Abstract
Objective
To determine if Functional Electrical Stimulation (FES) would improve ischemic pain, walking distance, and quality of life of patients with intermittent claudication.
Design
Single blind, randomized block, two factorial design.
Patients
Patients diagnosed with Peripheral Artery Disease (PAD) and intermittent claudication (IC). Ankle Brachial Index ranged 0.4–0.9 on at least one leg. Patients were randomly assigned to experimental (FES+Walk, N=13) or control (WALK, N=14) groups.
Intervention
Experimental group patients received FES to the dorsiflexor and plantarflexor muscles while walking for one hour/day, six days/week for eight weeks. Control group patients received similar intervention without FES. A Follow-up period of both groups lasted eight weeks.
Outcome Measures
Outcome measures were taken at baseline (T0), after intervention (T1), and after follow-up (T2). Primary measures included Perceived Pain Intensity (PPI), Six Minute Walk (6MW), and Peripheral Arterial Disease Quality of Life (PADQOL). Secondary measures included Intermittent Claudication Questionnaire (ICQ) and Timed Up and Go (TUG).
Results
Group by time interactions in PPI were significant (P<.001) with differences of 27.9 points at T1 and 36.9 points at T2 favoring the FES+Walk group. Groups difference in Symptoms and Limitations in Physical Function of the PADQOL reached significance (T1=8.9, and T2=8.3 improvements; P=.007). ICQ was significant (T1=9.3 and T2=13.1 improvements; P=.003). Improvement in 6MW and TUG tests were similar between groups.
Conclusions and Relevance
Walking with FES markedly reduced ischemic pain and enhanced QOL compared to just walking. FES while walking may offer an effective treatment option for the elderly with PAD and Intermittent Claudication.
Keywords: Peripheral Artery Disease, Intermittent Claudication, Functional Electrical Stimulation
INTRODUCTION
Over 8 million Americans suffer from peripheral arterial disease (PAD)1 and 7% experience intermittent claudication (IC), defined as ischemic leg pain with exertion that improves with rest.2–4 Yearly, 1.5 million patients with PAD receive medical care at a cost of more than 4 billion dollars5,6 including pharmaceutical intervention which has limited benefits.7,8 Endovascular surgeries, including atherectomy, angioplasty, stenting or drug-eluting stents, as well as surgical revascularization using bypass, can be successful in select patients but long-term benefits are limited.9 Although the complication rate and overall morbidity associated with endovascular intervention are lower when compared with surgical revascularization,10 the need for repeated surgical intervention remains a significant medical and financial burden.11 Additionally, most patients are likely to endure functional limitations in their daily activities, and their quality of life is adversely impacted.5 Limited mobility and de-conditioning exacerbates co-morbidities including hypertension, obesity, hyperlipidemia, and hyperglycemia.4 Spinal cord stimulation (SCS) is another surgical intervention that was shown to effectively diminish claudication and improve quality of life measures in patients with critical limb ischemia.12 However, clear indication to implant SCS is limited to patients with severe ischemia where other surgical interventions are not warranted or have failed.13
Dose-dependent exercise provides the best non-surgical treatment to decrease pain, improve walking distance, and enhance quality of life in patients with IC.2,14 Research suggests self-directed walking is associated with significantly less functional decline when performed at least three times/week compared with only one-two times/week.2,15 Supervised exercise, at sufficient intensity has been shown to increase blood flow to dorsiflexors.16,17 However, patients with co-morbidities and ischemic walking pain are often unable or unwilling to participate in regular exercise.18
To complement exercise, functional electrical stimulation (FES) while walking provides a novel treatment option. Non-invasive FES has been shown to minimize chronic pain, enhance muscle strength, increase muscle cross-sectional area and metabolism following trauma or disease of musculo-skeletal,19 peripheral vascular,20 cardio-pulmonary,21–24 and neurological systems.25,26 Likewise, FES has been shown to enhance arterial, venous, and lymphatic flow.27–29 Advanced biomedical electronics now allow electrical stimulation to be applied using wearable FES systems to help patients suffering from stroke, multiple sclerosis, and traumatic brain injury. To date, the efficacy of FES during walking has not been investigated in patients with PAD and IC.
We hypothesized that patients who walk using a new FES system will have a significant reduction in ischemic walking pain, increase walking abilities, and improve quality of life after eight weeks of training. We further hypothesized that such gains would be sustained following eight weeks of follow-up.
METHODS
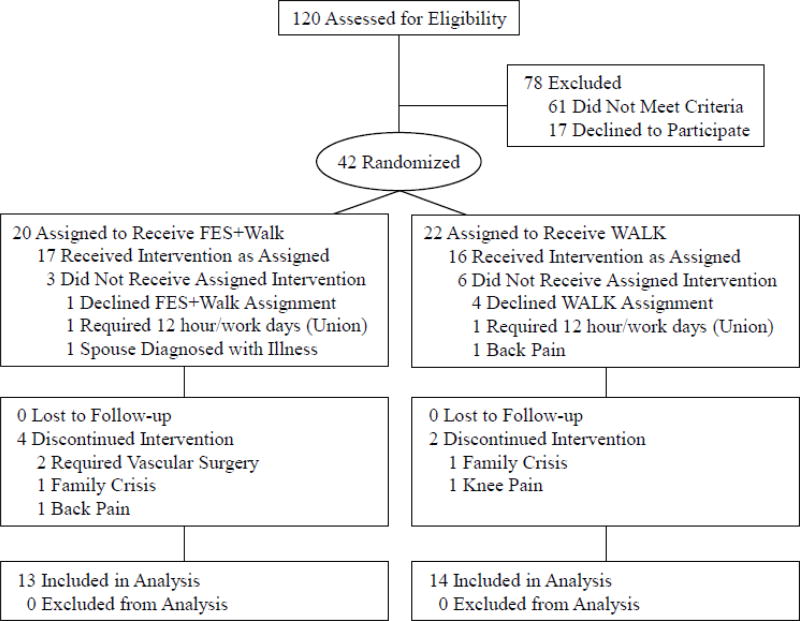
A single blind, randomized block, two factorial design compared outcomes of two cohorts: FES+Walk vs. WALK (walk only). Sample size determination was based on the effect size observed in a previous study of this FES system.23 Randomization occurred by drawing group assignments from sealed envelopes prepared by the principal investigator. A program director drew group assignments from the envelopes and informed the PI, who enrolled all patients. Patients were blocked according to claudication severity (mild=0/20, moderate=21/40, severe=41/60, or profound=61/100) using Perceived Pain Intensity (PPI). This study was approved by the MultiCare Institutional Review Board and all subjects gave informed consent. The CONSORT (Figure 1) diagram summarizes the recruitment, randomization, attrition, and risk factors.
Figure 1.
CONSORT flow chart of eligibility, randomization, and intervention.
Baseline (T0) measurements were collected prior to randomization. Post-treatment (T1) and carry-over (T2) measures were collected by clinicians blinded to group assignment and not otherwise involved with the patients. All patients received standard care by their physician, defined as conservative (non-surgical) management of signs and symptoms of PAD, including medications to improve circulation and manage pain.
Patients were ambulatory, community dwelling adults diagnosed with PAD and IC, had Ankle Brachial Index (ABI) between 0.4–0.9 on at least one leg, scored 23 or higher on the Folstein Mini Mental test, and demonstrated visible muscle contractions when receiving FES, within their level of comfort for the dorsiflexor and plantarflexor muscles. Patients were excluded if they had an implanted electronic stimulator, had arthritis or CNS damage resulting in neuromuscular limitations in either leg, or had skin lesions where the FES electrodes would be placed.
At study onset patients were instructed not to talk about their intervention or treatment assignment with anyone in the clinic. To assure that the changes in walking abilities and claudication were independent of receiving FES; all data were collected without the patients wearing the FES system.
Primary Outcome Measures
Walking distance was established by measuring meters traveled during a 6 Minute Walk Test (6MW). This test has been shown to be reliable for patients with PAD and IC.30
The Perceived Pain Intensity (PPI) was used to determine the magnitude of ischemic walking pain. Within 30 seconds after completing the 6MW, assessors asked patients to rate their ischemic pain in their lower legs by placing a hash mark on a 0–100 mm horizontal line. Patients were instructed that 0 meant “no pain” and 100 meant “the most intense pain ever experienced.”
Primary changes in lifestyle were measured with the Peripheral Arterial Disease Quality of Life (PADQOL) questionnaire. This is a well validated measure using 5 sub-scales: Social Relationships, Self-Concepts and Feelings, Symptoms and Limitations in Physical Functioning, Fear and Uncertainty, and Positive Adaptation.31
Secondary Outcome Measures
The Intermittent Claudication Questionnaire (ICQ) measured the impact of ischemic walking pain on quality of life. This measure has been shown to be a reliable and valid measure of quality of life for patients with IC.31
The Timed Up and Go (TUG) test32 measured short duration mobility to determine if patients improved their task performance speed when asked to stand from a chair, walk 3 meters, return to the chair and sit down. A video camera recorded this task to assure accurate data collection.
Intervention Procedures
Patients in the FES+Walk group were asked to walk six days/week for one hour/day, for eight weeks using FES, whereas patients in the WALK group were asked to ambulate the same amount without FES. Similar supervised walking regimens are often recommended for patients with PAD.14 All patients kept a daily log and attended weekly clinic visits to monitor and document walking duration, physician visits, medication changes, and unforeseen complications.
FES System
The FES system (Supplementary Figure 1S) is designed to provide electrical stimulation bilaterally to plantarflexors and dorsiflexors. Specifically, plantarflexors were stimulated during the middle portion of the stance phase (40% of the gait cycle). Dorsiflexors were stimulated during the remaining 60% of the cycle. Each FES unit contained a motion sensor which automatically triggered muscle contractions of the plantarflexors and dorsiflexors during walking. A software program allowed adjustments in the intensity (µcoul) and pulses/sec (PPS) parameters. This FES system delivered symmetrical biphasic waveform pulses, at a rate of 25 PPS to the dorsiflexors and 8–15 PPS to the plantarflexors. Stimulation intensity for each patient was adjusted by increasing the phase charge (µcoul) of each channel within the patient’s comfort level. Muscle contraction timing was monitored by the FES motion sensor and adjusted automatically to the patient’s walking cadence.
Bilaterally, two 5×5 cm electrodes were placed over the dorsiflexors while one 8×12 cm electrode and one 5×9 cm electrode were placed over the plantarflexors. The electrodes were fabricated with a carbon graphite base and hydrated absorbent microfiber material. Electrodes were affixed with Velcro to the inside of a neoprene cuff which housed the FES system. Patients used a remote control for each leg, allowing them to turn the stimulation on/off and to increase/decrease dorsiflexor and plantarflexor stimulation intensity. A “walk” mode triggered stimulation according to the patient’s walking cadence. When patients stopped walking, a motion sensor paused stimulation; then resumed when the patient began walking again. FES was applied bilaterally as PAD typically causes decreased blood flow in both legs even though complaints of IC may be unilateral.
Prior to the intervention, two training sessions occurred for patients in both groups to determine the optimal intensity and PPS (most comfortable stimulation with the best muscle contraction). In accordance with the inclusion criteria all patients demonstrated muscle contractions within their level of comfort. After these training sessions, patients were randomly assigned to either the FES+Walk or WALK group.
After eight weeks of the FES+Walk and WALK interventions (T1), all outcome measures were administered by a clinician blinded to group assignment. Next, an eight-week follow-up phase (with no weekly visits, daily log, or FES) was completed for all patients to determine if any benefits carried over. Finally, all outcome measures were administered again (T2).
Data Analysis
A 2 × 3 (group by time) mixed ANOVA was conducted for each measure by testing and examining the first order interaction of each group across time. Group represents the between-subjects factor and time represents the within-subjects factor. For significant two-way interactions, simple effects analyses were performed. In the event of a non-significant interaction, main effects were inspected. Means for each data collection pair (T0 vs. T1, T0 vs. T2, and T1 vs. T2) were compared to understand changes in metrics experienced within each group. Finally, between group differences were compared to identify significant differences in means at any of the three data collection points and to confirm that significant differences did not exist between the groups at baseline. All assumptions were closely examined, including sphericity and homogeneity of variance/covariance matrices, and significance was assessed at the P≤.05 level. Data analysis was conducted in SPSS (Armonk, New York) and the R Statistical Computing (Vienna, Austria).
RESULTS
Forty-three patients were randomized and 27 completed the study. The resultant cohort consisted of 13 FES+Walk and 14 WALK patients. Distribution of baseline (T0) PPI severity (mild, moderate, severe, profound) did not differ between groups, confirming the successful randomized blocking strategy (Supplementary Table 1). Mean age was 67.2 ± 7.4 years for the FES+Walk group and 68.7 ± 4.5 years for the WALK group, with age ranging from 58 to 79 years. Demographic data, including body mass index, ABI, and baseline PPI were comparable (Table 1). Eight of 13 and 7 of 14 patients in the FES+Walk and WALK groups respectively underwent endarterectomy and/or stenting procedures at lease 6 months prior to enrollment in the current investigation. Other potential confounding variables were similar for the two groups (FES+Walk/WALK) including: active smokers = 6/6, Diabetes = 8/8, and COPD = 3/4. There were no between groups significant differences in medication use (Table 1). Mean duration of walking/day monitored at weekly visits over the eight weeks of intervention showed a differential of 4.4 minutes (FES+Walk = 51.2 vs. WALK = 46.8).
Table 1.
Demographic characteristics of patients by study group
| FES+Walk (N=13) | WALK (N = 14) | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Mean (SD) | Median | Range | p-Value | |
| Age | 67.2 (7.4) | 67 | 58–79 | 68.7 (4.5) | 70.0 | 61–75 | 0.54 |
| BMI | 30.3 (4.2) | 28.1 | 25.7–36.9 | 27.9 (6.1) | 28.0 | 16.2–42.1 | 0.14 |
| Min ABI | 0.68 (0.11) | 0.68 | 0.50 – .86 | 0.60 (0.14) | 0.67 | 0.34–0.77 | 0.12 |
| Baseline PPI | 45.9 (24.4) | 40 | 16–85 | 48.3 (21.7) | 47 | 15–75 | 0.79 |
| Risk Factors* | |||||||
| Smoking | 6 (46%) | 6 (43%) | |||||
| Diabetes | 8 (61%) | 8 (57%) | |||||
| COPD | 3 (23%) | 4 (29 %) | |||||
| Medications* | |||||||
| • Vasodilators | 10 (77%) | 10 (71%) | |||||
| • Narcotic-Pain | 6 (46%) | 4 (28%) | |||||
| • Cholesterol / Hypertension | 13 (100%) | 13 (92%) | |||||
| • Anti-Diabetic | 9 (69%) | 7 (50%) | |||||
| • Anti-Depressant | 4 (30%) | 5 (35%) | |||||
| • Antiplatelet / Coagulant | 6 (46%) | 3 (21%) | |||||
no significant differences between groups
Primary and secondary interaction outcome data are found in Table 2 and Table visual representation of claudication pain (PPI) is found in Figure 2. PPI data showed a significant group × time interaction in the mixed ANOVA (P<.001) with a large effect size (η2 = .20). The WALK group had a fairly stable mean PPI across the three time points, while the FES+Walk group’s mean PPI decreased between T0-T1 (mean difference 26 points, 95% CI: 12.5–39.5, P=.001). Mean PPI of the FES+Walk group continued to decrease during the follow-up as noted between T0-T2 (mean difference 30.2, 95% CI: 13.0–47.3, P=.001). At T0 the two groups did not differ, yet significant between group differences favoring the FES+Walk group were observed at T1 with a mean difference of 27.9 points (95% CI: 25.8–29.9; P=.02) and at T2 with an estimated mean difference of 36.9 points (95% CI: 34.8–38.9; P<.001).
Table 2.
Primary (a–c) and secondary (d, e) interaction outcome ANOVA and between group analysis results
| Group × Time Interaction |
Between Groups | ||||
|---|---|---|---|---|---|
|
| |||||
| Measure | F2,50 | p | Time | Estimate (95% CI) | p |
| (a) Perceived Pain Index | 10.3 | < .001** | T0 | 2.8 (.7, 4.9) | .76 |
| T1 | 27.9 (25.8, 29.9) | .02* | |||
| T2 | 36.9 (34.8, 38.9) | < .001** | |||
|
| |||||
| (b) Six Minute Walk | 1.6 | .22 | T0 | −2.5 (−4.5, −.4) | .93 |
| T1 | −28.2 (−30.2, −26.1) | .28 | |||
| T2 | −20.3 (−22.4, −18.3) | .44 | |||
|
| |||||
| (c) PADQOL Symptoms | 6.5 | .007** | T0 | −0.5 (−2.5, 1.6) | .85 |
| T1 | −8.9 (−10.9, −6.8) | .01* | |||
| T2 | −8.3 (−10.4, −6.3) | .05 | |||
|
| |||||
| (d) Timed Up and Go | 2.5 | .07 | T0 | .2 (−1.8, 2.3) | .75 |
| T1 | 1.4 (−.7, 3.4) | .11 | |||
| T2 | 1.2 (−.9, 3.2) | .16 | |||
|
| |||||
| (e) Intermittent Claudication Questionnaire | 8.1 | .003** | T0 | −2.0 (−4.0, .1) | .68 |
| T1 | −9.3 (−11.4, −7.3) | .08 | |||
| T2 | −13.1 (−15.2, −11.6) | .02* | |||
Degrees of Freedom adjusted using the Greenhouse-Geisser correction for the violation of sphericity; T0: baseline; T1: end of intervention; T2: end of follow up; PADQOL = Peripheral Artery Disease Quality of Life
Figure 2.
Primary and secondary measure box plots
No statistically significant differences between groups were observed for the primary outcome of walking distance (Figure 2) measured by the 6MW (group × time interaction P=.22). Power analysis indicated that a sample size n=68 per group is needed to reach 80% power at α=0.05. However, for the within-group analysis (Supplementary Table 2), the FES+Walk group demonstrated a significant mean increase of 43.8 meters walked (95% CI: 8.2–79.3 meters, P=.02) between T0-T1 and 42.2 meters walked (95% CI: 1.2–83.8 meters, P=.04) between T0-T2. In contrast, the WALK group did not reach significance (P=.28 and P=.15) on either T0-T1 or T0-T2.
Four of the five PADQOL Factors did not reach group × time interaction significant differences. However, the Symptoms and Limitations in Physical Functioning (Symptoms) Factor achieved a significant difference (P=.007). Both groups saw mean increases in this subscale over time (Figure 2 and Table 2) and the main effect for time was significant (P<.001). The mean score for the experimental group increased significantly between T0-T1, with an estimated mean difference of 10.5 points (95% CI: 4.0–17.0; P=.002) and between T0-T2 with a mean difference of 12.9 points (95% CI: 5.1–20.7; P=.002). The WALK group also experienced a significant increase in this subscale but only between T0 and the end of T2 with an estimated mean difference of 5.1 points (95% CI: 0.6–9.5; P=.02). At the T1 testing the between groups difference of 8.9 points in mean symptoms score was significant (95% CI: 6.8–10.9, P=.01). At T2 the groups difference was 10.8 points (95% CI:6.3–10.4, P=.05).
Secondary outcome data regarding quality of life measures (Table 2) are similar to the significant findings on the PADQOL Symptoms Factor, the ICQ demonstrated a significant (Figure 2) group × time interaction (P =.003) with insignificant difference in mean ICQ score between the two groups at T0 while increasing differences in FES+Walk vs. WALK group means at T1 and T2. The FES+Walk group saw significant differences in ICQ between T0-T1 (10.8 points, 95% CI: 3.7–17.8 points; P=.003) and between T0 and T2 (14.0 points, 95% CI: 5.6–2.4 points; P=.002). No between groups difference was recorded at T1 but at T2 a significant between group difference in means of 13.1 points (95% CI:11.6–15.2) was observed (P=.02).
The mean TUG score of the WALK group remained nearly unchanged for the entire study and the group × time interaction was not significant (P=.08; Figure 2e). The main effect of time was significant (P=.01), with both groups demonstrating an initial decrease in TUG seconds during the intervention phase (T1) followed by an increase in the follow-up phase (T2) , resulting in a significant quadratic effect for time (P=.03). The FES+Walk group experienced a significant 1.4 second decrease in mean TUG between T0 and T1 (95% CI: 0.3–2.5 sec; P=.01).
Because Cilostazol was prescribed for two FES+WALK and four WALK patients (Table 1) a sensitivity analysis was performed to examine the group × time interaction without these patients. Sensitivity findings were similar to our initial results, with significant interactions for the PPI (F2,38=16.3, P<.001), PADQOL (F2,38=10.3, P=.001), and ICQ (F2,38=11.4, P=.001) and non-significant findings in the group × time interaction for the other measures (all P>.05).
DISCUSSION
Applying FES during walking is a novel approach to the management of IC with no adverse events occurring during this study. As patients in both groups had similar IC at study onset, the findings suggest significant benefits in PPI of the FES+Walk group whose ischemic pain dropped 57% after intervention and 66% after follow-up, while the WALK group PPI scores increased 8% during the study. In contrast, these findings indirectly appear to show superior improvement compared to a 33% reduction in IC during 12 weeks of a phase III clinical study injecting DP-R202 (a new sarpogrelate hydrochloride) to patients with chronic PAD.33
Although the 6MW measure did not reach significance in group × time interaction, the FES+Walk within group increase T0-T2 and T1-T2 was significant (P=.02) with a mean improvement of 43.8 meters after eight weeks with FES (Supplementary Table 2). The FES+Walk data represent improvement that is more than twice the distance achieved by PAD patients in a 2009 study, who underwent six months of walking on a treadmill, gaining a mean of 20.9 meters,34 an improvement similar to our control group (18.1 meters) which was not significant (P=.28). It is interesting to note that IC severity as measured with PPI is poorly correlated with the 6MW distance.35 Our findings that 6MW distance did not correlate with PPI, (r=−0.35 and r=0−.11 for FES+Walk and WALK respectively) corroborate these published findings.
In the current study, the significant improvements in quality of life were reflected by a marked increase of 10.8 points on the ICQ. This is a far better outcome compared to a 12-week supervised exercise program.36 Using the more comprehensive PADQOL,37 only the Symptoms Factor improved significantly. Both questionnaires reflect patients’ own view of quality of life.31 The apparent interrelatedness of improving one’s quality of life by enhancing physical functioning can be attributed to the FES-induced marked reduction in IC. This supports the original hypothesis that combining FES with walking is significantly superior to structured walking alone.
Explaining the mechanism that governs the effects of FES on ischemic walking pain is beyond the scope of this study. Yet, three interdependent mechanisms may exist. First, FES may have reduced IC via the activation of inhibiting sensory pathways. This supposition is based on the observation that 12 of 13 patients in the FES+WALK group showed marked decrease in perceived pain while using FES. A second mechanism is based on the repeated activation of the plantarflexors and dorsiflexors which is likely to promote peripheral blood flow, increase perfusion, and tissue oxygenation.20,29,38 A third hypothesis is that with diminished blood supply to the plantarflexors and dorsiflexors these muscles may become atrophic and lead to sarcopenia.19 Indirect evidence that the FES helped strengthen these muscles is extrapolated from the observation that FES+Walk patients maintained the ability to walk further compared to the WALK group patients even at the end of the follow-up phase. Taken together, we hypothesized that a combination of pain reduction, improved circulation, and muscle strengthening concurrently contribute to helping patients with PAD and IC.
Study Limitations
An unavoidable limitation is the inability to blind subjects to the treatment offered because the FES provides muscle contraction and skin sensation which are clearly felt by the patient. Another limitation is the high attrition rate (27/43 patients finished the study). This highlights the difficulty of conducting studies involving patients with multiple co-morbidities and complex social needs. Of the 16 patients who withdrew, four (25%) were randomized into the WALK without FES group and refused to continue due to this assignment. Despite the small sample size, the significant data reported for 27 patients suggests a robust treatment effect particularly the marked reduction of the perceived claudication pain. To increase statistical power, a larger sample size is needed to confirm the value of FES in improving quality of life measures and walking distance. Another limitation is the relatively brief intervention and follow-up periods, of only eight weeks. The decision to restrict the intervention period to 16 weeks was a practical decision influenced largely by budgetary constraints. It is conceivable that the inability to eliminate completely the claudication PPI after 8 weeks of walking with FES reflects a possibility of a plateau effect or a failure to restore adequate peripheral circulation. Larger, multi-site investigations over longer intervention period are recommended before generalizing these findings.
CONCLUSION
These results demonstrate the use of FES during daily walking can significantly reduce IC and enhance quality of life for elderly patients with intermittent claudication. Larger, multi-site investigations of considerably longer duration are recommended before the results can be fully demonstrated.
Supplementary Material
FES system worn bilaterally
Distribution of patients to blocking groups
Primary and secondary outcomes within group analysis
Acknowledgments
Research reporting in this publication was supported by the National Institute on Aging of the National Institutes of Health under grant #1R21AG048001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors acknowledge Dr. Vinay Malhotra for his cardiology expertise and critique of the manuscript.
SPONSOR’S ROLE
NIH Program Officer provided: guidance in study design, study oversight, recruitment protocols, review of reports, budget expenditures, and monitoring adverse events (none).
ABBREVIATIONS
- 6MW
6 Minute Walk
- ABI
Ankle Brachial Index
- FES
Functional Electrical Stimulation
- FES+Walk
Experimental Group (FES plus walking)
- IC
Intermittent Claudication
- ICQ
Intermittent Claudication Questionnaire
- PAD
Peripheral Artery Disease
- PADQOL
Peripheral Arterial Disease Quality of Life
- PPI
Perceived Pain Intensity
- QOL
Quality of Life
- TUG
Timed Up and Go
- WALK
Control Group (Walk only)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
TRIAL REGISTRATION: NIH-NIA 1R21AG048001
https://clinicaltrials.gov/ct2/show/NCT02384980?term=David+Embrey&rank=1
AUTHOR CONTRIBUTIONS
David G. Embrey, PhD (conception and design, acquisition of data, drafting the article, intellectual content, and final approval)
Gad Alon, PhD (conception and design, analysis and interpretation of data, drafting the article, intellectual content, and final approval)
Brenna A. Brandsma, DPT (conception and design, acquisition of data, drafting the article, intellectual content, and final approval)
Felix Vladimir, MD (drafting the article, intellectual content, and final approval)
Angela Silva, DBA (conception and design, and final approval)
Bethann M. Pflugeisen (analysis and interpretation of data, drafting the article, intellectual content, and final approval)
Paul J. Amoroso, MD (conception and design, analysis and interpretation of data, drafting the article, intellectual content, and final approval)
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
References
- 1.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54(4):1021–1031. e1021. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep. 2013;15(4):347. doi: 10.1007/s11886-013-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egberg L, Andreassen S, Mattiasson AC. Living a demanding life--spouses’ experiences of living with a person suffering from intermittent claudication. J Adv Nurs. 2013;69(3):610–618. doi: 10.1111/j.1365-2648.2012.06043.x. [DOI] [PubMed] [Google Scholar]
- 4.Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs. 2012;30(1):5–10. doi: 10.1016/j.jvn.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13(1):15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13(3):209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 7.Stevens JW, Simpson E, Harnan S, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. The British journal of surgery. 2012;99(12):1630–1638. doi: 10.1002/bjs.8895. [DOI] [PubMed] [Google Scholar]
- 8.Squires H, Simpson E, Meng Y, et al. A systematic review and economic evaluation of cilostazol, naftidrofuryl oxalate, pentoxifylline and inositol nicotinate for the treatment of intermittent claudication in people with peripheral arterial disease. Health Technol Assess. 2011;15(40):1–210. doi: 10.3310/hta15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinecke H, Unrath M, Freisinger E, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36(15):932–938. doi: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 10.Jones WS, Patel MR, Dai D, et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. 2013;165(5):809–815. 815 e801. doi: 10.1016/j.ahj.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Jones WS, Mi X, Qualls LG, et al. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. Journal of the American College of Cardiology. 2015;65(9):920–927. doi: 10.1016/j.jacc.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Tshomba Y, Psacharopulo D, Frezza S, Marone EM, Astore D, Chiesa R. Predictors of improved quality of life and claudication in patients undergoing spinal cord stimulation for critical lower limb ischemia. Ann Vasc Surg. 2014;28(3):628–632. doi: 10.1016/j.avsg.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–550. doi: 10.1111/ner.12208. discussion 550. [DOI] [PubMed] [Google Scholar]
- 14.Gardner AW. Exercise rehabilitation for peripheral artery disease: An exercise physiology perspective with special emphasis on the emerging trend of home-based exercise. Vasa. 2015;44(6):405–417. doi: 10.1024/0301-1526/a000464. [DOI] [PubMed] [Google Scholar]
- 15.Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home-based exercise in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;44(6):569–575. doi: 10.1016/j.ejvs.2012.09.009. discussion 576. [DOI] [PubMed] [Google Scholar]
- 16.Jakubseviciene E, Vasiliauskas D, Velicka L, Kubilius R, Milinaviciene E, Vencloviene J. Effectiveness of a new exercise program after lower limb arterial blood flow surgery in patients with peripheral arterial disease: a randomized clinical trial. Int J Environ Res Public Health. 2014;11(8):7961–7976. doi: 10.3390/ijerph110807961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner AW, Ritti-Dias RM, Stoner JA, Montgomery PS, Khurana A, Blevins SM. Oxygen uptake before and after the onset of claudication during a 6-minute walk test. J Vasc Surg. 2011;54(5):1366–1373. doi: 10.1016/j.jvs.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidon M, McGee H. Recruitment to clinical trials of exercise: challenges in the peripheral arterial disease population. Physiotherapy. 2013;99(4):305–310. doi: 10.1016/j.physio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Kern H, Barberi L, Lofler S, et al. Electrical stimulation counteracts muscle decline in seniors. Frontiers in aging neuroscience. 2014;6:189. doi: 10.3389/fnagi.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsang GM, Green MA, Crow AJ, et al. Chronic muscle stimulation improves ischaemic muscle performance in patients with peripheral vascular disease. European journal of vascular surgery. 1994;8(4):419–422. doi: 10.1016/s0950-821x(05)80960-0. [DOI] [PubMed] [Google Scholar]
- 21.Iwatsu K, Yamada S, Iida Y, et al. Feasibility of neuromuscular electrical stimulation immediately after cardiovascular surgery. Archives of physical medicine and rehabilitation. 2015;96(1):63–68. doi: 10.1016/j.apmr.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Vivodtzev I, Debigare R, Gagnon P, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest. 2012;141(3):716–725. doi: 10.1378/chest.11-0839. [DOI] [PubMed] [Google Scholar]
- 23.Giavedoni S, Deans A, McCaughey P, Drost E, MacNee W, Rabinovich RA. Neuromuscular electrical stimulation prevents muscle function deterioration in exacerbated COPD: a pilot study. Respiratory medicine. 2012;106(10):1429–1434. doi: 10.1016/j.rmed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Abdellaoui A, Prefaut C, Gouzi F, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;38(4):781–788. doi: 10.1183/09031936.00167110. [DOI] [PubMed] [Google Scholar]
- 25.Embrey DG, Holtz SL, Alon G, Brandsma BA, McCoy SW. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Archives of physical medicine and rehabilitation. 2010;91(5):687–696. doi: 10.1016/j.apmr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation (FES) may modify the poor prognosis of stroke survivors with severe motor loss of the upper extremity: a preliminary study. Am J Phys Med Rehabil. 2008;87(8):627–636. doi: 10.1097/PHM.0b013e31817fabc1. [DOI] [PubMed] [Google Scholar]
- 27.Corley GJ, Breen PP, Birlea SI, Serrador JM, Grace PA, Olaighin G. Hemodynamic effects of habituation to a week-long program of neuromuscular electrical stimulation. Med Eng Phys. 2012;34(4):459–465. doi: 10.1016/j.medengphy.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Morita H, Abe C, Tanaka K, Shiratori M, Oguri M, Shiga T. Neuromuscular electrical stimulation and an ottoman-type seat effectively improve popliteal venous flow in a sitting position. The journal of physiological sciences : JPS. 2006;56(2):183–186. doi: 10.2170/physiolsci.SC002006. [DOI] [PubMed] [Google Scholar]
- 29.Clover AJ, McCarthy MJ, Hodgkinson K, Bell PR, Brindle NP. Noninvasive augmentation of microvessel number in patients with peripheral vascular disease. J Vasc Surg. 2003;38(6):1309–1312. doi: 10.1016/s0741-5214(03)00895-4. [DOI] [PubMed] [Google Scholar]
- 30.Addison O, Ryan AS, Prior SJ, et al. Changes in Function After a 6-Month Walking Intervention in Patients With Intermittent Claudication Who Are Obese or Nonobese. J Geriatr Phys Ther. 2016 doi: 10.1519/JPT.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conijn AP, Jens S, Terwee CB, Breek JC, Koelemay MJ. Assessing the quality of available patient reported outcome measures for intermittent claudication: a systematic review using the COSMIN checklist. Eur J Vasc Endovasc Surg. 2015;49(3):316–334. doi: 10.1016/j.ejvs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Gine-Garriga M, Guerra M, Mari-Dell’Olmo M, Martin C, Unnithan VB. Sensitivity of a modified version of the ‘timed get up and go’ test to predict fall risk in the elderly: a pilot study. Arch Gerontol Geriatr. 2009;49(1):e60–66. doi: 10.1016/j.archger.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Lee HC, Lee SR, Han KR, et al. Efficacy and Safety of DP-R202 in Patients with Chronic Artery Occlusive Disease: Multicenter Randomized Double-blind Active-controlled Parallel Group Phase III Clinical Study. Clin Ther. 2016;38(3):557–573. doi: 10.1016/j.clinthera.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 34.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixit S, Chakravarthy K, Reddy RS, Tedla JS. Comparison of two walk tests in determining the claudication distance in patients suffering from peripheral arterial occlusive disease. Adv Biomed Res. 2015;4:123. doi: 10.4103/2277-9175.158036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guidon M, McGee H. One-year effect of a supervised exercise programme on functional capacity and quality of life in peripheral arterial disease. Disabil Rehabil. 2013;35(5):397–404. doi: 10.3109/09638288.2012.694963. [DOI] [PubMed] [Google Scholar]
- 37.Treat-Jacobson D, Lindquist RA, Witt DR, et al. The PADQOL: development and validation of a PAD-specific quality of life questionnaire. Vasc Med. 2012;17(6):405–415. doi: 10.1177/1358863X12466708. [DOI] [PubMed] [Google Scholar]
- 38.Williams KJ, Moore HM, Davies AH. Haemodynamic changes with the use of neuromuscular electrical stimulation compared to intermittent pneumatic compression. Phlebology. 2015;30(5):365–372. doi: 10.1177/0268355514531255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FES system worn bilaterally
Distribution of patients to blocking groups
Primary and secondary outcomes within group analysis