Abstract
Despite the widespread treatment of motion sickness symptoms using drugs and the involvement of the vestibular system in motion sickness, little is known about the effects of anti-motion sickness drugs on vestibular perception. In particular, the impact of oral promethazine, widely used for treating motion sickness, on vestibular perceptual thresholds has not previously been quantified. We examined whether promethazine (25 mg) alters vestibular perceptual thresholds in a counterbalanced, double-blind, within-subject study. Thresholds were determined using a direction recognition task (left vs. right) for whole-body yaw rotation, y-translation (interaural), and roll tilt passive, self-motions. Roll tilt thresholds were 31 % higher after ingestion of promethazine (P = 0.005). There were no statistically significant changes in yaw rotation and y-translation thresholds. This worsening of precision could have functional implications, e.g., during driving, bicycling, and piloting tasks. Differing results from some past studies of promethazine on the vestibulo-ocular reflex emphasize the need to study motion perception in addition to motor responses.
Keywords: promethazine, motion sickness, human experiments, vestibular perception, anti-motion sickness drug, medication
Introduction
Anti-motion sickness drugs result in substantial quality-of-life improvements for a broad range of individuals, including automobile passengers, migraine sufferers, sailors, pilots, and astronauts. Given the prevalence of motion sickness and the significant contribution of the vestibular system to this condition (Graybiel et al. 1965; James 1982 Money and Cheung 1983; Yates et al. 2014), it is of scientific and clinical interest to evaluate how anti-motion sickness drugs may impact basic vestibular sensation and perception. In this study, we examined whether oral promethazine HCl impacted vestibular perceptual thresholds (i.e., self-motion perceptual thresholds in the dark). Promethazine has been judged to be amongst the most effective anti-motion sickness drugs in laboratory (e.g., Wood and Graybiel 1972), clinical (Brainard and Gresham 2014), and field (Davis et al. 1993a, b; Bagian and Ward 1994) studies. Promethazine is known to block H1 receptor sites, but not the release of histamine, and to exhibit anti-cholinergic and anti-emetic effects, but the precise mechanisms and side effects are not completely understood (Wyeth 2004). We used the FDA-approved dose for treatment of motion sickness of 25 mg (Wyeth 2004). This is also a common intramuscular dose to treat motion sickness caused by spaceflight (Davis et al. 1993a, b; Bagian and Ward 1994).
To our knowledge, this is the first study to investigate whether promethazine impacts any aspect of vestibular perception, including vestibular thresholds. One previous study examining the impact of other anti-motion sickness drugs (dimenhydrinate and scopolamine) on vestibular perception suggested changes in thresholds for detection of angular acceleration, although statistical significance was not reported (Brandt et al. 1974). However, most studies of the impact of promethazine on vestibular sensorimotor responses have found no significant effects. One double-blind, crossover study (Dai et al. 2003) found no effect of 25 mg oral promethazine, administered 50–60 min prior to testing, on vestibulo-ocular reflex (VOR) gain, time constant, or adaptation in humans in response to Earth-vertical axis rotation. Similarly, vestibular evoked myogenic potentials (Colebatch et al. 1994) did not significantly change with 25 mg promethazine + 5 mg dextroamphetamine, nor with meclizine (50 mg), baclofen (10 mg), or cinnarizine (20 mg) + dimenhydrinate (40 mg) (Vanspauwen et al. 2011; Weerts et al. 2013). Miller and Graybiel (1969) also found little or no effect of several anti-motion sickness drugs on ocular counterrolling. However, a recent study found decreased ocular counterrolling and VOR gain during Earth vertical axis rotation after administration of 25 mg promethazine (Weerts et al. 2012).
The absence of promethazine vestibular perceptual studies motivated this work, especially since vestibular perception and sensorimotor responses involve different processing (Merfeld et al. 2005a, b). Many individuals, including astronauts, use promethazine to reduce motion sickness symptoms, highlighting the importance of investigating how this anti-motion sickness drug affects motion perception, since veridical motion perception is often critical in tasks like driving and piloting. Likewise, further understanding of promethazine’s vestibular perceptual impacts could help elucidate the mechanism underlying the finding that promethazine permits improved habituation to provocative stimuli (Wood and Graybiel 1972; Lackner and Graybiel 1994). We studied one aspect of vestibular perception: direction recognition thresholds (Benson et al. 1986; Benson et al. 1989; Grabherr et al. 2008). These are the smallest motions whose direction can be reliably perceived. In this experiment, subjects seated on a motorized platform in the dark were randomly moved either left or right, then reported their perceived motion direction. Since the predominant contribution of vestibular sensory input to this task has been demonstrated (Valko et al. 2012), we often refer to self-motion perceptual thresholds as vestibular perceptual thresholds.
We tested the hypothesis that 25 mg oral promethazine impacts vestibular perceptual thresholds. These tests assay perception, in contrast to previous studies of vestibular motor reflexes. We tested vestibular perceptual thresholds for three motion axes: yaw rotation, y-translation, and roll tilt, which primarily stimulate the semicircular canals, the otoliths, and both the canals and otoliths, respectively.
METHODS
Subjects
Ten healthy volunteers (seven males, three females; 26.5 ± 4.0 years old, range 20–33 years old; 77.8 ± 8.3 kg of body weight, range 64–95 kg; values are mean ± standard deviation) participated in the study. All subjects underwent clinical examination and vestibular diagnostic testing to screen for vestibular disorders, including Hallpike testing, electronystagmography, and sinusoidal vertical-axis angular VOR evoked via rotation. Subjects also answered a questionnaire to indicate any history of dizziness or vertigo, back/neck problems, cardiovascular, neurological, and other physical problems, which were exclusion criteria. Finally, a physician screened subjects for potential contraindications to promethazine including respiratory conditions like asthma and emphysema. Informed consent was obtained from all subjects prior to participation. The study was approved by the Institutional Review Board (IRB) at the Massachusetts Eye and Ear Infirmary (MEEI) and the Committee on the Use of Humans as Experimental Subjects (COUHES) at Massachusetts Institute of Technology (MIT) in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki, as revised in 2000. The consent form listed common side effects of promethazine (e.g., blurred vision, dizziness, confusion, disorientation, dry mouth, drowsiness, sensitivity to light, and either fast or slow heart rate) and did not state a hypothesis or expected outcome of the study.
Experimental Design
We performed a counterbalanced, placebo-controlled, double-blind experiment. Following a within-subject design, each subject underwent two sessions of vestibular perceptual threshold testing, once after administration of promethazine and once after administration of placebo. The order of the two sessions (promethazine vs. placebo) was counterbalanced across subjects. Since the half-life of promethazine is 15–20 h (Paton and Webster 1985; Strenkoski-Nix et al. 2000), the two sessions were separated by at least 4 days. A standard oral dose (Wyeth 2004) of 25 mg promethazine (NDC 65162-0521-10) and corn starch placebo were used. Both were prepared in a gelatin capsule to prevent identification. Drug preparation and blinding were performed by the pharmacy at MEEI. This study was registered on clinicaltrials.gov as NCT02136420. Each testing session took approximately 1.5 h. Given that promethazine plasma concentration peaks between 3 and 5 h after administration (Paton and Webster 1985; Strenkoski-Nix et al. 2000), subjects received the drug capsule (either promethazine or placebo) 2 h before the beginning of the experiment. We asked subjects whether they thought had ingested placebo or promethazine; no subjects were certain, which confirmed that blindness was maintained.
Motion Stimuli
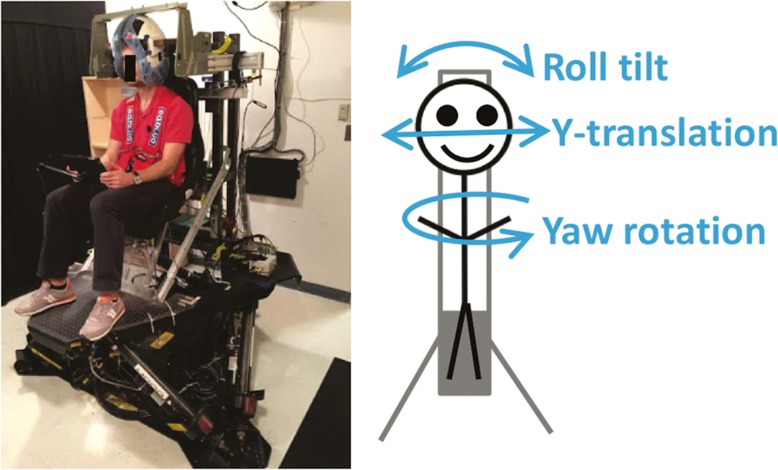
Direction recognition perceptual thresholds were measured as in other recent studies (Grabherr et al. 2008; Valko et al. 2012; Karmali et al. 2014). Subjects sat in the dark on a chair mounted on a computer-controlled Stewart-type hexapod motion platform with six electric motor actuated legs (MOOG CSA Engineering, Mountain View CA, Model 6DOF2000E, see Fig. 1). On each trial, the chair made a small movement, either to the left or to the right, and the subjects’ task was to report whether they moved left or right. Compared to a motion detection (i.e., did I move or not move?) task (Brandt et al. 1974; Guedry 1974), this is less susceptible to error from an arbitrary decision boundary (Green and Swets 1966; Merfeld 2011) and from subjects utilizing other cues such as chair vibration (Chaudhuri et al. 2013). Specifically, while detection and recognition thresholds are not significantly different on a low-vibration rotator, detection thresholds on a motion device with higher vibration are substantially lower (Chaudhuri et al. 2013), suggesting that detection thresholds reflect non-vestibular cues (i.e., vibration cues from the motion platform as to whether there was a motion). Moreover, it has been shown that subjects with bilateral vestibular ablation have significantly higher thresholds than subjects with normal vestibular function (2–50× depending upon the motion type) (Valko et al. 2012), demonstrating the predominance of vestibular sensory input to self-motion perception thresholds compared to other sensory modalities (e.g., somatosensory, proprioceptive, and tactile).
FIG. 1.
Six-degree-of-freedom MOOG platform device used to measure perceptual motion recognition thresholds. Three motion directions were tested: yaw rotation, y-translation, and roll tilt.
In each one of the two testing sessions, subjects underwent three separated testing blocks, corresponding to three different types of motion, in the following order: (1) “yaw rotation” about an Earth-vertical axis, which provides dynamic stimuli to the semicircular canals (primarily the horizontal canals), (2) “y-translation” along an Earth-horizontal, interaural axis, which provides dynamic stimuli to the otolith organs (primarily the utricular maculae), and (3) “roll tilt” about an Earth-horizontal, naso-occipital axis through head center at the level of the vestibular organs, which provides dynamic stimuli to the semicircular canals and static and dynamic stimuli to the otolith organs (utricule). This “head-centered” motion was chosen to minimize linear motion cues, as in previous studies (Lewis et al. 2011; Valko et al. 2012; Karmali et al. 2014; Lim et al. 2017).
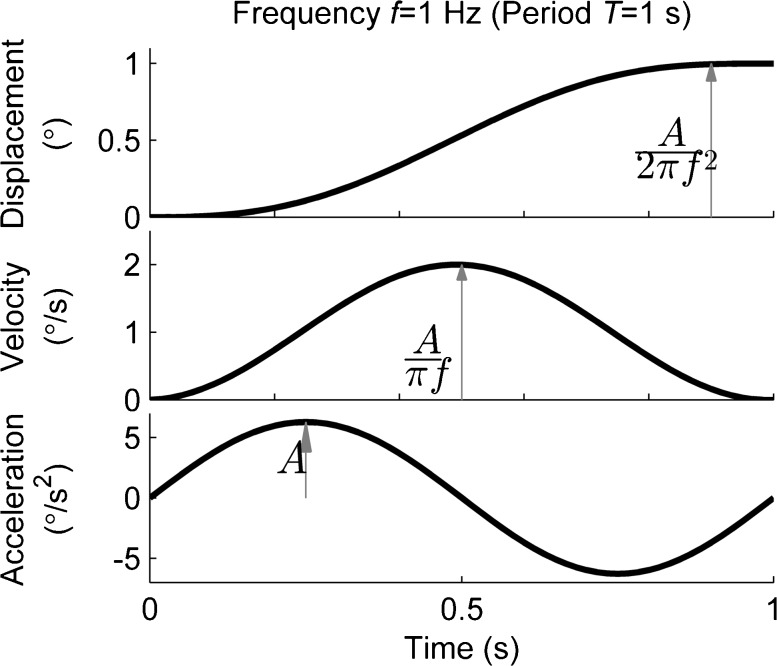
Motion stimuli (Fig. 2) consisted of single cycles of sinusoidal acceleration, which are widely used (Benson et al. 1986; Grabherr et al. 2008; Butler et al. 2010; Soyka et al. 2011; Crane 2012). This corresponds to cosine bell velocity and sigmoidal displacement. We define motion frequency f as the inverse of the period of one cycle, though we note that frequency is strictly defined only for infinite-duration sinusoids. The equations defining the motion are: angular acceleration a(t) = A sin(2πft), angular velocity v(t) = [A/(2πf)] [1 − cos(2πft)], and displacement Δ∆p(t) = [A/(2πf)] [t − (1/(2πf)) sin(2πft)]. For yaw rotation and roll tilt motions, these correspond to angular motions. Although truncation of sinusoids to finite durations causes some distortion of frequency content, the practical consequences are small (Merfeld et al. 2016). Frequencies used were 1 Hz for yaw rotation and y-translation and 0.2 Hz for roll tilt. While vestibular perceptual thresholds are known to be frequency dependent (Mah et al. 1989; Grabherr et al. 2008; Haburcakova et al. 2012; Valko et al. 2012; Karmali et al. 2014), we focused on 1 Hz as a well-studied frequency (Grabherr et al. 2008; Zupan and Merfeld 2008; Valko et al. 2012; Chaudhuri et al. 2013; Karmali et al. 2014) that does not overly tax the subjects’ attention. Shorter motions might be “missed” by an inattentive subject, while longer motions require sustained attention. Furthermore, these shorter duration motions permit moderate total testing time (~10 min per block). For roll tilt, we selected 0.2 Hz as previous studies suggest that this frequency requires integration of semicircular canal rotation cues and otolith tilt cues (Lewis et al. 2011; Karmali et al. 2014; Lim et al. 2017).
FIG. 2.
During each one of the three testing motions (yaw rotation, y-translation, and roll tilt), participants were subjected to motion stimuli corresponding to single cycles of sinusoidal acceleration. An example of one cycle of sinusoidal acceleration of yaw rotation is shown in the bottom figure, and this corresponds to cosine bell velocity and sigmoidal displacement profiles. The example shown shows a 1-Hz motion (frequency used in yaw rotation and y-translation motions) with a displacement of 1 °.
Experimental Procedure
The experimental procedures were nearly identical to those previously used (Valko et al. 2012). Subjects were seated upright in a chair on the MOOG hexapod motion platform (Fig. 1) in a completely dark, light-tight room. The subjects’ torso was secured with a five-point harness. Their head was also secured with a foam-lined helmet that was fixed relative to the chair and platform (i.e., whole-body motions), and it could be tightened until snug. To minimize the influence of haptic cues caused by air motion, we covered all skin surfaces (long sleeves, gloves, socks), and auditory cues were masked with noise-canceling headphones. Furthermore, white noise (approximately 60 dBA) was played through the headphones starting just before each motion trial and lasting until the end. This also served to alert the subject that the trial was starting and when it was completed, while masking other sounds during the motion.
On each trial, the motion direction (i.e., left or right) was randomized. After each motion, subjects reported whether they moved to the left or right by pressing the left or right half of the screen of an iPad, respectively. The iPad was mounted in front of the subject and the screen and backlight were off, maintaining complete darkness. This procedure is referred to as a one-interval, two-alternative, forced-choice task (Treutwein 1995; Leek 2001), meaning that each trial consisted of a single stimulus with two possible categories (e.g., left or right) and that subjects were required to give a response. Subjects were instructed to make their best guess if they were unsure. Only for roll tilt, subjects were returned to the upright position after each trial using a subthreshold motion. Several training trials were provided before each block to ensure that subjects were familiar with the task and procedures. On a very small fraction of trials (<0.5 %), subjects reported that they did not pay attention, in which case, the trial was repeated with the direction re-randomized. While the platform actuators produced some vibration during motion, it was similar for left and right motions and thus did not provide a useful motion direction cue, which we have previously confirmed by comparing recognition thresholds on the MOOG with those measured on a low-vibration rotator (Chaudhuri et al. 2013).
As with recent studies (Butler et al. 2010; Soyka et al. 2011; Roditi and Crane 2012; Valko et al. 2012), we chose an adaptive staircase algorithm because it allowed us to efficiently and precisely determine thresholds (Taylor and Creelman 1967; Karmali et al. 2016a, b). Specifically, a standard three-down, one-up staircase paradigm (Leek 2001) was used, in which the stimuli magnitude reduced after three consecutive correct responses and increased after one incorrect response. Testing started at stimuli magnitudes well above typical thresholds (4 °/s for yaw rotation, 4 cm/s for y-translation, 2 °/s for roll tilt). Each motion type was tested in a block of contiguous trials, and they were always tested in the same order (yaw rotation, y-translation, and roll tilt). As is standard practice (Benson et al. 1986; Grabherr et al. 2008; Butler et al. 2010; Merfeld 2011; Soyka et al. 2011; Crane 2012; Haburcakova et al. 2012; Karmali and Merfeld 2012; Merfeld et al. 2016; Bermúdez Rey et al. 2016), each trial consisted of a motion, which lasted a fixed amount of time, and a response, in which subjects had as much time as needed to report a perceived direction. For yaw rotation and y-translation, subjects completed 150 trials and the motion had a frequency of 1 Hz. This frequency (1 Hz) and the total number of trials (150) were selected to balance between managing testing time and a reasonable coefficient of variation of approximately 15 % (Karmali et al. 2016). For roll tilt, subjects completed 75 trials and the motion had a frequency of 0.2 Hz. Since the motion of each trial takes more time for 0.2 vs. 1 Hz (i.e., 5 s versus 1 s), 75 trials provided an appropriate balance between managing testing time and a reasonable coefficient of variation on the threshold estimate of approximately 23 % (Karmali et al. 2016).
Threshold Determination
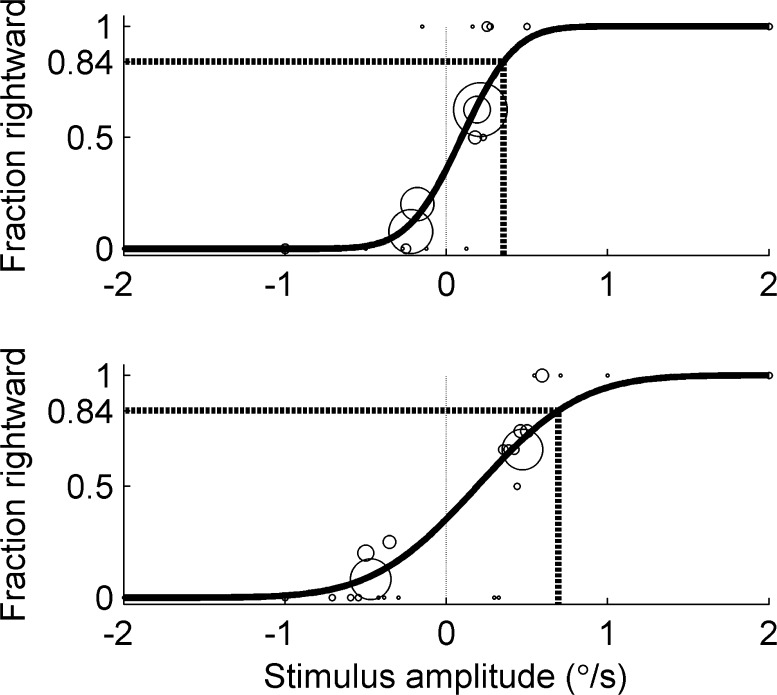
Thresholds were determined for each of the three motion directions (yaw rotation, y-translation, and roll tilt) using a psychometric curve fit to a Gaussian cumulative distribution function defined by two parameters: standard deviation (σ) and mean (μ) (McCullagh and Nelder 1989; Merfeld 2011; Valko et al. 2012). The mean corresponds to the perceptual bias or the point of subjective equality at which the subject has an equally likely probability of responding left vs. right. We define threshold as being equal to the standard deviation (i.e., the “one-sigma” threshold (Green and Swets 1966; Merfeld 2011)), such that the subject correctly perceives 84 % of trials at this stimuli level, after adjusting for the mean. Fits were determined using a generalized linear model (GLM) and probit link function, including the use of a recent innovation to improve the accuracy of parameter estimation (Chaudhuri et al. 2013) when fitting serially dependent data (Leek et al. 1992; Treutwein and Strasburger 1999; Kaernbach 2001; Leek 2001). These bias-reduced GLM fits were performed using the brglmfit.m function (Chaudhuri et al. 2013) in Matlab 2014a (The Mathworks, MA, USA). Figure 3 shows an example of the Gaussian cumulative distribution psychometric functions corresponding to roll tilt testing (placebo vs. promethazine) from one subject.
FIG. 3.
Example of subject responses fit with Gaussian cumulative distribution psychometric functions for roll tilt testing from one subject (top: placebo; bottom: promethazine). Circles represent the fraction rightward out of all responses at a given amplitude, where the size of the circle indicates the number of responses. In some cases, responses for stimuli within a 0.03 °/s interval were pooled for display only, but not for fitting analysis. The dashed line indicates the level where the subject perceived 84 % of the motions to be rightward, which is equal to the one-standard-deviation threshold after adjusting for the mean. These results show that, for this particular subject, promethazine increased the standard deviation and, therefore, the roll tilt threshold.
Statistical Analysis
Prior studies have found that human vestibular thresholds are consistent with a lognormal distribution across subjects (Benson et al. 1986; Benson et al. 1989; Bermúdez Rey et al. 2016). Thus, as in previous studies (Grabherr et al. 2008; Valko et al. 2012; Karmali et al. 2014), statistical calculations across subjects were performed after taking the logarithm of the threshold (for each case, both Kolmogorov-Smirnov and Shapiro-Wilk tests confirmed the log-transformed data to be consistent with normal distributions). For each motion case, paired, two-sided t-tests were used to compare the thresholds measured with placebo versus promethazine. Statistical tests were performed using SYSTAT 13 Version 13.00.05 (SYSTAT Software Inc. 2009). Since three motion conditions were tested, we used a Bonferroni correction for multiple testing and used alpha = 0.05/3 = 0.017.
RESULTS
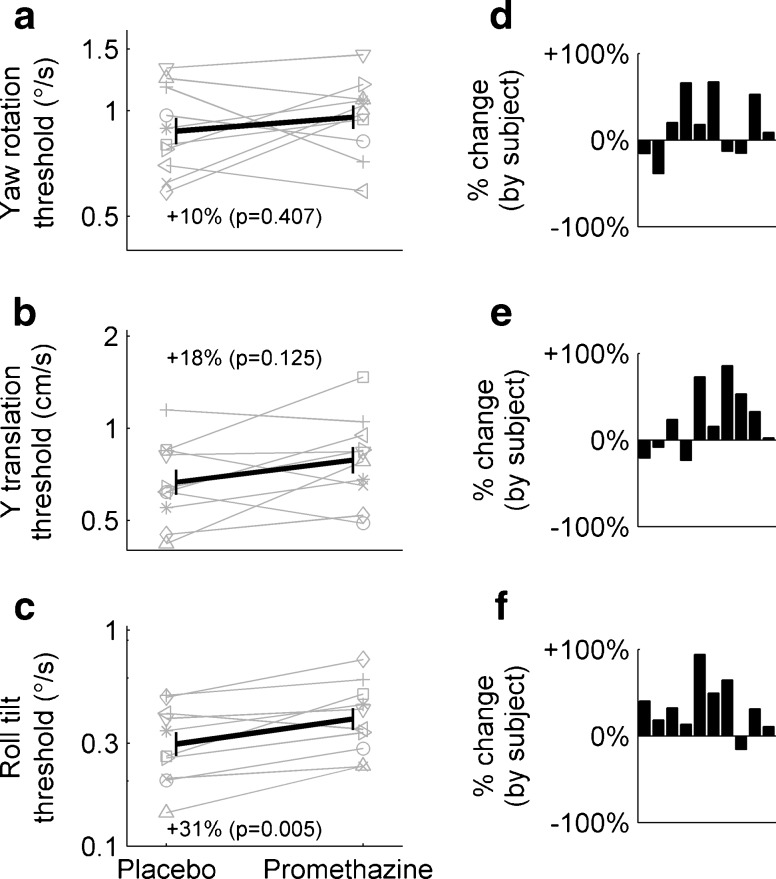
Figure 4 compares motion thresholds after dosage of placebo and promethazine for each of the three motion types. Gray lines show individual subject effects, and black lines show average effects across subjects (geometric mean ± SD). For example, the roll tilt threshold (Fig. 4c) for one subject, represented with a triangle (△), was 0.14 °/s with placebo (corresponds to the subject recognizing the roll tilt direction correctly 84 % of the time when presented with a motion with a peak velocity of 0.14 °/s). The subject’s roll tilt threshold increased to 0.23 °/s with promethazine. While there was substantial interindividual variability, average roll tilt thresholds (Fig. 4c) were 0.30 °/s with placebo and 0.39 °/s with promethazine. All but one subject had increased thresholds with promethazine (Fig. 4f). This increase in threshold of 31 % was statistically significant (t(9) = 3.663, P = 0.005) and indicates a decrease in roll tilt perceptual precision after administration of promethazine. Average yaw rotation thresholds (Fig. 4a) were 0.87 °/s with placebo and 0.96 °/s with promethazine. Statistical testing did not reveal a significant difference between conditions (t(9) = 0.870, P = 0.41). Average y-translation thresholds (Fig. 4b) were 0.67 cm/s with placebo and 0.79 cm/s with promethazine. Similarly, this difference was not statistically significant (t(9) = 1.691, P = 0.13). We confirmed that testing session order (i.e., promethazine first vs. placebo first) did not cause any significant effects on thresholds using paired, two-sided t-tests grouping by session day (yaw rotation: t(9) = −0.470, P = 0.65; y-translation: t(9) = 1.294, P = 0.23; roll tilt: t(9) = 0.694, P = 0.51). Mean (±SD) velocity thresholds are shown in Table 1. We also found no evidence of a correlation between motion types and the effect of promethazine on individual subjects. This suggests that individual differences in the impact of promethazine do not transfer across motion types. Also, to control for the fact that we administered a fixed dose of promethazine to subjects with different body weights, we confirmed that there is not a significant correlation between subject body weight and the change in thresholds caused by promethazine (for each of yaw rotation, y-translation, or roll tilt). All subjects were able to complete all tests, and none experienced any symptoms of motion sickness, which was not surprising given the small motions that subjects experienced.
FIG. 4.
Vestibular perceptual thresholds (a yaw rotation, b y-translation, and c roll tilt) measured for the same ten subjects in two conditions: with placebo and with promethazine. Gray lines represent individual subjects, and black lines represent the average, calculated as geometric mean, and standard deviation of the thresholds across subjects. Threshold data are presented using a logarithmic scale. There was a statistically significant change of +31 % in roll tilt thresholds (see c) after the intake of 25 mg of promethazine. Yaw rotation and y-translation thresholds showed an increase of +10 and +18 % with promethazine, although these changes were not statistically significant. d–f % Change in thresholds due to promethazine relative to placebo for each of the subjects (subjects are presented in the same order in each of the three panels).
TABLE 1.
Velocity thresholds for yaw rotation, y-translation, and roll tilt (mean ± SD)
| Treatment | Units | Mean velocity | Upper SD | Lower SD | |
|---|---|---|---|---|---|
| Yaw rotation (1 Hz) | Placebo | °/s | 0.87 | 0.27 | 0.21 |
| Promethazine | °/s | 0.96 | 0.26 | 0.21 | |
| Y-translation (1 Hz) | Placebo | cm/s | 0.67 | 0.23 | 0.17 |
| Promethazine | cm/s | 0.79 | 0.29 | 0.21 | |
| Roll tilt (0.2 Hz) | Placebo | °/s | 0.30 | 0.15 | 0.10 |
| Promethazine | °/s | 0.39 | 0.17 | 0.12 |
Statistics computed using results from all ten subjects. Note that standard deviations are not symmetric with respect to the mean when expressed in velocity units. This is because the mean thresholds and standard deviations were calculated in logarithmic units and transformed back to velocities
DISCUSSION
To our knowledge, this is the first study of the effects of 25 mg oral promethazine HCl on vestibular perception, which we assayed using vestibular perceptual direction recognition thresholds. We found that roll tilt perceptual thresholds significantly increased by 31 % after a standard dose of promethazine, as compared to after a placebo control. This means that promethazine worsens subjects’ ability to perceive roll tilt motions. Drawing parallels between sensory systems, this result is consistent with studies showing reduced visual perceptual performance after administration of promethazine (Wood et al. 1985; Hindmarch et al. 2002) as well as other anti-motion sickness drugs (Weerts et al. 2014, 2015), despite substantial differences in the perceptual tasks used. There was no statistically significant effect of promethazine on yaw rotation or y-translation thresholds, although they show a positive trend towards increased thresholds with promethazine (+10 % in yaw rotation and +18 % in y-translation). We note that these effect sizes are smaller than the effect size for roll tilt. Power analyses with the existing data showed that, to identify a significant effect of promethazine (assuming a power of 0.80), we would expect to require a total of 40 and 141 subjects for y-translation and yaw rotation, respectively. Finally, as we found no correlation between subject body weight and the effect of promethazine on thresholds, this suggests that the standard 25-mg dose did not deferentially affect individuals based upon their size.
While we do not know yet the functional implications of a 31 % increase of perceptual roll tilt thresholds, a comparison with other functional errors suggests that it may be significant. For example, 0.2-Hz perceptual roll tilt thresholds are correlated with the risk of falling on the Romberg balance test with the subjects standing on foam with eyes closed (Bermúdez Rey et al. 2016). Specifically, fallers had roll tilt thresholds approximately double than those of non-fallers, and even after age adjustment, there was a 5.6-fold increase in the odds of falling for each 2.71× increase in roll tilt threshold. Using a rough extrapolation of their results, a 31 % increase in threshold would correspond to a 61 % increase in the odds of falling. Also, it was found that 0.2-Hz roll tilt thresholds increase by 35 % per decade of aging after the age of ~40 (Bermúdez Rey et al. 2016). Similarly, postural errors worsen approximately 20 % in older subjects compared to younger ones (Lin et al. 2008), when measured using root-mean-squared error of center of pressure, and this increase in postural sway results in an increased risk of falling (Fernie et al. 1982). Thus, our measured changes in vestibular precision are similar to those found during aging, which have significant functional outcomes. Finally, we recently studied whether vestibular thresholds underlie performance in a manual control task in which a group of normal subjects, seated in a moving chair and who did not take any drugs, had to keep themselves upright using a joystick by responding to random perturbations. Analysis of the group results found that individuals with 30 % higher roll tilt thresholds performed 30 % worse in their ability to null chair motion (Rosenberg et al. 2016). This indicates that a higher vestibular threshold directly impacts functional performance in motion nulling/control tasks. Thus, an increased vestibular threshold due to promethazine could impact tasks such as driving a car, riding a bicycle, or flying or landing an aircraft or spacecraft. Finally, the reduced susceptibility to motion sickness after ingestion of promethazine could be a result of an increased threshold masking provocative motion stimuli (Wood and Graybiel 1972; Lackner and Graybiel 1994). These previous studies highlight that the effect of promethazine on roll tilt thresholds that we observed herein could have concrete functional impacts, which remain to be determined.
Our roll tilt perceptual result differs from most prior studies of the impact of promethazine on the VOR (Miller and Graybiel 1969; Dai et al. 2003; Weerts et al. 2012) and vestibular evoked myogenic potential (Vanspauwen et al. 2011; Weerts et al. 2013), since they found no significant effect. However, methodological differences could also explain the discrepancy. For example, we aimed to determine the subjects’ threshold (i.e., precision errors) with small motions, in comparison to studies that measured systematic errors in the VOR (i.e., accuracy errors) with larger, supra-threshold motions. Other differences included motion directions and frequencies. More broadly, there is evidence for different mechanisms for vestibular motor reflexes (e.g., VOR) and perception (Merfeld et al. 2005a, b), a primary motivation for our study, which may explain the differences seen. Future studies could use an approach that studies multiple responses and multiple drugs to determine neural sites of action. For example, Weerts et al. (2012) found that the angular and linear VORs were differentially affected depending on the sites of action of each drug. This approach could also be extended to determine whether VOR and perceptual effects arise from a common site of action. While our results do not provide any new insights into the neural site of action, many of the potential sites that have previously been suggested (Vanspauwen et al. 2011; Weerts et al. 2012) also apply to our findings. This includes muscarinic, nicotinic, and H1 receptors in the vestibular nucleus of rodents (Rotter et al. 1979; Wamsley et al. 1981; Burke and Fahn 1985; Clarke et al. 1985; Schwartz 1986; Zanni et al. 1995) as well as broader central effects.
A recent study found no evidence that vestibular perceptual thresholds, measured using techniques similar to those in the present study, are correlated with the subject’s sleepiness, quantified using both objective and subjective measures (Galvan-Garza 2016). Subjects were repeatedly tested at least 20 times on different days over 8 months using two of the motion conditions of our current study (yaw rotation at 1 Hz and roll tilt at 0.2 Hz). Although sleepiness was not a manipulated variable in Galvan-Garza’s study, these results suggest that thresholds are relatively unaffected by sleepiness. Thus, it is unlikely that the potential sleepiness (Weerts et al. 2014) caused by promethazine impacted our results. Furthermore, previous studies that showed cognitive performance decrements caused by promethazine used tasks with a high workload (Wood et al. 1985; Hindmarch et al. 2002), whereas our study used a relatively easy task in which subjects had unlimited time to respond and the option to repeat trials if needed. Thus, we conclude that increased roll tilt thresholds with promethazine are not predominantly due to sleepiness side effects.
Thresholds varied significantly across subjects, both with promethazine and placebo. We note that this is consistent with previous studies that have found roughly a 10-fold variation across subjects (Benson et al. 1986; Benson et al. 1989; Valko et al. 2012). Additionally, our results show no evidence that promethazine changed intersubject variability. We also note that our vestibular thresholds are very similar to those recently reported for a different sample of normal subjects (Valko et al. 2012).
While speculative, a few explanations may underlie the difference in effect size in roll tilt thresholds versus yaw rotation and y-translation thresholds. First, roll tilt thresholds rely on the integration of cues from the semicircular canal and otolith organs. Specifically, while semicircular canal cues are more reliable at higher frequencies and otolith cues are more reliable at lower frequencies, there is a range between approximately 0.1 and 0.4 Hz where the two cues have similar reliability. The brain performs sensory integration to reduce the threshold to less than that of either cue individually (Lewis et al. 2011; Lim et al. 2017). A disruption by promethazine of sensory integration could result in increased thresholds. Statistically optimal (Bayesian) sensory integration of two equally reliable cues predicts a reduction in threshold of 29.3 % compared to either of the individual cues (e.g., Ernst and Banks 2002; Gu et al. 2008; Karmali et al. 2014). If the canal and otolith cues were equally reliable in our 0.2-Hz roll tilt threshold task, complete disruption of this sensory integration by promethazine, such that the subject relies upon only one of the cues, would predict a relative increase in threshold by 29.3 %, which compares well with the 31 % observed. In contrast, yaw rotation and y-translation thresholds rely on primarily only the semicircular canals and the otolith organs, respectively, and thus sensory integration, at least within the vestibular system, does not play a significant role in determining these thresholds. Second, roll tilt thresholds were tested at a different frequency compared to yaw and y-translation thresholds (0.2 vs. 1 Hz) and differences in dynamics could influence neural processing (Valko et al. 2012 Merfeld et al. 2016). Finally, since the testing order of motion types was held constant between sessions and roll tilt thresholds were always tested last, it is possible that the temporal pharmacokinetics and pharmacodynamics of promethazine (Wood et al. 1985) might have resulted in smaller drug effect sizes for yaw rotation and y-translation than for roll tilt. Our data do not allow us to definitively conclude whether the differential effect of promethazine on vestibular perceptual thresholds for roll tilt at 0.2 Hz vs. yaw rotation and y-translation at 1 Hz is due to vestibular sensory integration, motion duration, pharmacodynamics, or some other explanations. Future studies should investigate several motion conditions (e.g., rotations, translations, and tilts in multiple axes) across a range of motion frequencies (Grabherr et al. 2008; Valko et al. 2012; Karmali et al. 2014).
This is the first study to report the impact of 25 mg of promethazine on vestibular perceptual thresholds. In particular, our findings show that roll tilt thresholds significantly increase by 31 %. This may have important functional implications in tasks relying upon vestibular perception, particularly roll tilt perception. In addition, vestibular thresholds in other motion directions (yaw rotation, and y-translation), although not statistically significant, did show a tendency to increase with promethazine. These results will guide future studies on anti-motion sickness drugs on the vestibular perception, which could impact their future clinical and field use.
Acknowledgements
We appreciate the participation of our anonymous subjects. We thank the Jenks Vestibular Physiology Lab for the use of the MOOG device and Dr. Dan Merfeld for his scientific insight and assistance using his MOOG device. We appreciate the assistance of Christine Finn at the Massachusetts Eye and Ear Infirmary pharmacy. This research was supported by the National Space Biomedical Research Institute through NASA NCC 9-58 and by the National Institutes of Health through NIDCD DC013635 (FK). Preliminary results have been presented at a conference (Karmali et al. 2016b).
Compliance with Ethical Standards
Informed consent was obtained from all subjects prior to participation. The study was approved by the Institutional Review Board (IRB) at the Massachusetts Eye and Ear Infirmary (MEEI) and the Committee on the Use of Humans as Experimental Subjects (COUHES) at Massachusetts Institute of Technology (MIT) in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki, as revised in 2000.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Ana Diaz-Artiles, Phone: +1 (607) 255-3249, Email: ad877@cornell.edu.
Adrian J. Priesol, Email: Adrian_Priesol@meei.harvard.edu
Torin K. Clark, Email: torin.clark@colorado.edu
David P. Sherwood, Email: dsherwoo@mit.edu
Charles M. Oman, Email: coman@mit.edu
Laurence R. Young, Email: lry@mit.edu
Faisal Karmali, Email: Faisal_Karmali@meei.harvard.edu.
References
- Bagian JP, Ward DF. A retrospective study of promethazine and its failure to produce the expected incidence of sedation during space flight. J Clin Pharmacol. 1994;34(6):649–651. doi: 10.1002/j.1552-4604.1994.tb02019.x. [DOI] [PubMed] [Google Scholar]
- Benson AJ, et al. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med. 1986;57(11):1088–1096. [PubMed] [Google Scholar]
- Benson AJ, et al. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60(3):205–213. [PubMed] [Google Scholar]
- Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM (2016). Vestibular perceptual thresholds increase above the age of 40. Front Neurol 7:162. doi:10.3389/fneur.2016.00162 [DOI] [PMC free article] [PubMed]
- Brainard A, Gresham C. Prevention and treatment of motion sickness. Am Fam Physician. 2014;90(1):41–46. [PubMed] [Google Scholar]
- Brandt T, et al. Drug effectiveness on experimental optokinetic and vestibular motion sickness. Aerosp Med. 1974;45(11):1291–1297. [PubMed] [Google Scholar]
- Burke RE, Fahn S. Choline acetyltransferase activity of the principal vestibular nuclei of rat, studied by micropunch technique. Brain Res. 1985;328(1):196–199. doi: 10.1016/0006-8993(85)91344-7. [DOI] [PubMed] [Google Scholar]
- Butler JS, et al. Bayesian integration of visual and vestibular signals for heading. J Vis. 2010;10(11):23. doi: 10.1167/10.11.23. [DOI] [PubMed] [Google Scholar]
- Chaudhuri SE, et al. Whole-body motion-detection tasks can yield much lower thresholds than direction-recognition tasks: implications for the role of vibration. J Neurophysiol. 2013;110(12):2764–2772. doi: 10.1152/jn.00091.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, et al. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5(5):1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, et al. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT. Fore-aft translation aftereffects. Exp Brain Res. 2012;219(4):477–487. doi: 10.1007/s00221-012-3105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, et al. The relation of motion sickness to the spatial-temporal properties of velocity storage. Exp Brain Res. 2003;151(2):173–189. doi: 10.1007/s00221-003-1479-4. [DOI] [PubMed] [Google Scholar]
- Davis JR, et al. Comparison of treatment strategies for space motion sickness. Acta Astronaut. 1993;29(8):587–591. doi: 10.1016/0094-5765(93)90074-7. [DOI] [PubMed] [Google Scholar]
- Davis JR, et al. Treatment efficacy of intramuscular promethazine for space motion sickness. Aviat Space Environ Med. 1993;64(3 Pt 1):230–233. [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415(6870):429–433. doi: 10.1038/415429a. [DOI] [PubMed] [Google Scholar]
- Fernie GR, et al. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age Ageing. 1982;11(1):11–16. doi: 10.1093/ageing/11.1.11. [DOI] [PubMed] [Google Scholar]
- Galvan-Garza R. Enhancement of perception with the application of stochastic vestibular stimulation. Department of Aeronautics & Astronautics, Cambridge, MA: Massachusetts Institute of Technology; 2016. [Google Scholar]
- Grabherr L, et al. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186(4):677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Graybiel A, et al. Effects of exposure to a rotating environment (10 rpm) on four aviators for a period of twelve days. Aerosp Med. 1965;36:733–754. [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Gu Y, et al. Neural correlates of multisensory cue integration in macaque MSTd. Nat Neurosci. 2008;11(10):1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedry FE. Psychophysics of vestibular sensation. In: Kornhuber HH, editor. Vestibular system part 2: psychophysics, applied aspects and general interpretations. Berlin, Heidelberg: Springer Berlin Heidelberg; 1974. pp. 3–154. [Google Scholar]
- Haburcakova C, et al. Frequency dependence of vestibuloocular reflex thresholds. J Neurophysiol. 2012;107(3):973–983. doi: 10.1152/jn.00451.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I, et al. An evaluation of the effects of high-dose fexofenadine on the central nervous system: a double-blind, placebo-controlled study in healthy volunteers. Clin Exp Allergy. 2002;32(1):133–139. doi: 10.1046/j.0022-0477.2001.01245.x. [DOI] [PubMed] [Google Scholar]
- James W. The sense of dizziness in deaf-mutes. Am J Otol. 1982;4:239–254. [Google Scholar]
- Kaernbach C. Slope bias of psychometric functions derived from adaptive data. Percept Psychophys. 2001;63(8):1389–1398. doi: 10.3758/BF03194550. [DOI] [PubMed] [Google Scholar]
- Karmali, F. and D. M. Merfeld (2012). A distributed, dynamic, parallel computational model: the role of noise in velocity storage. J Neurophysiol 108(2):390–405 [DOI] [PMC free article] [PubMed]
- Karmali F, et al. Visual and vestibular perceptual thresholds each demonstrate better precision at specific frequencies and also exhibit optimal integration. J Neurophysiol. 2014;111(12):2393–2403. doi: 10.1152/jn.00332.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali F, et al. Determining thresholds using adaptive procedures and psychometric fits: evaluating efficiency using theory, simulations, and human experiments. Exp Brain Res. 2016;234(3):773–789. doi: 10.1007/s00221-015-4501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali, F., et al. (2016b) Development of a countermeasure to enhance sensorimotor adaptation to altered gravity level. IEEE Aerospace Conference, Big Sky, MT
- Lackner JR, Graybiel A. Use of promethazine to hasten adaptation to provocative motion. J Clin Pharmacol. 1994;34(6):644–648. doi: 10.1002/j.1552-4604.1994.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63(8):1279–1292. doi: 10.3758/BF03194543. [DOI] [PubMed] [Google Scholar]
- Leek MR, et al. Estimation of psychometric functions from adaptive tracking procedures. Percept Psychophys. 1992;51(3):247–256. doi: 10.3758/BF03212251. [DOI] [PubMed] [Google Scholar]
- Lewis RF, et al. Abnormal motion perception in vestibular migraine. Laryngoscope. 2011;121(5):1124–1125. doi: 10.1002/lary.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, et al. Perceptual precision of passive body tilt is consistent with statistically optimal cue integration. J Neurophysiol: jn. 2017;00073:02016. doi: 10.1152/jn.00073.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, et al. Reliability of COP-based postural sway measures and age-related differences. Gait Posture. 2008;28(2):337–342. doi: 10.1016/j.gaitpost.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Mah, R. W., et al. (1989). Threshold perception of whole-body motion to linear sinusoidal stimulation. AIAA Conference on Motion Cues in Flight Simulation and Simulator Induced Sickness, Boston, MA
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1989. [Google Scholar]
- Merfeld DM. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp Brain Res. 2011;210(3–4):389–405. doi: 10.1007/s00221-011-2557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, et al. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005;94(1):186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, et al. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt&Translation. J Neurophysiol. 2005;94(1):199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, et al. Dynamics of individual perceptual decisions. J Neurophysiol. 2016;115(1):39–59. doi: 10.1152/jn.00225.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EF, II, Graybiel A. Effect of drugs on ocular counterrolling. Clin Pharmacol Ther. 1969;10(1):92–99. doi: 10.1002/cpt196910192. [DOI] [PubMed] [Google Scholar]
- Money KE, Cheung BS. Another function of the inner ear: facilitation of the emetic response to poisons. Aviat Space Environ Med. 1983;54(3):208–211. [PubMed] [Google Scholar]
- Paton DM, Webster DR. Clinical pharmacokinetics of H1 receptor antagonists (the antihistamines) Clin Pharmacokinet. 1985;10(6):477–497. doi: 10.2165/00003088-198510060-00002. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Suprathreshold asymmetries in human motion perception. Exp Brain Res. 2012;219(3):369–379. doi: 10.1007/s00221-012-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M., et al. (2016) Sensory precision limits vehicle control performance. Human Research Program Investigator’s Workshop, Galveston, TX
- Rotter A, et al. Muscarinic receptors in the central nervous system of the rat. II. Distribution of binding of [3H]propylbenzilylcholine mustard in the midbrain and hindbrain. Brain Res. 1979;180(2):167–183. doi: 10.1016/0165-0173(79)90003-1. [DOI] [PubMed] [Google Scholar]
- Schwartz RD. Autoradiographic distribution of high affinity muscarinic and nicotinic cholinergic receptors labeled with [3H]acetylcholine in rat brain. Life Sci. 1986;38(23):2111–2119. doi: 10.1016/0024-3205(86)90210-9. [DOI] [PubMed] [Google Scholar]
- Soyka F, et al. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res. 2011;209(1):95–107. doi: 10.1007/s00221-010-2523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenkoski-Nix LC, et al. Pharmacokinetics of promethazine hydrochloride after administration of rectal suppositories and oral syrup to healthy subjects. Am J Health Syst Pharm. 2000;57(16):1499–1505. doi: 10.1093/ajhp/57.16.1499. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Creelman CD. PEST: efficient estimates on probability functions. The Journal of the Acoustical Society of America. 1967;41(4A):782–787. doi: 10.1121/1.1910407. [DOI] [Google Scholar]
- Treutwein B. Adaptive psychophysical procedures. Vis Res. 1995;35(17):2503–2522. doi: 10.1016/0042-6989(95)00016-X. [DOI] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61(1):87–106. doi: 10.3758/BF03211951. [DOI] [PubMed] [Google Scholar]
- Valko Y, et al. Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci. 2012;32(39):13537–13542. doi: 10.1523/JNEUROSCI.2157-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanspauwen R, et al. No effects of anti-motion sickness drugs on vestibular evoked myogenic potentials outcome parameters. Otol Neurotol. 2011;32(3):497–503. doi: 10.1097/MAO.0b013e31820d94d0. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, et al. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. 1981;1(2):176–191. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts AP, et al. Pharmaceutical countermeasures have opposite effects on the utricles and semicircular canals in man. Audiol Neurootol. 2012;17(4):235–242. doi: 10.1159/000337273. [DOI] [PubMed] [Google Scholar]
- Weerts AP, et al. Baclofen affects the semicircular canals but not the otoliths in humans. Acta Otolaryngol. 2013;133(8):846–852. doi: 10.3109/00016489.2013.782615. [DOI] [PubMed] [Google Scholar]
- Weerts AP, et al. Evaluation of the effects of anti-motion sickness drugs on subjective sleepiness and cognitive performance of healthy males. J Psychopharmacol. 2014;28(7):655–664. doi: 10.1177/0269881113516201. [DOI] [PubMed] [Google Scholar]
- Weerts AP, et al. Restricted sedation and absence of cognitive impairments after administration of intranasal scopolamine. J Psychopharmacol. 2015;29(12):1231–1235. doi: 10.1177/0269881115598414. [DOI] [PubMed] [Google Scholar]
- Wood CD, Graybiel A. Theory of anti-motion sickness drug mechanisms. Aerospace Medicine. 1972;43(3):249–252. [PubMed] [Google Scholar]
- Wood CD, et al. Evaluation of antimotion sickness drug side effects on performance. Aviat Space Environ Med. 1985;56(4):310–316. [PubMed] [Google Scholar]
- Wyeth (2004). Oral Phenergan (promethazine HCl) prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/07935s030lbl.pdf.
- Yates BJ, et al. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness. Exp Brain Res. 2014;232(8):2455–2469. doi: 10.1007/s00221-014-3937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanni M, et al. Distribution of neurotransmitters, neuropeptides, and receptors in the vestibular nuclei complex of the rat: an immunocytochemical, in situ hybridization and quantitative receptor autoradiographic study. Brain Res Bull. 1995;36(5):443–452. doi: 10.1016/0361-9230(94)00193-5. [DOI] [PubMed] [Google Scholar]
- Zupan LH, Merfeld DM. Interaural self-motion linear velocity thresholds are shifted by roll vection. Exp Brain Res. 2008;191(4):505–511. doi: 10.1007/s00221-008-1540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]