was more specifically targeted by the intervention. While a number of studies have explored the role of mindfulness in the context of weight loss, the findings are mixed.12-15 The role of self-efficacy in relation to weight loss has also been examined in other studies. Findings from the SUCCEED trial indicated that improvements in self-efficacy accompanied a decrease in BMI following a lifestyle intervention.18 While there has been no specific examination of mindfulness, self-efficacy and their relationship to weight loss in EC patients, Rejeski and colleagues have previously found that changes in the Weight Efficacy Lifestyle Questionnaire partially mediated the effects of their lifestyle intervention on weight loss.63 There is also some evidence that coping self-efficacy may mediate the relationship between aspects of mindfulness and emotion regulation,64 and that mindfulness still predicts depression, anxiety, stress, and well-being even when accounting for self-efficacy.65 This may suggest that mindfulness can still offer unique benefits beyond those found in more traditional behavioral interventions focused on improving self-efficacy.
The most interesting findings were related to dietary quality. The FHQ has been shown to be positively correlated with more robust 3-day food records,37 but may be limited in its capacity to capture the details of dietary patterns. However, in general, we expected to see poorer scores in most categories and that they would improve as a result of the intervention. One possibility is that women had an awareness of the importance of a healthy diet (eg, fruit and vegetable intake) and this led to them self-reporting higher than expected scores at baseline. Once they had been taught to identify healthy foods and patterns of eating, they may then have more accurately reported on their dietary patterns, and this was reflected as a worsening of scores over time. Despite this, we did see women self-report in the poor category for both “fats and oils” and the “other” category that reflects fast food and high-fat cooking methods, which would confirm poor eating habits. In a similar fashion, we did not see PA, as measured objectively, improve significantly. We did, however, find some women who increased their walking and MVPA a great deal. Furthermore, self-report did show a modest increase in PA overall. Again, this intervention talked about the importance of MVPA but did not specifically aim to increase this in a structured way, as with the dietary counseling. The introduction of gentle yoga and the mention of PA may have resulted in the improved physical functioning we saw reflected in the improved SPPB, even though none of the scores indicated risk for future disability (<10). The primary driver of this change seemed to be the chair stand time. This may have been a reflection of improved technique or simply an increased motivation on the part of the participants. In terms of the HRQL and QOL broadly, we again saw high scores at baseline, meaning it was unlikely we would see much improvement. However, there were still some women who reported low levels initially and who did respond positively, and although not significant, Cohen’s d did indicate a small positive effect. Sleep quality is particularly responsive to mindfulness training24 and that may have been the case here. Despite not improving to a level below the cutoff for poor sleep quality (>5 indicates a poor quality of sleep), sleep quality still improved when looking at the completers’ analysis and effect size estimates. This finding warrants further examination in this population, as many health problems and comorbidities, including fatigue, depression, hypertension, and CVD risk, are associated with poor sleep quality.66,67
There were a number of limitations to the current study. First, we conducted multiple statistical tests on a limited data set and therefore the likelihood of making a type 1 error was high. Because this was an exploratory study, primarily to test feasibility, we felt it was valuable to explore the potential effects of the intervention. Additionally, we were underpowered to detect significant changes in our outcomes, and a larger number of women would have allowed a more robust examination of the interventions effects. We also lacked either a no-treatment or different treatment control group for comparative purposes. We had initially proposed such an intervention, but limitations in the recruitment schedule meant we focused on a single-arm intervention. We did not have a large number of minority participants, which is typical of many research studies, but in the future, there should be a significant effort to include a representative proportion of survivors in order to refine recommendations and determine which programs will be of most benefit based on cultural norms and expectations of supportive care. We would urge some caution with regard to the generalizability of the findings of the current study. Specifically, the enrollment challenges indicate that large numbers of EC survivors may not be interested in this type of intervention. Hard-to-reach populations require a multipronged approach to recruitment that includes both mail and in-person contact, providing access to information sessions to generate interest and especially by garnering support of the clinical providers. If possible and feasible, providing incentives to participation with the use of travel vouchers or gift cards can also be of help. Another limitation of this study related to recruitment was a lack of adequate surveys to determine why women were not interested in this study. This could have provided important information that would serve to improve enrollment in future studies. Another limitation was that the same person conducted the assessments and delivered part of the intervention, which could have biased some of the results. Finally, adherence to home-based mindfulness training and dietary logs was self-reported and impossible to verify.
Strengths of this study were that we began by conducting a theory-based examination of PA and dietary behavior in the same population to refine and target the intervention. The Mindfulness in Motion and the Dietary counselling portions of the intervention have both been previously validated in research studies.24,26,68 However, this was the first time they have been combined to target a unique population of cancer survivors in order to improve lifestyle behaviors and potentially relevant health outcomes. Most traditional approaches to weight loss (increasing PA and improving diet) may be seen as particularly challenging for women who are dealing with a diagnosis of cancer, obesity, and trying to manage multiple comorbidities. This approach therefore aimed to reduce the impact of psychological barriers (anxiety, depression) to making healthy lifestyle choices by increasing survivors’ ability to self-regulate their behavior (eg, improving diet). This skill set would then hypothetically “prime” participants to be more receptive to making healthy eating choices and engaging in PA. It should be noted that a recent study by Zhang et al examined the proportion of EC patients that would be safely able to exercise based on their medical charts.69 Only 14% of patients were deemed safely able to exercise unsupervised, which points to the need for programs where lifestyle change can be gradual and/or staged. Mindfulness training that incorporates gentle yoga and movement first may then pave the way for more substantial behavior change efforts, such as starting an exercise program.
Conclusions
Despite the limitations of the current study, a number of which related to challenges with recruiting an adequate sample, there does seem to be some potential for developing and disseminating novel behavioral lifestyle programs for EC patients and survivors. There is a significant need to improve diet and increase PA in this population and developing approaches that (a) are acceptable and achievable by a large number of women dealing with a diagnosis of cancer and comorbidity concurrently and (b) can enhance the capacity of women to self-regulate their behavior and successfully maintain healthy lifestyles even in the face of challenging psychosocial barriers, such as anxiety and depression, that could lead to improved supportive care.
Mindfulness training is increasingly advocated as part of integrative medical care and has a number of significant benefits. It is a life skill that can be taught and learned by anyone, is not expensive, and does not result in adverse side effects. Newer approaches to teaching mindfulness are being translated into eHealth and mHealth settings, which means a greater capacity for dissemination and therefore reach. We need to make sure that investigations of this type of lifestyle training are done within the context of well-designed, theory-based scientific trials if we hope to see greater uptake and incorporation of mindfulness-based approaches into evidence-based practice for health management.
Acknowledgments
Ashley Harris, RD, provided support for the dietary counseling intervention, and Amanda Goode, MS, provided help with manuscript editing.
Footnotes
Authors’ Note: Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Pelotonia Fellowship Program. This project was also supported in part by a Food Innovation Center (FIC): Doctoral Research Grant of the Ohio State University. Alexander Lucas’s work on this article was also supported in part by a Cancer Control Traineeship, National Cancer Institute/National Institute of Health (NCI/NIH; R25CA122061).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365-1374. [DOI] [PubMed] [Google Scholar]
- 4. Secord AA, Hasselblad V, Von Gruenigen VE, et al. Body mass index and mortality in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2016;140:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [DOI] [PubMed] [Google Scholar]
- 6. Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176-179. [DOI] [PubMed] [Google Scholar]
- 7. von Gruenigen VE, Waggoner SE, Frasure HE, et al. Lifestyle challenges in endometrial cancer survivorship. Obstet Gynecol. 2011;117:93-100. [DOI] [PubMed] [Google Scholar]
- 8. Laskey RA, McCarroll ML, von Gruenigen VE. Obesity-related endometrial cancer: an update on survivorship approaches to reducing cardiovascular death. BJOG. 2016;123:293-298. [DOI] [PubMed] [Google Scholar]
- 9. Allison DB, Downey M, Atkinson RL, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring). 2008;16:1161-1177. [DOI] [PubMed] [Google Scholar]
- 10. Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. New York, NY: Hyperion; 1994. [Google Scholar]
- 11. Shennan C, Payne S, Fenlon D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology. 2011;20:681-697. [DOI] [PubMed] [Google Scholar]
- 12. Olson KL, Emery CF. Mindfulness and weight loss: a systematic review. Psychosom Med. 2015;77:59-67. [DOI] [PubMed] [Google Scholar]
- 13. Godsey J. The role of mindfulness based interventions in the treatment of obesity and eating disorders: an integrative review. Complement Ther Med. 2013;21:430-439. [DOI] [PubMed] [Google Scholar]
- 14. Forman EM, Shaw JA, Goldstein SP, et al. Mindful decision making and inhibitory control training as complementary means to decrease snack consumption. Appetite. 2016;103:176-183. [DOI] [PubMed] [Google Scholar]
- 15. Kearney DJ, Milton ML, Malte CA, McDermott KA, Martinez M, Simpson TL. Participation in mindfulness-based stress reduction is not associated with reductions in emotional eating or uncontrolled eating. Nutr Res. 2012;32:413-420. [DOI] [PubMed] [Google Scholar]
- 16. Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11 suppl):S547-S552. [DOI] [PubMed] [Google Scholar]
- 17. Blanchard CM, Courneya KS, Stein K; American Cancer Society’s SCS-II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198-2204. [DOI] [PubMed] [Google Scholar]
- 18. McCarroll ML, Armbruster S, Frasure HE, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2014;132:397-402. [DOI] [PubMed] [Google Scholar]
- 19. McCarroll ML, Armbruster S, Pohle-Krauza RJ, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137:508-515. [DOI] [PubMed] [Google Scholar]
- 20. Koutoukidis DA, Knobf MT, Lanceley A. Obesity, diet, physical activity, and health-related quality of life in endometrial cancer survivors. Nutr Rev. 2015;73:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975-991. [DOI] [PubMed] [Google Scholar]
- 22. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305-312. [PMC free article] [PubMed] [Google Scholar]
- 23. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York, NY: Guilford Press; 2012. [Google Scholar]
- 24. Klatt MD, Buckworth J, Malarkey WB. Effects of low-dose mindfulness-based stress reduction (MBSR-ld) on working adults. Health Educ Behav. 2009;36:601-614. [DOI] [PubMed] [Google Scholar]
- 25. Klatt M, Steinberg B, Duchemin AM. Mindfulness in Motion (MIM): an onsite mindfulness based intervention (MBI) for chronically high stress work environments to increase resiliency and work engagement. J Vis Exp. 2015;(101):e52359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: a randomized trial. Brain Behav Immun. 2013;27:145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klatt M. Mind-body wellness and integrative medicine. Personalized approach to cancer survivorship. In: Lester J, Schmidt P. eds. Cancer Rehabilitation and Survivorship: Transdisciplinary Approaches to Personalized Care. Pittsburg, PA: Oncology Nursing Society; 2011:317-323. [Google Scholar]
- 28. Campbell MK, Carr C, Devellis B, et al. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Ann Behav Med. 2009;38:71-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [DOI] [PubMed] [Google Scholar]
- 30. World Cancer Research Fund. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 31. Carels RA, Darby L, Cacciapaglia HM, et al. Using motivational interviewing as a supplement to obesity treatment: a stepped-care approach. Health Psychol. 2007;26:369-374. [DOI] [PubMed] [Google Scholar]
- 32. Rejeski WJ, Brawley LR, Ambrosius WT, et al. Older adults with chronic disease: benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22:414-423. [DOI] [PubMed] [Google Scholar]
- 33. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27-45. [DOI] [PubMed] [Google Scholar]
- 34. Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84:822-848. [DOI] [PubMed] [Google Scholar]
- 35. Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15:329-342. [DOI] [PubMed] [Google Scholar]
- 36. Carlson LE, Brown KW. Validation of the Mindful Attention Awareness Scale in a cancer population. J Psychosom Res. 2005;58(1):29-33. [DOI] [PubMed] [Google Scholar]
- 37. Silverstein L, Scott B, Zahrt H. Dietary quality: the Food Habits Questionnaire. In: Jeor S., St ed. Obesity Assessment: Tools, Methods, Interpretations. New York, NY: Chapman & Hall; 1997:281-291. [Google Scholar]
- 38. Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60-70. [DOI] [PubMed] [Google Scholar]
- 39. Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235-243. [DOI] [PubMed] [Google Scholar]
- 40. Ayabe M, Brubaker PH, Mori Y, et al. Self-monitoring moderate-vigorous physical activity versus steps/day is more effective in chronic disease exercise programs. J Cardiopulm Rehabil Prev. 2010;30:111-115. [DOI] [PubMed] [Google Scholar]
- 41. McClain JJ, Craig CL, Sisson SB, Tudor-Locke C. Comparison of Lifecorder EX and ActiGraph accelerometers under free-living conditions. Appl Physiol Nutr Metab. 2007;32:753-761. [DOI] [PubMed] [Google Scholar]
- 42. Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, Lee RE. Validity of a modified CHAMPS physical activity questionnaire among African-Americans. Med Sci Sports Exerc. 2003;35:1537-1545. [DOI] [PubMed] [Google Scholar]
- 43. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. [DOI] [PubMed] [Google Scholar]
- 44. Peters C, Lotzerich H, Niemeier B, Schule K, Uhlenbruck G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994;14:1033-1036. [PubMed] [Google Scholar]
- 45. Peters C, Lotzerich H, Niemeir B, Schule K, Uhlenbruck G. Exercise, cancer and the immune response of monocytes. Anticancer Res. 1995;15:175-179. [PubMed] [Google Scholar]
- 46. Reboussin BA, Rejeski WJ, Martin KA, et al. Correlates of satisfaction with body function and body appearance in middle- and older aged adults: the Activity Counseling Trial (ACT). Psychol Health. 2000;15:239-254. [Google Scholar]
- 47. Rejeski WJ, Ettinger WH, Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157-167. [DOI] [PubMed] [Google Scholar]
- 48. Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49:71-75. [DOI] [PubMed] [Google Scholar]
- 49. Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350-357. [DOI] [PubMed] [Google Scholar]
- 50. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 51. Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009;114:121-127. [DOI] [PubMed] [Google Scholar]
- 52. von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107:2786-2791. [DOI] [PubMed] [Google Scholar]
- 53. Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17:441-446. [DOI] [PubMed] [Google Scholar]
- 54. Cook LS, Nelson HE, Cockburn M, Olson SH, Muller CY, Wiggins CL. Comorbidities and endometrial cancer survival in Hispanics and non-Hispanic whites. Cancer Causes Control. 2013;24:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. von Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gynecol Oncol. 2008;109:19-26. [DOI] [PubMed] [Google Scholar]
- 56. von Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health Qual Life Outcomes. 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125:699-704. [DOI] [PubMed] [Google Scholar]
- 58. Basen-Engquist K, Carmack C, Brown J, et al. Response to an exercise intervention after endometrial cancer: differences between obese and non-obese survivors. Gynecol Oncol. 2014;133:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soliman PT, Bassett RL J., Wilson EB, et al. Limited public knowledge of obesity and endometrial cancer risk: what women know. Obstet Gynecol. 2008;112(4):835-842. [DOI] [PubMed] [Google Scholar]
- 60. Park JH, Lee J, Oh M, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121:2740-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jernigan AM, Tergas AI, Satin AJ, Fader AN. Obesity management in gynecologic cancer survivors: provider practices and attitudes. Am J Obstet Gynecol. 2013;208:e401-e408. [DOI] [PubMed] [Google Scholar]
- 62. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [DOI] [PubMed] [Google Scholar]
- 63. Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: the cooperative lifestyle intervention program. J Gerontol B Psychol Sci Soc Sci. 2011;66:279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luberto CM, Cotton S, McLeish AC, Mingione CJ, O’Bryan EM. Mindfulness skills and emotion regulation: the mediating role of coping self-efficacy. Mindfulness. 2014;5:373-380. [Google Scholar]
- 65. Soysa CK, Wilcomb CJ. Mindfulness, self-compassion, self-efficacy, and gender as predictors of depression, anxiety, stress, and well-being. Mindfulness. 2013;6:217-226. [Google Scholar]
- 66. Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lehrer S, Green S, Ramanathan L, Rosenzweig KE. Obesity and deranged sleep are independently associated with increased cancer mortality in 50 US states and the District of Columbia. Sleep Breath. 2013;17:1117-1118. [DOI] [PubMed] [Google Scholar]
- 68. Focht BC, Lucas AR, Grainger E, Simpson C, Thomas-Ahner JM, Clinton SK. The Individualized Diet and Exercise Adherence Pilot Trial (IDEA-P) in prostate cancer patients undergoing androgen deprivation therapy: study protocol for a randomized controlled trial. Trials. 2014;15:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang X, Haggerty AF, Brown JC, et al. The prescription or proscription of exercise in endometrial cancer care. Gynecol Oncol. 2015;139:155-159. [DOI] [PMC free article] [PubMed] [Google Scholar]


