Abstract
Background
Some people are highly motivated to seek aggressive encounters, and among those who have been incarcerated for such behavior, recidivism rates are high. These observations echo two core features of drug addiction: high motivation to seek addictive substances, despite adverse consequences, and high relapse rates. Here we used established rodent models of drug addiction to determine whether they would be sensitive to “addiction-like” features of aggression in CD-1 mice.
Methods
In Exp. 1–2, we trained older CD-1 mice to lever-press for opportunities to attack younger C57BL6/J mice. We then tested them for relapse to aggression seeking after forced abstinence or punishment-induced suppression of aggression self-administration. In Exp. 3, we trained a large cohort of CD-1 mice, and tested them for choice-based voluntary suppression of aggression seeking, relapse to aggression seeking, progressive ratio responding, and punishment-induced suppression of aggression self-administration. We then used cluster analysis to identify patterns of individual differences in compulsive “addiction-like” aggressive behavior.
Results
In Exp. 1–2, we observed strong motivation to acquire operant self-administration of opportunities to aggress, and relapse vulnerability during abstinence. In Exp. 3, cluster analysis of the aggression-related measures identified a subset of “addicted” mice (~19%) that exhibited intense operant-reinforced attack behavior, decreased likelihood to select an alternative reinforcer over aggression, heightened relapse vulnerability and progressive ratio responding, and resilience to punishment-induced suppression of aggressive behavior.
Conclusion
Using procedures established to model drug addiction, we showed that a subpopulation of CD-1 mice demonstrate “addiction-like” aggressive behavior, suggesting an evolutionary origin for pathological aggression.
Keywords: Aggression, reward, motivation, addiction, relapse, mice
Introduction
Aggression is evolutionarily advantageous, critical to survival, and well-conserved across species (1, 2). However, voluntarily seeking aggression against members of one’s own species, and finding the experience reinforcing, has been described as an almost entirely human occurrence, representing a “perversion” of a hunting instinct that can be unmasked in the general population under permissive circumstances and may thereafter be repeated compulsively (3–5). The phenomenology of this appetitive aggression is similar to that of other rewarding experiences, such as sexual pleasure or drug intoxication; accordingly, aggression is sometimes pursued despite immediate or long-term adverse consequences (6, 7). Unsurprisingly, relapse (recidivism) rates among violent offenders are as high as relapse rates in drug addiction (7–9). Thus, appetitively driven human aggression appears to mimic core features of drug addiction: high motivation to seek the rewarding stimulus, often despite adverse consequences, and high relapse rates. Additionally, like drug addiction, which develops in only about 20% of people who use addictive drugs (10), pathological aggression develops only in a minority of people who engage in aggressive encounters during their lifetime (11–13).
If appetitive aggression against conspecifics is modulated by reward mechanisms similar to those that drive drug addiction, it may be observable across species in an analogous manner (14). There is already some evidence for this. Dominant mice will lever press or nose-poke for the opportunity to attack subordinate intruder mice (15–17), as well as form persistent conditioned place preference (CPP) to aggression-paired contexts (18, 19). However, self-administration and CPP are not sufficient to show that a rewarding stimulus is being sought maladaptively, addictively or pathologically (14, 20, 21). In models of drug addiction, those criteria have been operationalized as self-administration despite adverse consequences (compulsive drug self-administration) and relapse to drug seeking during abstinence (20, 22). To our knowledge, no published reports have evaluated these behaviors in preclinical studies on aggressive behavior.
In the present study, we first determined relapse to aggression seeking by combining a mouse operant model of self-administration of aggression (15, 23) with rodent drug relapse models in which drug seeking is assessed after prolonged forced abstinence in the homecage (24, 25) or after punishment-induced abstinence (26), or after choice-based voluntary abstinence (27). We then used an experimental procedure inspired by a DSM-IV-based rat model of addiction (14, 20), in which we trained a large cohort of male CD-1 mice for aggression self-administration, and then tested them for choice-based voluntary suppression, relapse to aggression seeking, responding under a progressive ratio reinforcement schedule, and aggression self-administration despite adverse consequences (punishment).
The results of our first set of experiments demonstrate robust relapse to aggression seeking (operationally defined as persistent lever-pressing under extinction conditions) after prolonged abstinence or suppression of aggression seeking, whether forced, punished or choice-based, in nearly all aggressive mice. The results of our follow-up experiment, using previously established metrics for an “addicted” rodent (14, 20), identify a subset of “aggression-addicted” mice (~19%) that exhibited intense operant-reinforced attack behavior, decreased likelihood to select an alternative palatable food reward over aggression, heightened relapse vulnerability and progressive ratio responding, and resistance to punishment-induced suppression of aggression self-administration.
Materials and Methods
A detailed description of experimental subjects, apparatus, and procedures are provided in the Supplementary Online Methods. Below, we describe the specific experiments.
Exp. 1: Relapse to aggression seeking after forced abstinence (Figure 1A)
Figure 1. Relapse to aggression seeking after forced abstinence.
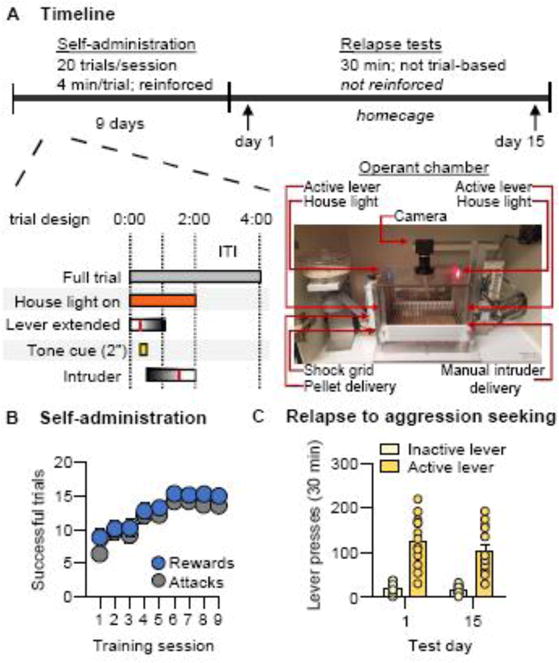
(A) Time course schematic for training and testing (top) and for individual trials (bottom). Vertical red lines within the “lever extended” and “intruder” bars indicate an active lever press and removal of an intruder following an attack bout, respectively, relative to total possible durations. (B) Number of rewarded and attack trials over 9 days (80-min session/d) of aggression self-administration under a trial-design fixed-ratio-1 (FR1) reinforcement schedule (n=26). (C) Number of nonreinforced “active”-lever and inactive-lever presses during a 30-min relapse to aggression seeking test under extinction conditions on Day 1 (n=13) or Day 15 (n=13) of forced abstinence. Individual data denoted with circles. Data are mean±SEM.
The goal of Exp. 1 was to determine the persistence of non-reinforced aggression seeking (relapse) after cessation of self-administration. We trained 32 mice for self-administration, of which we excluded 6 “non-aggressive” mice (their data are not shown) that either failed to acquire self-administration or attacked less than 6 times on average across the 9 training days. We tested the 26 remaining mice for relapse to aggression seeking (30-min tests) after 1 day (n=13) or 15 days (n=13) of forced abstinence in their homecage (between-subjects design). During the relapse tests, the mice were returned to the self-administration chamber, thereby re-exposing them to the contextual cues associated with aggression self-administration during training. Lever-presses led to contingent delivery of the discrete cue previously paired with the delivery of submissive C57 mice.
Exp. 2: Relapse to aggression seeking after punishment-induced suppression (Figure 2A)
Figure 2. Relapse to aggression seeking after punishment-induced suppression.
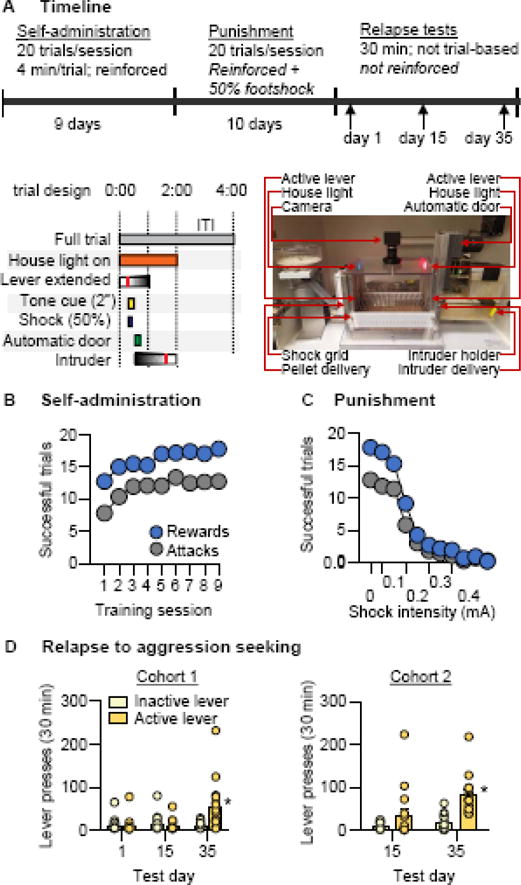
(A) Time course schematic for training, punishment, and testing (top) and for individual trials (bottom). (B) Number of rewarded and attack trials over 9 days (80-min session/d) of aggression self-administration under a trial-design FR1 reinforcement schedule (n=31). (C) Number of rewarded and attack trials over 10 days (80-min session/d) of aggression self-administration during the punishment phase (n=31). The mice received response-contingent shocks in sessions 1–6 (0.1 mA increase every other day), and 0.4 mA on sessions 7–10 on 50% of reinforced active-lever presses. (D) Number of nonreinforced “active”-lever and inactive-lever presses during a 30-min test of relapse to aggression seeking in cohort 1 (left, n=16) and cohort 2 (right, n=15) under extinction conditions. Individual data denoted with circles. * Different from day 1 and 15 (left) or day 15 (right), p < 0.05. Data are mean±SEM.
The goal of Exp. 2, in which we ran two cohorts of mice in succession, was to measure relapse to aggression seeking after suppression induced by punishment rather than after forced abstinence. For the two cohorts, we trained a total 54 mice for aggression self-administration, of which we excluded 23 “non-aggressive” mice. In the first cohort, we trained 16 mice for aggression self-administration; after punishment-induced suppression, we repeatedly tested them for relapse 1 day, 15 days, and 35 days after the last punishment session. Because relapse rates were very low on days 1 and 15 and increased on day 35, we repeated the experiment with a second cohort of mice (n=15), testing them at only 15 and 35 days to independently replicate the time-dependent increase in relapse to aggression seeking. During the forced-abstinence days, we kept the mice in the animal facility.
Exp. 3: Relapse to aggression seeking after choice-based voluntary suppression and examination of individual differences (Figures 3A and 4A)
Figure 3. Relapse to aggression seeking after choice-based voluntary suppression, and differences in propensity to aggression in a large cohort of CD-1 mice.
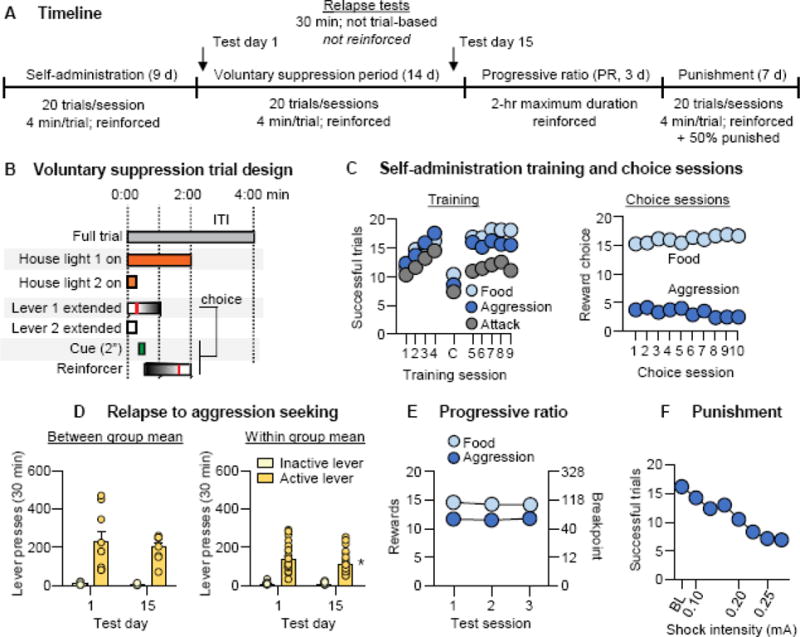
(A) Schematic of overall the time course for training, choice-based voluntary suppression, progressive-ratio testing, and punishment testing. (B) Schematic for individual trials. Self-administration trials and punishment trials were identical to those described in Fig. 1A and 2A. (C) Left: Number of food rewards, aggression rewards, and attack trials (left) over 9 days (80-min session/d) of food and aggression self-administration under an FR1 reinforcement schedule (n=43). Right: Number of food and aggression rewards earned over 10 days (80-min session/d) of the voluntary suppression choice phase (n=43). (D) Number of nonreinforced “active”-lever and inactive-lever presses during a 30-min test of relapse to aggression seeking. Left: between-subjects comparison, day 1 (n=9) versus day 15 (n=8). Right: within-subjects comparison (n=26). (E) Number of rewards earned during three 2-h progressive-ratio tests for aggression (left) and palatable food (right) (n=43). (F) Number of rewarded trials over 10 days (80-min session/day) of aggression self-administration during the punishment phase (n=43). The mice received response-contingent shocks (0.1 mA on days 1–3, 0.2 mA on days 4–5, and 0.25 mA on days 6–7) on 50% of reinforced active-lever presses. Individual data denoted with circles in corresponding panels. * Different from day 1, p<0.05; C = choice test. Data are mean±SEM.
Figure 4. Cluster analysis of aggression-related behaviors.
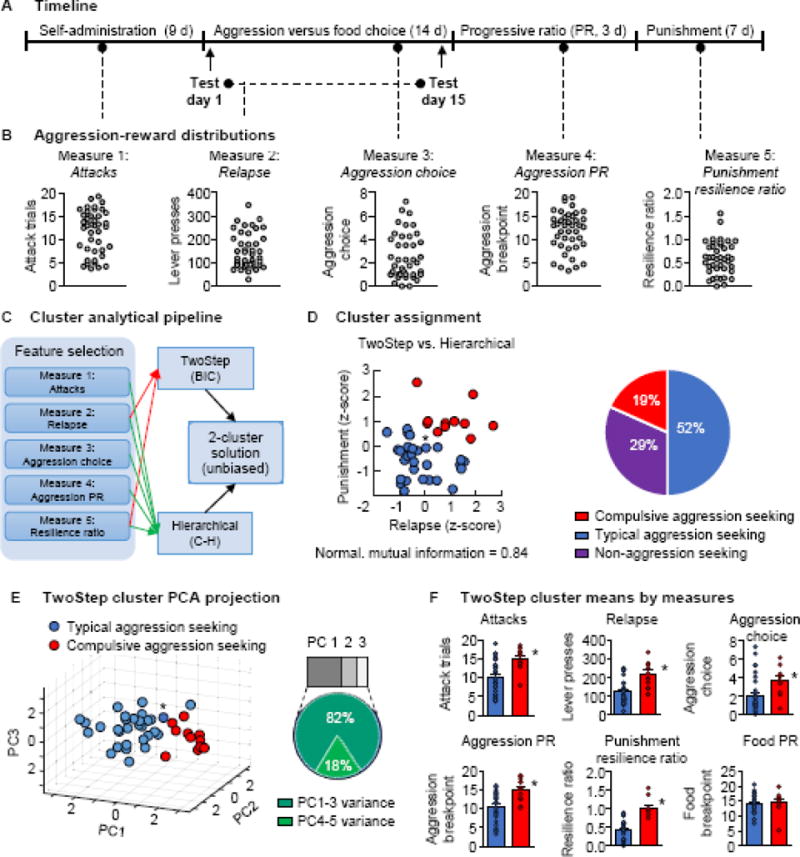
(A) Schematic of measures used in cluster analysis. (B) Distributions of individual responses (n=41) for each of the five measures: attacks, relapse, aggression choice, aggression progressive ratio, and punishment (see text). (C) Cluster analytical pipeline. Two clustering algorithms were used independently to provide unbiased optimal group assignments and establish cluster assignment overlap. (D) Scatterplot of normalized punishment and resilience ratio scores, colored by cluster assignment. Red circles denote compulsive aggression seeking mice (Cluster 1, n=11) and blue circles denote typical aggression seeking mice (Cluster 2, n=30). Right: Final phenotype assignments under TwoStep clustering. (E) Visual representation of clusters using projection of data onto the first 3 principal components of the 5 original measures. Asterisk denotes the single mouse differentially assigned between clustering algorithms in 4D and 4E. Right: Pie chart showing the variance of the 3 principal components by which the data are displayed. (F) Breakdown, by cluster, of the measures shown in 4B. Individual data denoted with circles. * Different from the typical aggression group, p < 0.05; PC = principal component; BIC = Bayesian Information Criterion; C–H = Calinski-Harabasz criterion; MAD; mean absolute deviation. Group data are mean±SEM.
In Exp. 3 we tested the persistence of aggression seeking following voluntary suppression by availability of a mutually exclusive alternative reward, palatable food. We also used a larger sample so we could examine individual differences. We trained 60 mice for aggression self-administration, of which we excluded 17 “non-aggressive” mice. We divided the remaining 43 mice into two cohorts, each of which we first trained for self-administration of both palatable food and aggression for 9 days (counterbalanced order each day), followed by tests for relapse to aggression seeking 1 day and/or 15 days after training; in between the tests, we exposed the mice to the choice-based voluntary-suppression procedure (see above). In the first cohort (n=17), we tested the mice for relapse to aggression seeking on both day 1 and day 15 (n=9) or on day 15 only (n=8) (within- and between-subjects assessment). We then tested all mice under the progressive-ratio schedule and for punishment-induced suppression of aggression self-administration. In the second cohort (n=26), we used the same procedure and tested all mice for relapse to aggression seeking on day 1 and day 15. The data from Exp. 3 were used for cluster analysis as described in the Supplementary Online Methods and results.
Results
Relapse to aggression seeking after forced abstinence
In Experiment (Exp.) 1 we used a rodent model of drug relapse after forced abstinence (28) to determine whether the propensity to relapse to aggression seeking would decrease, increase, or remain stable at extended time points following the cessation of voluntary aggression (Fig. 1A). We trained sexually experienced, 4–6-month-old male CD-1 mice (n=32) to self-administer encounters with an intruder (a younger submissive C57BL/6J (C57) male mouse; see Supplementary Online Methods). We excluded 6 “non-aggressive” mice that either failed to acquire self-administration or attacked less than 6 times per day during the training phase (data not shown). The remaining 26 mice increased their aggression self-administration over days, as measured by the daily number of rewarded trials (F8,200=10.3, p<0.001) and the number of trials on which they attacked the intruder (F8,200=10.1, p<0.001) (Fig. 1B; see Supplemental Table S1 for complete statistical results). We then assigned aggressive mice to two groups and tested them for relapse to non-reinforced aggression seeking on either Day 1 (n=13) or Day 15 (n=13) of forced abstinence. On both days, the mice showed high rates of responding on the previously active but not the inactive lever (F1,24=97.4, p<0.001), indicating persistent aggression seeking that was independent of the duration of forced abstinence (p>0.1) (Fig. 1C).
Relapse to aggression seeking after punishment-induced abstinence
In Exp. 2 we used a rodent model of drug relapse after punishment-induced suppression of self-administration (26) to determine whether aggression seeking would resume after termination of punishment (Fig. 2A). We trained sexually experienced, 4–6-month-old CD-1 mice (n=54) to self-administer encounters with an intruder C57 mouse. We excluded 23 “non-aggressive” mice that failed to acquire self-administration. The remaining 31 mice increased their aggression self-administration over days, as measured by the daily number of rewarded trials (F8,240=9.7, p<0.001) and attack trials (F8,240=6.6, p<0.001) (Fig. 2B). After self-administration training, we exposed the aggressive mice to 10 days of punished responding, in which 50% of the reinforced lever-presses were paired with footshock; we gradually increased the shock intensity over days (see Supplementary Online Methods). During the punishment phase, the mice decreased their responding for aggression with increasing shock intensity, as measured by the number of rewarded daily trials (F6,180=234.1, p<0.001) and attack trials (F6,180=82.3, p<0.001) (Fig. 2C). Next, we split these mice into 2 groups for relapse testing. We tested the first group (n=16) on Day 1, Day 15, and Day 35 of forced abstinence after termination of the punishment phase. We found that aggression seeking was significantly higher on Day 35 than on Day 1 and Day 15 (Test day × Lever interaction, F2,30=6.2, p=0.006) (Fig. 2D, left). Because relapse rates were very low on Day 1 and Day 15 and increased on Day 35, we repeated the experiment with the second group of mice (n=15), testing them only on Day 15 and Day 35 to independently replicate the time-dependent increase in relapse to aggression seeking after termination of punishment-imposed abstinence. We found that aggression seeking was significantly higher and Day 35 than on Day 15 (Test day × Lever interaction, F1,14=17.3, p=0.001) (Fig. 2D, right).
Relapse to aggression seeking after choice-based voluntary suppression
In Exp. 3 we used a rodent model of drug relapse after choice-based voluntary abstinence or suppression of drug self-administration (27) to determine whether aggression seeking would subsequently decrease, increase, or remain stable after voluntary suppression of aggression self-administration (Fig. 3A). Voluntary suppression of aggression self-administration was achieved by providing a mutually exclusive palatable-food reward during repeated daily choice sessions (see Supplementary Online Methods). We also used a larger sample so we could examine individual differences in propensity to aggression seeking, using different measures of motivation to seek an appetitive reward (see below). We trained 60 CD-1 mice for self-administration of access to aggression and food for 9 days (1 session/d for each reward). We excluded 17 mice because they failed to acquire aggression self-administration. The remaining aggressive mice (n=43) acquired self-administration of both food (F8,336=23.2, p<0.001) and aggression (rewarded trials: F8,336=9.0, p<0.001; attack trials: F8,336=3.7, p<0.001) across 9 daily counterbalanced training sessions (Fig. 3C, left). We exposed the mice to one choice session between the 4th and 5th training sessions to determine initial choice during training. We then tested the mice for voluntary suppression of aggression seeking using the palatable food as the alternative reward, observing a robust preference for food (F1,42=260.1, p<0.001) that increased over the choice sessions (F9,378=4.3, p<0.001) (Fig. 3C, right).
Prior to choice-based voluntary suppression, we split the mice into two cohorts for both between- and within-subjects assessment of relapse. In the first cohort (n=17), we used a between-subjects design and compared two groups of mice for relapse before (Group 1, n=9, Day 1) versus after voluntary suppression of aggression self-administration (Group 2, n=8, Day 15). We also exposed the mice in Group 1 to the choice-based voluntary suppression procedure and retested them for relapse on Day 15. In the second cohort (n=26), we used a within-subjects design and tested all mice for relapse repeatedly on Day 1 and Day 15 under conditions identical to those of Group 1 in cohort 1. In each cohort of mice, we observed high rates of responding on the active but not the inactive lever on Day 1 and Day 15, indicating persistent aggression seeking that was only minimally suppressed by choice (between group: F1,15=63.6, p<0.001; within group: F1,25=118.2, p<0.001); the within-group cohort showed a modest decrease in responding across the two test days (F1,25=6.8, p=0.015), possibly due to extinction learning (Fig. 3D).
We next tested all mice for 3 days for their motivation to seek aggression and palatable food under a progressive-ratio reinforcement schedule (see Supplementary Online Methods), a measure of the reinforcing efficacy of positive reinforcers. Responding was higher for food than for aggression (F1,42=14.8, p<0.001) (Fig. 3E). Last, we tested the mice in a modified punishment procedure where the shock was increased across days up to a maximum value of 0.25 mA, a shock level that would allow us to detect individual differences in punishment sensitivity (see Supplementary Online Methods), based on the results of Exp. 2. The mean number of reward trials decreased across sessions as shock level increased (F7,294=30.0, p<0.001) (Fig. 3F).
Cluster analysis of individual differences in aggression-seeking behaviors
The data from Exp. 3 were then used to identify individual differences in aggression motivation. We used unsupervised cluster analyses to obtain an unbiased estimate of the number of aggression-seeking phenotypes within the population, and to classify individual aggressive mice into the identified subpopulations. Before clustering the aggression motivation data, we first excluded two mice that were more than 2.5 standard deviations and 3 median absolute deviations from the 5-dimensional centroid/medoid of the sample, leaving a reduced sample of 41 aggressive mice. We chose the following 5 dimensions for cluster analysis (Fig. 4A–B): (1) attacks, (2) relapse, (3) aggression choice, (4) aggression progressive ratio, and (5) punishment resilience ratio (see Supplemental Online Methods for a detailed description).
We then used an SPSS classification procedure, TwoStep clustering, both to determine the number of clusters in the dataset and to assign every mouse to a cluster. We chose to use relapse and punishment resilience ratio (resistance to punishment) as the variables for TwoStep clustering because (1) relapse propensity (presumably reflecting persistent craving) and disregard of adverse consequences are cardinal features of addiction, and (2) clustering routines will perform better on small sample sizes with reduced dimensionality (in our case, n=41 observations). TwoStep clustering identified an optimal cluster number of 2 in the two-dimensional distribution of relapse and punishment resilience ratio values and classified 30 mice into one cluster that we termed typical aggression seeking and 11 mice into the second cluster that we termed compulsive aggression seeking (Fig. 4C).
We then ran a parallel cluster analysis to validate the results of the TwoStep analysis. For this secondary cluster analysis, we used all five measures in an agglomerative hierarchical algorithm (Ward’s method). The results were like those of the TwoStep routine, finding an optimal cluster number of 2 with Calinski-Harabasz validation and producing cluster assignments that differed by only one mouse from those of the TwoStep routine (typical aggression seeking, n=29; compulsive aggression seeking, n=12; Normalized Mutual Information=0.84, p<0.001). This mouse is indicated by an asterisk in Fig. 4D–E. For all subsequent analyses, we use the cluster assignments from the initial TwoStep classification (see Supplemental Table S2 for direct comparisons of the group means for the 5 measures between the two clustering methods).
To display the clusters, we represented 5-dimensional data from each mouse in a reduced 3-dimensional space spanned by the first 3 principal components of the data (proportion of explained variance: 82%) (Fig. 4E, right, Supplemental Fig. S1). Subsequent comparison of the cluster means confirmed that the 11 mice classified as “compulsive aggression seeking” were significantly higher on all 5 aggression-seeking measures (attacks: F1,40=10.5, p=0.002; relapse: F1,40=15.8, p<0.001; aggression choice: F1,40=6.3, p=0.016; aggression progressive ratio: F1,40=45.8, p=0.002; and punishment-resilience ratio: F1,40=10.5, p=0.002) (Fig. 4F). The mice in the two clusters did not differ in their motivation to seek palatable food under the progressive-ratio schedule (p>0.1) (Fig. 4F).
Discussion
We used established animal models of drug addiction and relapse to characterize motivated aggression behavior in male CD-1 mice. We report three main findings. First, we observed that ~70% of older, sexually experienced outbred CD-1 mice will rapidly learn to lever-press for an opportunity to attack younger subordinate male C57 mice. Second, using models of drug relapse after forced abstinence (25), punishment-induced abstinence (26), or choice-based voluntary abstinence (27), we showed relapse to aggression seeking that persisted long after the last aggressive act. Third, and perhaps most important, our cluster analysis of the aggression-related measures identified a subset of mice (~19%) that met criteria previously developed to denote addiction in rodent models (14, 20, 21, 29): intense operant-reinforced attack behavior, decreased likelihood to select an alternative food reward over aggression, heightened relapse vulnerability and progressive ratio responding, and resistance to punishment-induced suppression of aggressive behavior. Below we discuss these findings.
Aggression self-administration in mice
Our findings of reliable aggression self-administration in CD-1 mice confirm and extend work with other strains of mice, starting with studies by Fish et al. (15, 23), the originators of the operant aggression self-administration procedure. They showed that adult male sexually experienced outbred CFW resident mice would nose-poke for access to attack adult male subordinate sexually naïve CFW intruders under any of several reinforcement schedules. Subsequently, Couppis et al. (16) and May et al. (30) showed that outbred adult male sexually experienced Swiss-Webster resident mice would nose-poke for access to subordinate adult sexually-naïve Swiss-Webster intruders. Most recently, Falkner et al. (17) showed that outbred adult male sexually-experienced Swiss Webster resident mice would nose-poke for access to subordinate sexually-naïve inbred Balb/c intruders.
Together, these results and our training-phase results indicate that the act of aggression can be rewarding to some mice, in a manner analogous to that seen in some people under both typical circumstances (6, 31–33) and pathological/clinical circumstances (34, 35).
Relapse to aggression reward
The aggressive CD-1 mice exhibited robust relapse to aggression seeking, as assessed in models of relapse after forced abstinence (25) or voluntary abstinence (27). One difference, however, was that relapse to aggression seeking did not appear to progressively increase or incubate over time (Fig. 1 & 3), as is the case with addictive drugs (24, 36). The reasons for this difference are unknown, but we suspect that it was due to the very high rates of lever-pressing on day 1 of abstinence—several-fold higher than what we and others have observed in studies of incubation of drug seeking (24, 36). This suggests that the motivation to seek aggression during forced or voluntary abstinence remains high in a time-independent manner. In contrast, we found that while punishment suppressed aggression-seeking behavior for the initial two-weeks post-punishment, aggression seeking gradually recovered in a subset of the mice, who ultimately showed a high propensity to relapse. This is like our earlier findings with punished seeking of methamphetamine or palatable food (26).
Our discrete-choice voluntary suppression procedure significantly decreased aggression self-administration in most mice. This finding extend results from previous studies by our group and others, in which discrete-choice procedures (palatable food versus drug) were used to decrease self-administration of cocaine (37), heroin (38, 39), or methamphetamine (27) in food-sated rats. However, in those studies, we observed complete suppression of drug intake in essentially all rats, even after extended long-term access to methamphetamine or heroin (39, 40). In contrast, suppression of aggression self-administration was more variable across mice, and the availability of an alternative food reward rarely caused complete cessation of aggression during the choice sessions (Fig. 3C).
Addiction-like aggressive behavior
Variability across mice was one of the key features of our findings, and we used this feature to emulate methods that other investigators have used to characterize addiction-prone subpopulations of laboratory animals (14, 21, 41, 42). Using those methods, we found that ~19% of our large cohort of mice met criteria for an “aggression-addicted” rodent. This subpopulation showed intense operant-reinforced attack behavior, decreased likelihood to select an alternative food reward over aggression, heightened relapse vulnerability and progressive ratio responding, and resistance to punishment-induced suppression of aggressive behavior. In drug-addiction research, these are the hallmarks of the addicted rodent (20, 41–43).
To identify subpopulations in our mice, we performed unbiased clustering on aggression-related measures to reveal two distributions in the population, which we termed compulsive aggression seeking and typical aggression seeking mice. We corroborated our results with a second clustering routine, which gave almost identical results and reassigned only a single subject between clusters. We take the existence of two subpopulations to reflect the fact that aggression can be highly adaptive, even critical for survival; in stable closed colonies of feral-derived mice, aggression has a clear hereditary natural-selection bias (44). It may have been evolutionarily advantageous for aggression to be neurally linked with reward. However, like other reward-driven behaviors (45), aggression can become maladaptive. For example, in mice bred for aggressiveness, repeated victories lead to increasingly severe and unusually aggressive attacks over subsequent resident-intruder pairings, even when the subordinate displays clear submission signals (46). Understanding how this transition occurs, and even the basic neural mechanisms guiding adaptive aggression seeking, will have important implications for the development of treatment strategies across neuropsychiatric disorders that are co-morbid with maladaptive aggression (47).
However, there are some caveats in the interpretation of the present data. One issue is that our data were derived from male, sexually experienced genetically outbred CD-1 mice, a strain known for its innately high aggression levels. Thus, whether the data from the current sample generalizes to other mice strains and to female mice is unknown. Another issue is that the ethology of aggression, while often conserved across species, is evolutionarily guided to each species’ niche environment. Thus, we do not know whether the ‘compulsive-aggression seeking’ observed in our study is pathological or maladaptive in mice. However, we argue that such behavior in humans is detrimental and that modeling the ’compulsive’ appetitive aggression in mice may be relevant to understanding the neural circuits underlying human appetitive aggression, which is often both pathological and maladaptive.
Clinical implications
Inappropriate aggression is the direct cause of suffering and death for millions of people around the world (48). Like addictive drugs, aggression can be highly rewarding, pursued despite immediate or long-term adverse consequences (6, 33), and sought anew after lengthy enforced abstinence (7). We take the general commonality of aggression seeking between rodents and humans, and more importantly the shared vulnerability for compulsive addiction-like aggression seeking within a smaller subpopulation in each species, to reflect a biologically conserved correlate of compulsive aggression seeking across species. This observation suggests that this type of aggression in humans—appetitive aggression (3)—can be viewed within the context of addiction, and that neurobiological and behavioral tools for the study of compulsive drug seeking and relapse should be used to study brain mechanisms of this type of aggression, both preclinically and clinically. Our finding of a distinct subpopulation of compulsively aggression-seeking male CD-1 mice suggests an evolutionary origin for pathological aggression and support theories that explain human aggression in terms of its appetitive rewarding properties (31). Finally, an appetitively motivated compulsion toward aggression might be an important endophenotype to include in dimensional formulations of psychopathology such as the National Institute of Mental Health’s Research Domain Criteria (RDoC). The current formulation of the RDoC mentions aggression only within the domain called Negative-Valence Systems (49). RDoC workgroups have considered adding non-defensive aggression as a unit of behavioral analysis within the domain called Social Processes, but only in terms of its instrumental role in establishing social hierarchies (50). Our findings suggest that these conceptualizations of aggression are fundamentally incomplete. Whether viewed dimensionally (as a trait that every individual possesses to some degree) or categorically (as a pathological excess in a discrete subpopulation), some forms of aggression may be best understood as strong appetitive reinforcers, carrying the attendant risks of addiction.
Supplementary Material
Acknowledgments
Funding
The research was supported by the Intramural Research Program of NIDA (YS) and PRAT 1FI2GM117583-01 (SAG). We thank Andrew Huang for assisting in running the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Thomas AL, Davis SM, Dierick HA. Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom? PLoS Genet. 2015;11:e1005416. doi: 10.1371/journal.pgen.1005416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grether GF, Anderson CN, Drury JP, Kirschel AN, Losin N, Okamoto K, et al. The evolutionary consequences of interspecific aggression. Ann N Y Acad Sci. 2013;1289:48–68. doi: 10.1111/nyas.12082. [DOI] [PubMed] [Google Scholar]
- 3.Weierstall R, Elbert T. The Appetitive Aggression Scale-development of an instrument for the assessment of human’s attraction to violence. Eur J Psychotraumatol. 2011;2 doi: 10.3402/ejpt.v2i0.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crombach A, Elbert T. The benefits of aggressive traits: a study with current and former street children in Burundi. Child Abuse Negl. 2014;38:1041–1050. doi: 10.1016/j.chiabu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Meyer-Parlapanis D, Weierstall R, Nandi C, Bambonye M, Elbert T, Crombach A. Appetitive Aggression in Women: Comparing Male and Female War Combatants. Front Psychol. 2015;6:1972. doi: 10.3389/fpsyg.2015.01972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chester DS, DeWall CN. The pleasure of revenge: retaliatory aggression arises from a neural imbalance toward reward. Soc Cogn Affect Neurosci. 2016;11:1173–1182. doi: 10.1093/scan/nsv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durose M, Cooper A, Synder H. In: Recidivism Of Prisoners Released In 30 States In 2005: Patterns From 2005 To 2010. Statistics BoJ., editor. 2014. (Recidivism of Prisoners Released Series). [Google Scholar]
- 8.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Drug Alcohol Depend. 1994;2:244–268. [Google Scholar]
- 11.Tremblay RE. Early development of physical aggression and early risk factors for chronic physical aggression in humans. Curr Top Behav Neurosci. 2014;17:315–327. doi: 10.1007/7854_2013_262. [DOI] [PubMed] [Google Scholar]
- 12.Provencal N, Booij L, Tremblay RE. The developmental origins of chronic physical aggression: biological pathways triggered by early life adversity. J Exp Biol. 2015;218:123–133. doi: 10.1242/jeb.111401. [DOI] [PubMed] [Google Scholar]
- 13.Lacourse E, Cote S, Nagin DS, Vitaro F, Brendgen M, Tremblay RE. A longitudinal-experimental approach to testing theories of antisocial behavior development. Dev Psychopathol. 2002;14:909–924. doi: 10.1017/s0954579402004121. [DOI] [PubMed] [Google Scholar]
- 14.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 15.Fish EW, De Bold JF, Miczek KA. Aggressive behavior as a reinforcer in mice: activation by allopregnanolone. Psychopharmacology (Berl) 2002;163:459–466. doi: 10.1007/s00213-002-1211-2. [DOI] [PubMed] [Google Scholar]
- 16.Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl) 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 17.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D. Hypothalamic control of male aggression-seeking behavior. Nat Neurosci. 2016;19:596–604. doi: 10.1038/nn.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1mice. Genes Brain Behav. 2016 doi: 10.1111/gbb.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 22.Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fish EW, DeBold JF, Miczek KA. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology (Berl) 2005;182:116–127. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- 24.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 30.May ME, Kennedy CH. Aggression as positive reinforcement in mice under various ratio- and time-based reinforcement schedules. J Exp Anal Behav. 2009;91:185–196. doi: 10.1901/jeab.2009.91-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bushman BJ, Baumeister RF, Phillips CM. Do people aggress to improve their mood? Catharsis beliefs, affect regulation opportunity, and aggressive responding. J Pers Soc Psychol. 2001;81:17–32. [PubMed] [Google Scholar]
- 32.Moran JK, Weierstall R, Elbert T. Differences in brain circuitry for appetitive and reactive aggression as revealed by realistic auditory scripts. Front Behav Neurosci. 2014;8:425. doi: 10.3389/fnbeh.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan G, Preston-Campbell RN, Moeller SJ, Steinberg JL, Lane SD, Maloney T, et al. Reward vs. Retaliation-the Role of the Mesocorticolimbic Salience Network in Human Reactive Aggression. Front Behav Neurosci. 2016;10:179. doi: 10.3389/fnbeh.2016.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glenn AL, Raine A. Psychopathy and instrumental aggression: Evolutionary, neurobiological, and legal perspectives. Int J Law Psychiatry. 2009;32:253–258. doi: 10.1016/j.ijlp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Boccardi M, Bocchetta M, Aronen HJ, Repo-Tiihonen E, Vaurio O, Thompson PM, et al. Atypical nucleus accumbens morphology in psychopathy: another limbic piece in the puzzle. Int J Law Psychiatry. 2013;36:157–167. doi: 10.1016/j.ijlp.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacolgy. 2017 doi: 10.1038/npp.2016.287. accepted pending revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol. 2015;20:913–926. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Vanderschuren LJM, Minnaard AM, Smeets JAS, Lesscher HMB. Punishment models of addictive behavior. Cur Opinion Behav Sci. 2017;13:77–84. [Google Scholar]
- 44.Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;47:1008–1019. doi: 10.1007/BF01923336. [DOI] [PubMed] [Google Scholar]
- 45.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 46.Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Anderson DJ. Optogenetics, sex, and violence in the brain: implications for psychiatry. Biol Psychiatry. 2012;71:1081–1089. doi: 10.1016/j.biopsych.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumner SA, Mercy JA, Dahlberg LL, Hillis SD, Klevens J, Houry D. Violence in the United States: Status, Challenges, and Opportunities. JAMA. 2015;314:478–488. doi: 10.1001/jama.2015.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.RDoC. 2016 https://www.nimh.nih.gov/research-priorities/rdoc/constructs/frustrative-nonreward.shtml.
- 50.RDoC. 2012 https://www.nimh.nih.gov/research-priorities/rdoc/social-processes-workshop-proceedings.shtml.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


