Abstract
Natural sounds can be characterized by their fluctuations in amplitude and frequency. Ageing may affect sensitivity to some forms of fluctuations more than others. The present study used individual differences across a wide age range (20–79 years) to test the hypothesis that slow-rate, low-carrier frequency modulation (FM) is coded by phase-locked auditory-nerve responses to temporal fine structure (TFS), whereas fast-rate FM is coded via rate-place (tonotopic) cues, based on amplitude modulation (AM) of the temporal envelope after cochlear filtering. Using a low (500 Hz) carrier frequency, diotic FM and AM detection thresholds were measured at slow (1 Hz) and fast (20 Hz) rates in 85 listeners. Frequency selectivity and TFS coding were assessed using forward masking patterns and interaural phase disparity tasks (slow dichotic FM), respectively. Comparable interaural level disparity tasks (slow and fast dichotic AM and fast dichotic FM) were measured to control for effects of binaural processing not specifically related to TFS coding. Thresholds in FM and AM tasks were correlated, even across tasks thought to use separate peripheral codes. Age was correlated with slow and fast FM thresholds in both diotic and dichotic conditions. The relationship between age and AM thresholds was generally not significant. Once accounting for AM sensitivity, only diotic slow-rate FM thresholds remained significantly correlated with age. Overall, results indicate stronger effects of age on FM than AM. However, because of similar effects for both slow and fast FM when not accounting for AM sensitivity, the effects cannot be unambiguously ascribed to TFS coding.
Keywords: frequency modulation, amplitude modulation, temporal fine structure, age
Introduction
Dynamic changes in pitch are fundamental for communicating contour in speech and music. The ability to detect changes in pitch is in part related to the efficacy with which the cochlea and auditory nerve transduce sound into neural impulses. For sinusoidal frequency modulation (FM), two potential peripheral cues involve rate-place (tonotopic) and temporal (phase-locking) information. According to the rate-place coding theory, FM is detected by fluctuations in the firing rate of auditory neurons as the instantaneous frequency of the tone changes, resulting in shifts of the excitation pattern. In this way, FM is converted to amplitude modulation (AM) by cochlear filtering (Zwicker 1956, 1970; Maiwald 1967a, b; Moore and Sek 1992, 1994; Saberi and Hafter 1995). In contrast, the temporal code relies on neural spikes that are phase-locked to the vibrations of the basilar membrane, providing the auditory system with time-interval-based information relating to the temporal fine structure (TFS) to convey the presence of FM (e.g., Moore and Sek 1995, 1996).
It has been proposed that FM with a low-frequency carrier (f c < 4–5 kHz) at slow modulation rates (f m < ∼10 Hz) utilizes a temporal code (Demany and Semal 1989; Moore and Sek 1995, 1996; Lacher-Fougère and Demany 1998; Moore and Skrodzka 2002), whereas FM at faster rates (f m ≥ ∼10 Hz) for the same low-frequency carriers and higher carriers at all rates (up to rates at which the sidebands become spectrally resolved; e.g., Hartmann and Hnath 1982) utilizes a rate-place code (e.g., Moore and Sek 1992, 1994; Saberi and Hafter 1995). There is some evidence suggesting that performance in tasks relying on neural phase locking may degrade with age. For example, older listeners generally perform more poorly than younger listeners on interaural phase and time difference (IPD and ITDs, respectively) detection or discrimination (Grose and Mamo 2010; Füllgrabe 2013; King et al. 2014; Gallun et al. 2014; Füllgrabe et al. 2015). Age effects on IPDs are present even when controlling for audiometric thresholds between young and older participants (Füllgrabe et al. 2015) and may be present as early as middle age (Ross et al. 2007; Grose and Mamo 2010; Füllgrabe 2013). Discrimination of harmonic from inharmonic stimuli, believed to require TFS coding, is also poorer in older participants (Hopkins and Moore 2011; Füllgrabe 2013). Although there is consensus that phase locking to TFS is required for IPD/ITD-based tasks for pure tones, it remains possible that tasks involving frequency coding, including frequency discrimination and FM tasks, are coded via a rate-place mechanism (e.g., Oxenham et al. 2009, 2011; Micheyl et al. 2013), or some combination of rate-place and time coding (e.g., Loeb et al. 1983; Shamma 1985; Shamma and Klein 2000; Loeb 2005).
Several studies have found that older participants perform more poorly at low-carrier, slow-rate FM detection than do younger (He et al. 2007; Strelcyk and Dau 2009; Grose and Mamo 2012; Paraouty et al. 2016; Wallaert et al. 2016; Paraouty and Lorenzi 2017) or middle-aged participants (Grose and Mamo 2012), even when all participants have normal hearing (NH) at the carrier frequency (He et al. 2007; Strelcyk and Dau 2009; Grose and Mamo 2012; Paraouty et al. 2016; Wallaert et al. 2016; Paraouty and Lorenzi 2017). These results are also consistent with the theory that temporal coding of TFS degrades with age in the absence of audiometric loss at the test frequency. However, some of these studies did not include comparable measures (such as AM detection or FM detection at fast rates) that are not thought to involve temporal coding of TFS. The lack of such “control” measures makes it difficult to rule out more general effects of aging, such as changes in cortical sensory coding or cognitive function (e.g., attention or processing speed). For example, Grose and Mamo (2012) found that older NH adults were worse at slow-rate, low-carrier FM detection relative to younger adults, but it is unclear whether they would have found the same effect for fast-rate FM detection at the same carrier. Even among studies that have used exclusively slow-rate FM, the outcomes have not been completely consistent. For instance, Schoof and Rosen (2014) measured slow FM difference limens (FMDLs) (f m = 2 Hz; f c = 1 kHz) in young (range 19–29 years) and older (range 60–72 years) listeners, but found no difference between the age groups. In this respect, as well as in several other measures examined by Schoof and Rosen (2014), their results are unusual in finding no perceptual deficits associated with aging, perhaps in part because of their strict definition of NH for the older group.
A few recent studies have used correlational measures in NH listeners to examine what peripheral code may be responsible for low-frequency carrier FM and have found conflicting evidence for the presence of TFS coding (e.g., Whiteford and Oxenham 2015; Otsuka et al. 2016; Paraouty and Lorenzi 2017). These studies have revealed high multicollinearity across modulation-detection tasks, including those thought to use separate mechanisms (e.g., low-carrier, slow-rate FM, and slow-rate AM). For instance, Whiteford and Oxenham (2015) used binaural modulation tasks to assess the fidelity of TFS coding based on IPDs (slow-rate dichotic FM) and level cues (slow- and fast-rate dichotic AM and fast dichotic FM) and found that many pairs of tasks (e.g., slow-rate dichotic FM and slow-rate dichotic AM) were correlated as strongly as pairs thought to share the same peripheral code (e.g., slow-rate dichotic FM and slow-rate diotic FM). The non-specific correlations could indicate that the variability between young NH listeners is driven primarily by non-peripheral factors, or that FM and AM use the same peripheral code. Paraouty and Lorenzi (2017) used a large sample of listeners varying in age to potentially increase the variability in peripheral TFS coding and found that thresholds for low-carrier FM with a 5-Hz modulation rate and low-carrier AM of the same rate were no longer correlated once AM was added to FM in order to disrupt potential excitation pattern cues. This could suggest that slow FM uses a combination of temporal and place coding; however, it is also possible that even at 5 Hz, there may be less viable TFS cues, given that the upper limit of extracting TFS cues in low-carrier FM is estimated to be around 10 Hz (e.g., Moore and Sek 1995).
The present study measured slow-rate (f m = 1 Hz) and fast-rate (f m = 20 Hz) FM and AM detection in both diotic and dichotic conditions, along with a measure of frequency selectivity based on forward masking, in a large cohort of participants whose ages ranged from 20 to 79 years. The paradigm was similar to that used in our earlier study of only young NH listeners (Whiteford and Oxenham 2015). The purpose of the dichotic tasks was to assess performance for disparity detection when TFS cues are necessary to complete the task (slow dichotic FM) relative to performance on the same task when TFS cues are not thought to be available (slow dichotic AM, but also fast dichotic FM and fast dichotic AM). The prediction of the study was that a selective deficit in the temporal coding of TFS should lead to poorer detection of slow-rate FM in both diotic and dichotic conditions, in ways that are unrelated (or at least less related) to AM or fast-rate FM detection. It was expected that age effects would be less likely to occur for diotic AM detection, given that previous studies have found either no effect (for f m = 5 Hz; He et al. 2008; Paraouty and Lorenzi 2017) or small effects (Füllgrabe et al. 2015; Wallaert et al. 2016) of age on sinusoidal AM detection at modulation rates comparable to those used in the present study.
Methods
Participants
Eighty-five adults (25 males, 60 females, mean age of 48.5 years, range 20.1–79.5) from the University of Minnesota and surrounding community participated in this study. There were 15 participants from each decade of age between 20 and 69 years and 10 participants between 70 and 79 years. Audiometric thresholds were assessed at octave-spaced frequencies between 250 and 8000 Hz. All participants had NH at low frequencies, defined as a low-frequency pure - tone average (PTA) (250, 500, and 1000 Hz) ≤20 dB hearing level (HL) in both ears, with no low-frequency PTA asymmetries greater than 10 dB. Across-ear average low-frequency PTA tended to increase (worsen) with age (r = 0.56, P < 0.0001, two-tailed). Average audiometric thresholds for each decade are plotted in Figure 1. Participants provided written informed consent and were compensated with course credit or hourly payment for their time. The experimental protocols were approved by the Institutional Review Board of the University of Minnesota.
FIG. 1.
Average audiometric thresholds for each decade of age. Error bars represent ±1 standard deviation. Symbols are offset around the octave frequencies for clarity.
Stimuli
With the exception of the first task (absolute thresholds for the carrier frequency), all stimuli and procedures were identical to those used by Whiteford and Oxenham (2015) and are described below.
Absolute threshold for the 500-Hz carrier frequency was measured separately in each ear using a 500-ms pure tone with 10-ms raised-cosine onset and offset ramps. FMDLs and AM difference limens (AMDLs) were measured diotically at this same frequency (f c = 500 Hz) for a slow (f m = 1 Hz) and fast (f m = 20 Hz) modulation rate. Both the target and the reference tones were 2 s in duration with 50-ms raised-cosine onset and offset ramps. The reference tone was always a 500-Hz pure tone, and the target tone was modulated. On a given trial, the modulator starting phase was set so that the FM target began with either an increase or a decrease in frequency excursion from the carrier frequency, with 50 % a priori probability. For the diotic AM tasks, the AM target began at either an amplitude peak or an amplitude trough. Stimuli for the dichotic FM and dichotic AM tasks were similar to their diotic counterparts, except that both the reference and target intervals were modulated. The difference was that the reference interval consisted of diotic stimuli, whereas the target interval had a starting modulator phase that was inverted in one ear, leading to dichotic stimulation that created the percept of a moving inter-cranial image for the slow modulation rate, based on ITDs in the case of FM and ILDs in the case of AM. At the fast modulation rate of 20 Hz, the dynamic ITDs and ILDs were too fast to induce the perception of movement. Instead, the target spatial image was perceived as more diffuse and less punctate than that of the reference (diotic) stimulus. For all FM and AM tasks (diotic and dichotic), the target and the reference tones were separated by a 500-ms inter-stimulus interval (ISI). All FM and AM stimuli were presented at 60 dB sound pressure level (SPL) in each ear.
Detection thresholds were also measured for a brief tone (20-ms total duration) with frequencies of 400, 430, 460, 490, 510, 540, 570, and 600 Hz, both in quiet and in the presence of a 500-Hz, 500-ms, pure-tone forward masker. The target frequency was only presented to the right ear, but the forward masker was presented diotically to reduce potential “confusion effects” (e.g., Neff 1986). Both the target and the masker had 10-ms raised-cosine onset and offset ramps. The forward masker level was fixed at 70 dB SPL, while the level of the target varied adaptively. The onset of the target occurred directly after the offset of the masker, resulting in a 10-ms gap between the half-amplitude points of masker and target envelopes. The slope of the masking function (target threshold as a function of masker-target frequency difference in octaves, calculated separately for targets below and above the masker frequency) provided an estimate of frequency selectivity.
All stimuli were generated digitally, converted to analog at a sampling rate of 48 kHz via a LynxStudio L22 soundcard, and presented over open-ear headphones (Sennheiser HD650) in a sound-attenuating chamber.
Procedure
Participants completed 11 tasks across 3–4 sessions. Each session lasted no longer than 2 h, and most participants completed the entire study within 3 sessions. The only difference in the procedures from Whiteford and Oxenham (2015) was the inclusion of absolute thresholds for a 500-Hz, 500-ms pure tone in each ear. The purpose of including this task was to obtain a more accurate estimate of audibility of the carrier frequency. The first task was the measurement of absolute thresholds for the 500-ms tones at the carrier frequency. All tasks used a two-interval, two-alternative forced-choice procedure, with a 3-down 1-up adaptive tracking rule that tracks the 79.4 % correct point of the psychometric function (Levitt 1971). The target was randomly presented in either the first or second interval, and the task was to select the interval containing the target by clicking the corresponding virtual button on the screen (labeled “1” or “2”). Feedback (“Correct” or “Incorrect”) was provided after each response. Each task is described in the order it was presented to the listeners. The order is consistent with that used by Whiteford and Oxenham (2015).
Task 1: Absolute Threshold for 500-ms Tone
The target was a 500-ms, 500-Hz pure tone, while the reference was 500 ms silence. The target and reference were separated by a 400-ms ISI. Participants were instructed to indicate whether they heard a tone in the first or second time interval, marked by red lights on the virtual response box on the screen. The target was presented at 40 dB SPL in the first trial, and the target level varied by a step size of 8 dB for the first two reversals. The step size was reduced to 4 dB for the third and fourth reversals and then to 2 dB for the following six reversals. Absolute threshold was defined as the mean level of the target at the last six reversal points. At least three adaptive runs were measured for each ear. If the standard deviation (SD) across the first three runs was ≥4 dB, thresholds from an additional three runs were collected, and only the last three runs were used in analyses. The order of the presentation ear (left vs. right) was randomized between runs.
Tasks 2 and 3: Dichotic FM Detection
Slow (f m = 1 Hz) dichotic FM detection was completed first, followed by fast (f m = 20 Hz) dichotic FM detection. For slow dichotic FM, participants heard two tones, one at a time, and were instructed to pick the tone that sounded as though it were “moving in your head.” Participants were encouraged to view the feedback on the screen to ensure they were listening for the correct feature. The frequency excursion from the carrier (∆f) was varied adaptively in the same manner as in Whiteford and Oxenham (2015). Each run began with the peak-to-peak frequency excursion (2∆f) set to 0.4 %, just below most average diotic FMDLs. The maximum value of the tracking variable was 2∆f = 2 % so that pitch cues would not interfere with the task (i.e., so that the target was perceived as a moving pure tone rather than one that was modulated in pitch). If the adaptive procedure called for a value of 2∆f that exceeded the maximum allowable value in more than 10 trials within a run, no threshold was recorded and listeners had to complete three additional runs. The value of ∆f varied by a factor of 2 for the first two reversals and a factor of 1.4 for the third and fourth reversals. The step size for the final six reversals was reduced to a factor of 1.19. Threshold was defined as the geometric mean value of 2∆f at the last six reversal points. All subsequent FM tasks use the same series of step sizes. If the SD across the first three runs was greater than or equal to 0.4 log units, those runs were regarded as practice, and thresholds from three additional runs were collected. The same SD criterion was used for all following FM tasks. Two participants could not differentiate the target from the reference tone with the standard starting value. For both participants, the starting value of the tracking variable was adjusted to 2∆f = 1.2 %. One participant was able to complete the task with the higher starting value. The other participant was unable to complete the slow dichotic FM task, even with an adjusted start value (age = 68). This participant was able to complete every other task, and so the ceiling value (2∆f = 2 %) was used as their threshold for slow dichotic FM.
Next, participants completed fast (f m = 20 Hz) dichotic FM detection. The instructions were to select which interval contained the tone with the “broader auditory image.” Again, participants were instructed to look at the feedback to help them identify the correct feature. Each run began with 2∆f = 2 %, with 2∆f never exceeding 200 % throughout each run.
Tasks 4 and 5: Diotic FM Detection
For both slow and fast FM detection, participants were instructed to pick the tone that was “modulated,” and that the modulated tone would sound like it is “changing.” Slow FM was always measured before fast FM. The starting value of the tracking variable was 2∆f = 5.02 % and never exceeded 2∆f = 200 %. The adaptive step sizes and the number of reversals used to define threshold were the same as in tasks 2 and 3.
Task 6: Absolute Thresholds for 20-ms Tones
Participants completed one adaptive run at each target frequency (400, 430, 460, 490, 510, 540, 570, and 600 Hz), and the order of the target frequency was randomized between runs. These were the same target frequencies as used in the forward masking patterns task (Task 11) but without the presence of the pure-tone forward masker. The instructions were to indicate whether the first or second time interval, marked by lights on the virtual response box on the screen, “had a click in it.” The design was analogous to the forward masking patterns task so that the target interval was 500 ms silence, directly followed by the 20-ms target. The reference interval was 520 ms silence. The reference and target intervals were separated by a 400-ms ISI. The level of the target frequency was varied adaptively. The target was presented at 40 dB SPL during the first trial, and the level was varied by a step size of 8 dB for the first two reversals. The step size was reduced to 4 dB for the third and fourth reversals and then to 2 dB for the following six reversals. Absolute threshold was defined as the mean target level at the last six reversal points. If the SD within a given run was ≥4 dB, one additional run was completed for the corresponding frequency, and only the second run was used in analyses.
Tasks 7 and 8: Dichotic AM Detection
The instructions for dichotic AM detection tasks were the same as for the dichotic FM tasks. Slow (f m = 1 Hz) dichotic AM was always measured before fast (f m = 20 Hz) dichotic AM. The starting value of the tracking variable, in units of 20log(m), was −8 dB. The step size was 6 dB for the first two reversals, 2 dB for the next two reversals, and 1 dB for the final six reversals. Participants with SDs ≥ 4 dB across their first three runs completed three additional runs, and only the subsequent runs were used in analyses. The same SD criterion was used for diotic AM detection (tasks 9 and 10).
Tasks 9 and 10: Diotic AM Detection
The task instructions for AM detection were the same as FM detection. Slow (f m = 1 Hz) AM was always measured before fast (f m = 20 Hz) AM. The modulation depth (m) was varied adaptively in the same manner as the dichotic AM tasks.
Task 11: Forward Masking Patterns
The 500-Hz pure-tone forward masker was presented in both intervals. In one of the intervals, a 20-ms target directly followed the masker. The instructions were to pick the tone that had a “click” after it. The ISI was 400 ms. The level of the masker was fixed at 70 dB SPL, while the target level varied adaptively. The starting value of the target was 60 dB SPL. Initially, the step size was 8 dB for the first two reversal points. The step size was decreased to 4 dB for the following two reversals and then decreased to 2 dB for the last six reversals. Threshold was defined as the average target level at the final six reversal points.
Participants completed two runs for each of the eight target frequencies (400, 430, 460, 490, 510, 540, 570, and 600 Hz; 16 runs total), and the order of the target frequencies was randomized between runs. If the SD across any of the two runs was ≥4 dB, participants completed two additional runs for the given target frequencies. If a participant had to repeat runs for two or more target frequencies, the order of the subsequent target frequencies was randomized. The recorded threshold was the mean (in dB) of thresholds across the final two runs in each condition.
Results
Diotic and Dichotic Frequency and Amplitude Modulation Detection Thresholds
Boxplots of the FMDLs across listeners are presented in Figure 2a. A two-way repeated-measures analysis of variance (ANOVA) was conducted on the average log-transformed thresholds for each subject, with modulation rate (slow vs. fast) and task type (diotic vs. dichotic) as within-subject factors. Results indicated a main effect of modulation rate [F (1, 84) = 434, P < 0.0001, η p 2 = 0.838], a main effect of task-type [F (1, 84) = 63, P < 0.0001, η p 2 = 0.428], and a significant interaction [F (1, 84) = 268, P < 0.0001, η p 2 = 0.761]. Post hoc Bonferroni-corrected t tests (α = 0.0083) showed significant differences between all combinations of FM tasks, diotic and dichotic at both modulation rates, except for fast diotic and fast dichotic FM. There was a slight trend for better performance on fast diotic FM relative to fast dichotic FM, but this was not significantly different once correcting for multiple comparisons [t (84) = −2.11, P = 0.03]. Thresholds for slow dichotic FM detection were significantly lower (better) than those for slow diotic FM detection (P < 0.0001). This result is consistent with the idea that subjects were using phase-locking to detect dynamic IPDs in the dichotic task, as IPD thresholds for static 500-Hz tones (e.g., Yost 1974) are far smaller than those produced by detectable values of 2Δf in the diotic FM detection tasks. Slow diotic FM detection was also significantly better than fast diotic FM (P < 0.0001). These trends are consistent with a number of previous studies that have implicated the use of phase-locking for slow, but not fast, FM detection (e.g., Moore and Sek 1995, 1996; Lacher-Fougère and Demany 1998; Strelcyk and Dau 2009).
FIG. 2.
Boxplots of diotic and dichotic a FMDLs and b AMDLs. Solid lines within each box represent the median, and the whiskers are the lowest and highest data points within 1.5 times the lower and higher inter-quartile ranges. Crosses represent individual data points outside the whiskers considered outliers. All data points, including outliers, were included in the analyses.
Boxplots of the AMDLs are presented in Figure 2b. A two-way repeated-measures ANOVA with modulation rate (slow vs. fast) and task-type (diotic vs. dichotic) as within-subject factors revealed a main effect of modulation rate [F (1, 84) = 52.2, P < 0.0001, η p 2 = 0.383], a main effect of task-type [F (1, 84) = 518, P < 0.0001, η p 2 = 0.861], and a significant interaction [F (1, 84) = 63.6, P < 0.0001, η p 2 = 0.431]. Post hoc Bonferroni-corrected t tests (α = 0.0083) for all possible pairwise comparisons demonstrated significant differences between all AM tasks, diotic and dichotic, except for slow vs. fast dichotic AM [t (84) = 1.28, P = 0.203]. As has been found several times for sinusoidal AM with gated carriers (Viemeister 1979; Sheft and Yost 1990; Moore and Sek 1995; Whiteford and Oxenham 2015), thresholds for fast diotic AM were significantly better than those for slow diotic AM (P < 0.0001), possibly due to the increased number of cycles in fast-rate compared to slow-rate AM (e.g., 2 cycles in slow AM compared to 40 cycles in fast AM) (e.g., Wallaert et al. 2016). Both slow and fast diotic AM thresholds were significantly better than their dichotic counterparts (P < 0.0001 in both cases).
Correlations Between Age, FMDLs, and AMDLs
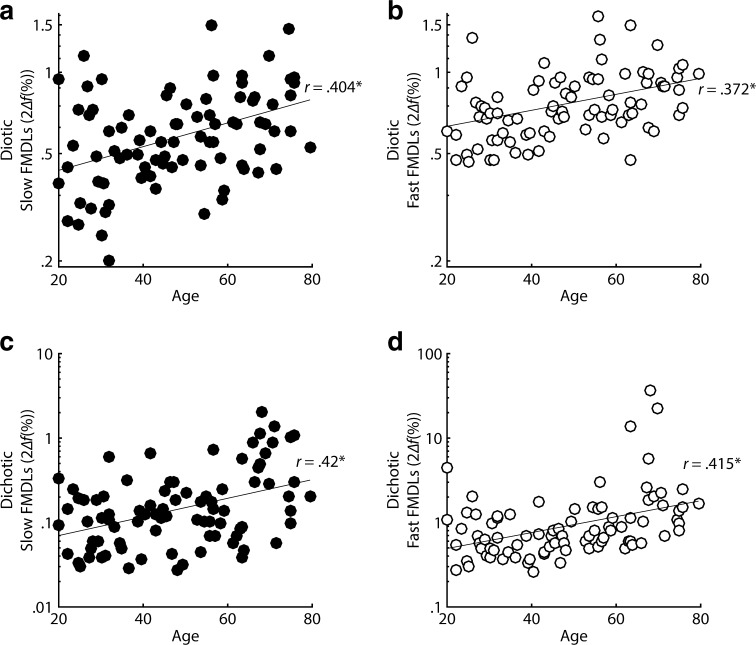
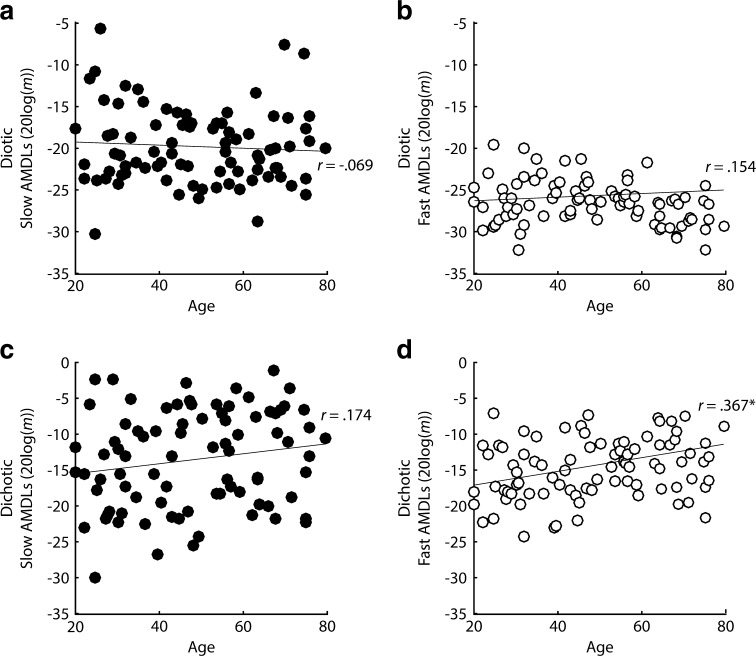
One way to examine the use of phase-locking in slow and fast FM is to correlate age with FM detection, given the large body of evidence suggesting TFS coding degrades with age (e.g., Hopkins and Moore 2011; Moore et al. 2012b; Füllgrabe 2013). One possibility is that performance on all modulation-detection tasks may degrade with age, regardless of the peripheral code involved. In fact, all of the FM detection tasks, diotic (slow FM: r = 0.404, P < 0.0001, one-tailed; fast FM: r = 0.372, P = 0.00025, one-tailed) and dichotic (slow FM: r = 0.42, P < 0.0001, one-tailed; fast FM: r = 0.42, P < 0.0001, one-tailed), were significantly correlated with age, even after using Bonferroni correction for running 16 multiple comparisons (i.e., all correlations run with age; α = 0.003; see Fig. 3). This was not the case for all of the AM tasks (see Fig. 4). Indeed, only fast dichotic AM was significantly correlated with age (r = 0.367, P = 0.0003, one-tailed), while slow dichotic AM (r = 0.174, P = 0.056, one-tailed) and slow (r = −0.069, P = 0.265, one-tailed) and fast diotic AM (r = 0.154, P = 0.08, one-tailed) were not.
FIG. 3.
Correlations between a, b diotic FM and age and c, d dichotic FM and age at slow (f m = 1 Hz; filled circles) and fast (f m = 20 Hz; open circles) modulation rates.
FIG. 4.
Correlations between a, b diotic AM and age and c, d dichotic AM and age at slow (f m = 1 Hz; filled circles) and fast (f m = 20 Hz; open circles) modulation rates.
The lack of correlation between diotic AM and age could not be accounted for by subclinical hearing loss and age co-varying (where reduced cochlear compression in older listeners might provide a benefit in AM detection), as neither diotic slow AM nor diotic fast AM correlated with age once partialling out average absolute thresholds at 500 Hz (slow AM: r p = −.148, p = 0.088, one-tailed; fast AM: r p = 0.173, p = 0.057, one-tailed). However, both slow and fast diotic FM still correlated with age after controlling for audibility at 500 Hz (slow FM: r p = 0.335, P = 0.0009, one-tailed; fast FM: r p = 0.33, P = 0.001, one-tailed). Even though fast diotic FM was significantly correlated with age and fast diotic AM was not, it is important to note that the difference between these two correlations, assessed using Steiger’s Z test (Steiger 1980), was not significant (Z = 1.75, P = 0.08, two-tailed), while the difference between the correlation of slow-rate diotic FM with age and the correlation of slow-rate diotic AM with age was highly significant (Z = 4.23, P < 0.0001, two-tailed).
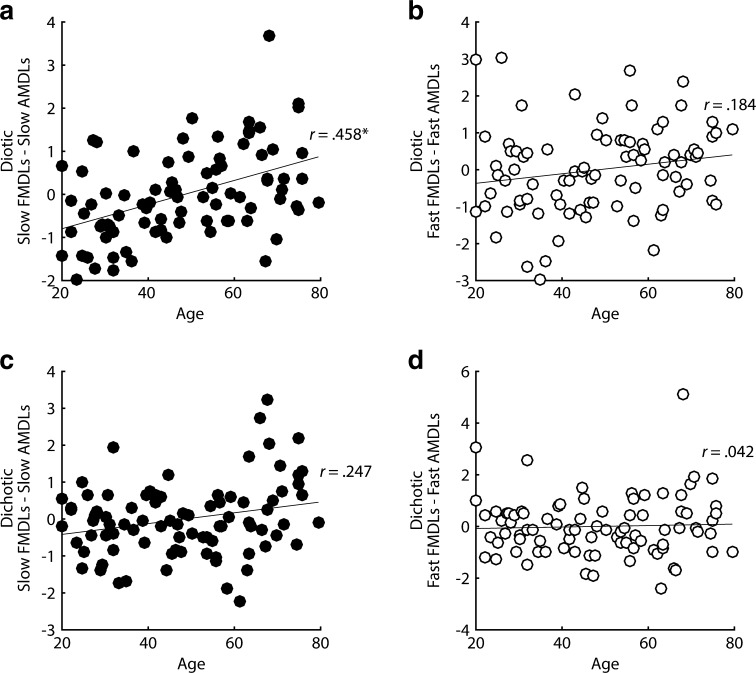
To control for any shared factors involved in modulation detection, including non-peripheral factors, we computed difference scores for each subject. First, the average FMDLs and AMDLs across subjects were z-transformed so that they were in the same units. Then, a difference score for each subject was calculated, subtracting their z-scored threshold in an AM detection task from their z-scored threshold in the corresponding FM detection task (e.g., slow diotic FM, slow diotic AM). Participants who perform better on FM detection relative to AM detection will have lower difference scores, and vice versa. Once controlling for sensitivity to diotic AM detection, slow diotic FM detection thresholds were still significantly correlated with age (r = 0.458, P < 0.0001, one-tailed), whereas fast diotic FM detection thresholds were not (r = 0.184, P = 0.046, one-tailed; see Fig. 5a, b). The difference between these two correlations was significant (Z = 2.34, P = 0.019, two-tailed). Assuming TFS coding degrades with age, this outcome provides some evidence for a role for TFS coding in slow diotic FM detection and perhaps no role, or a smaller role, for TFS coding in fast diotic FM.
FIG. 5.
Correlations between diotic (a, b) and dichotic (c, d) FM detection and age, once controlling for sensitivity to AM, using dimensionless z-scores.
If the variability in slow dichotic FM is driven by variability in TFS coding, then slow dichotic FM should be correlated with age, once controlling for sensitivity to slow dichotic AM. However, there was no significant correlation between the difference scores for slow dichotic FM once correcting for multiple comparisons (r = 0.247, P = 0.011, one-tailed; see Fig. 5c). As expected, there was also no correlation between fast dichotic FM difference scores and age (r = 0.042, P = 0.352, one-tailed; see Fig. 5d).
Correlations Between FM and AM Tasks
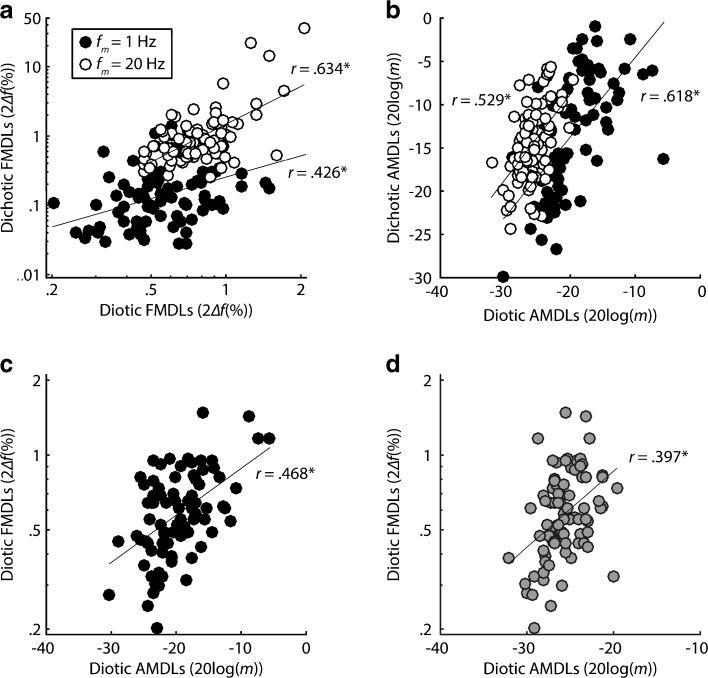
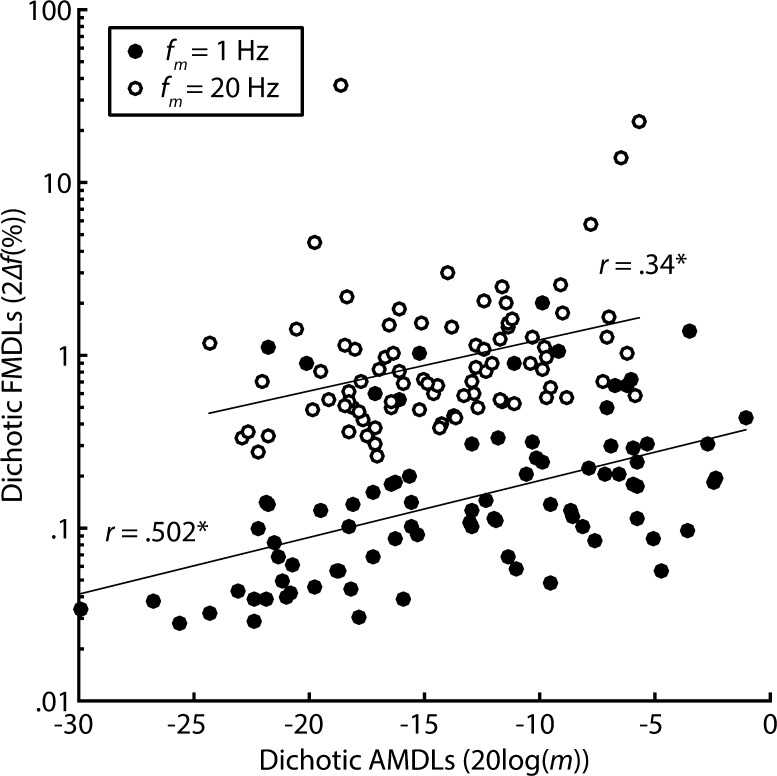
If the variability between participants in FM and AM diotic and dichotic tasks is largely driven by variability in peripheral coding, then thresholds in tasks that rely on similar peripheral codes should be highly correlated. Similarly, thresholds in tasks thought to rely on separate peripheral codes should not be highly correlated. In line with the peripheral coding hypothesis, slow diotic FM detection thresholds were correlated with slow dichotic FM detection thresholds (r = 0.426, P < 0.0001, one-tailed), a task that is believed to rely on TFS coding (see Fig. 6a). This correlation was significant even after using Bonferroni correction for seven multiple comparisons (α = 0.007). Tasks believed to reflect excitation pattern information, such as fast diotic FM and fast dichotic FM (r = 0.634, P < 0.0001, one-tailed) and fast diotic AM and fast dichotic AM (r = 0.529, P < 0.0001, one-tailed; see Fig. 6b), were also significantly correlated. However, tasks believed to reflect separate peripheral codes were also well correlated. For instance, thresholds in the slow diotic FM condition and the slow diotic AM condition were correlated (r = 0.468, P < 0.0001, one-tailed; see Fig. 6c). In the same vein, dichotic tests believed to reflect similar peripheral mechanisms, such as fast dichotic FM and fast dichotic AM (r = 0.34, P = 0.001, one-tailed), as well those thought to reflect separate mechanisms, such as slow dichotic FM and slow dichotic AM (r = 0.502, P < 0.0001, one-tailed), were also moderately correlated (see Fig. 7). Because thresholds in most of the modulated tasks were well correlated with each other, our correlational measures provide no strong evidence for either the use of place or temporal coding in FM detection at different rates.
FIG. 6.
Correlations between FM and AM, diotic and dichotic, tasks. For all panels, filled circles correspond to individual thresholds at slow rates (f m = 1 Hz) and open circles correspond to individual thresholds at fast rates (f m = 20 Hz). Gray circles correspond to conditions where the x-axis represents thresholds with the fast rate, and the y-axis represents thresholds with the slow rate. a, b Modulated tasks believed to measure similar peripheral codes are well correlated, but c, d tasks believed to measure different peripheral codes are also well correlated.
FIG. 7.
Correlations between dichotic FM and AM at two modulation rates. Filled circles represent individual thresholds with f m = 1 Hz, while open circles represent thresholds with f m = 20 Hz.
Frequency Selectivity and FM Detection
In order to estimate the steepness of the low- and high-frequency sides of the excitation pattern for each subject, we first calculated the threshold for each of the 20-ms target frequencies when preceded by the 500-Hz pure-tone masker. Then, two linear regression analyses were conducted to estimate the low and high slopes of the forward masking patterns. The low slope was based on a regression using the thresholds from the four lowest target frequencies (400, 430, 460, and 490 Hz), and the high slope was based on a regression using the thresholds from the four highest target frequencies (510, 540, 570, and 600 Hz). Under this arrangement, steep low slopes are increasingly positive, whereas steep high slopes are increasingly negative.
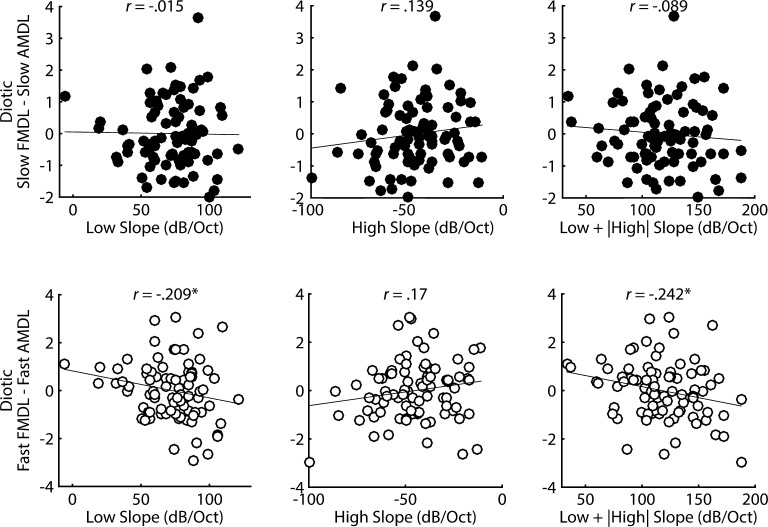
If fast, but not slow, diotic FM detection relies on place coding, then fast diotic FM thresholds should be correlated with the steepness of the filter slopes from the forward masking patterns but slow FM thresholds should not. However, neither slow (low slope: r = 0.003, P = 0.489; high slope: r = 0.039, P = 0.362; low slope + |high slope|: r = −0.02, P = 0.428) nor fast (low slope: r = 0.064, P = 0.28; high slope: r = 0.069, P = 0.265; low slope + |high slope|: r = 0.006, P = 0.478) diotic FMDLs were correlated with frequency selectivity. Subtractive measures controlling for the sensitivity to AM at the same modulation rate are plotted in Figure 8 (top row: slow FM; bottom row: fast FM). As can be seen from the top row in Figure 8, there was also no correlation between slow FMDLs and filter slopes once controlling for sensitivity to slow AMDLs (low slope: r = −0.015, P = 0.446; high slope: r = 0.139, P = 0.103; low slope + |high slope|: r = −0.089, P = 0.209). There was only a very weak correlation between fast FM and filter slopes (low slope: r = −0.209, P = 0.028; high slope: r = 0.17, P = 0.06; low slope + |high slope|: r = −0.242, P = 0.013; see bottom row of Fig. 8). Note that these correlations only reach significance without corrections for multiple comparisons.
FIG. 8.
Correlations between frequency selectivity and diotic FM detection, controlling for sensitivity to AM detection. The top row (filled circles) corresponds to f m = 1 Hz, while the bottom row (open circles) corresponds to f m = 20 Hz. Diotic fast FM was weakly, significantly correlated with the steepness of the filter slopes, while diotic slow FM was not.
Discussion
The Role of TFS Coding in FM Detection
Results from this study provided evidence both for and against the hypothesis that slow FMDLs require temporal coding of TFS, and that TFS coding degrades with age. The primary piece of supporting evidence was that slow, but not fast, diotic FMDLs were correlated with age, after controlling for sensitivity to AMDLs at the same rate. These two correlations were also significantly different from one another. AMDLs at the same rate make an ideal control for diotic FM because the task procedures and demands were very similar, with only the stimulus differing. Hence, any non-TFS factors should be well controlled. Second, as found in many previous studies (e.g., Moore and Sek 1996; Lacher-Fougère and Demany 1998; Whiteford and Oxenham 2015), detection of slow diotic FM was better than detection of fast diotic FM, while the opposite trend was found for AM. Together, the correlations with age and the patterns of average detection thresholds could suggest that slow, low-carrier FM uses a separate peripheral code, based on phase-locking to TFS cues. However, even a dissociation between AM and FM does not necessarily imply TFS processing, as these same results could be a product of differences in the correlational properties of cortical neurons (Micheyl et al. 2013). The present findings might also be explained by a combined place and temporal model for slow, low-carrier FM, as suggested by some studies measuring slow-rate FMDLs in the presence of an AM masker (e.g., Paraouty et al. 2016; Paraouty and Lorenzi 2017). For example, consistent with our findings, Paraouty and Lorenzi (2017) found that slow diotic FM and slow diotic AM were correlated across a large group of listeners. However, slow diotic FM in the presence of added AM, used to minimize place information, did not correlate with slow diotic AM but did correlate with slow diotic FM in quiet (with no added AM).
Other aspects of the results from the present study are less easily interpreted in terms of different coding mechanisms for FM and AM. First, slow dichotic FMDLs were not correlated with age, once slow dichotic AMDLs were factored out. Thus, for the one task where temporal coding of TFS must play a role (dichotic FM, or dynamic IPD, detection), no strong correlation with age was found, raising questions as to why it was found for the diotic FM, which may or may not be represented via temporal coding. One possibility is that performance in the binaural tasks was limited by more central constraints, during or following the binaural integration of information. Second, similar correlations were observed between age and both slow and fast diotic FM without controlling for sensitivity to AM. It could be that the variability was driven by non-TFS coding factors in fast-rate diotic FM (as suggested by the lack of correlation with age once controlling for sensitivity to fast diotic AM), but not slow-rate diotic FM. Alternatively, both slow- and fast-rate diotic FM detection may use a similar peripheral code. The moderate correlations between many of the modulation-detection tasks, even those thought to use separate peripheral codes, found both in the present data and several other studies (Whiteford and Oxenham 2015; Otsuka et al. 2016; Paraouty and Lorenzi 2017), could also suggest similar peripheral codes for slow and fast FM and AM. For example, Otsuka et al. (2016) interpreted significant correlations between slow FM, slow AM, and IPDs to mean that neural phase-locking to TFS cues might be used for both types of slow, low-carrier sinusoidal modulation detection. However, this interpretation would not explain why detection for slow rate FM is better than fast-rate FM, while the opposite trend exists for AMDLs.
A number of non-peripheral factors might also be responsible for the high multicollinearity between many FM and AM tasks. First, the primary limiting factor in diotic FMDLs and AMDLs may arise from central coding. For example, a shared cortical rate-place code for frequency and intensity (Micheyl et al. 2013) might account for correlations in behavioral FM/AM data, although the model has yet to be applied to FMDLs and AMDLs in the same manner as frequency and intensity difference limens. Second, all of the diotic and dichotic FM and AM tasks in the present study have very similar task demands, and some multicollinearity could be from cognitive aspects required to detect modulation, such as sustained attention (e.g., Füllgrabe et al. 2015). Given that more general cognitive measures were not assessed in the present study, their influence on the present measures remains speculative.
Effects of Age or High-Frequency Hearing Loss?
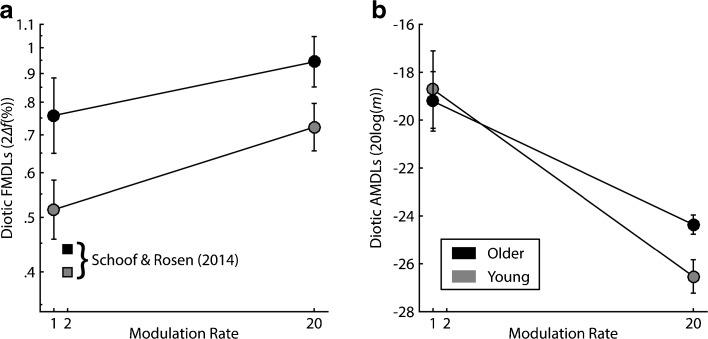
It is important to note that any effects of age found in the present study could be driven by high-frequency hearing loss. This is because age and average high-frequency (2, 4, and 8 kHz) audiometric thresholds were highly correlated (r = 0.741, P < 0.0001). In addition, Schoof and Rosen (2014) did not find a relation between poor slow, low-carrier FMDLs and age. One difference in their study is the more stringent NH criterion relative to the current study or previous studies (He et al. 2007; Strelcyk and Dau 2009; Grose and Mamo 2012; Paraouty et al. 2016). Schoof and Rosen (2014) required older and younger subjects to have audiometric thresholds in both ears ≤25 dB HL up to and including 4 kHz (two octaves above the carrier frequency, f c = 1 kHz), and then thresholds ≤25 dB HL in at least one ear at 6 kHz (two and a half octaves above the carrier). Figure 9 plots the present diotic FMDLs and AMDLs from a subset of our subjects, selected using the same age and analogous audiometric criteria. Average FMDLs are plotted for younger (age < 30; n = 15) and older (age ≥ 60; n = 11) participants. Average FMDLs from Schoof and Rosen for a 1-kHz carrier with a 2-Hz modulation rate are plotted for reference (squares). Even when using a similar, more stringent NH criterion, our results demonstrate significantly poorer FMDLs in the older adults [F (1,24) = 4.24, P = 0.046, η p 2 = 0.155], a main effect of modulation rate [F (1,24) = 15.754, P = 0.001, η p 2 = 0.396], but no interaction between modulation rate and group [F (1,24) = 0.693, P = 0.413, η p 2 = 0.028]. Importantly, the same group trends were not observed for AMDLs: There was no main effect of group [F (1,24) = 0.343, P = 0.563, η p 2 = 0.014], but there was a main effect of modulation rate [F (1,24) = 44.875, P < 0.0001, η p 2 = 0.652], and no group by rate interaction [F (1,24) = 1.9, P = 0.181, η p 2 = 0.073]. The fact that group differences remain in the FM but not the AM tasks, despite the more stringent criteria, is consistent with the poorer FMDLs being due to age rather than age-related high-frequency hearing loss.
FIG. 9.
Average a diotic FMDLs and b diotic AMDLs for young (age < 30; gray) vs. old (age ≥ 60; black) participants. Circles correspond to the present data, measured for a 500-Hz carrier at slow (f m = 1 Hz) and fast (f m = 20 Hz) modulation rates. Squares correspond to average FMDLs from Schoof and Rosen (2014), measured for a 1-kHz carrier at a 2-Hz modulation rate.
It remains to be explained why Schoof and Rosen (2014) did not find an age effect for slow, low-carrier FM. The average age of older and young adults was comparable between studies when using a similar NH criterion (Schoof and Rosen (2014): mean age young = 23.7, mean age older = 64.1; present study: mean age young = 25.1, mean age older = 66.3). Apart from a few methodological differences (e.g., 3AFC instead of 2AFC, f c = 1 kHz instead of f c = 500 Hz, and minor differences in the use of practice trials), their procedures were quite similar to those of the present study. Schoof and Rosen’s (2014) FMDLs are substantially better than were found in the current study, but this was expected, as FMDLs tend to be better at 1 kHz relative to 500 Hz when quantified as percent peak-to-peak frequency change (Demany and Semal 1989). It is possible that there may have been differences in audiometric thresholds at the test frequency between the current study and Schoof and Rosen (2014) accounting for these effects. However, this seems unlikely given that all but one participant in the present study had audiometric thresholds ≤20 dB HL at 500 Hz, and this participant had 25 dB HL in just one ear. One last explanation is that our group may have had greater absolute hearing loss at 6 kHz (not measured in the present study) or 8 kHz, and perhaps hearing loss specifically at 6 or 8 kHz may influence the results. This could be a meaningful difference if high-frequency hearing loss is a marker for age-related synaptopathy throughout the cochlea, including lower-frequency areas where absolute thresholds are normal, as was found in CBA/CaJ mice (Sergeyenko et al. 2013). However, there is currently no direct, physiological measure to confirm age-related synaptopathy in humans, so it remains unclear whether high-frequency hearing loss co-varies with a loss of synaptic terminals at lower-frequency places along the basilar membrane. Furthermore, simulations using signal detection theory suggest that very severe synaptopathy may be necessary to noticeably affect frequency coding, whether or not a temporal code is used (Oxenham 2016).
The Role of Frequency Selectivity in FM Detection
Even after controlling for sensitivity to AM, fast-rate diotic FM was only very weakly correlated with the steepness of the auditory filter slopes but slow diotic FM was not, suggesting a possible small role for frequency selectivity specific to fast diotic FM. This trend was only significant when no corrections were made for multiple comparisons. However, if cochlear filtering was responsible for the average trends observed in Figure 3, then one might expect moderate-to-strong correlations between fast-rate diotic FM and frequency selectivity. Hence, the evidence for a role of place coding in fast FM detection is weak. These results are consistent with the findings of Whiteford and Oxenham’s (2015) study of young, NH listeners, where they found similarly weak correlations between FMDLs and filter slopes. It may be that stronger correlations will be observed in a population that spans a wide range of sensorineural hearing loss at the carrier frequency and, hence, a wide range of frequency selectivity. According to the initial hypothesis of this study, increasing the variability in place coding should have a greater influence on fast, but not slow, FM detection.
Changes in Between-Subject Variability with Age
The current study examined low-carrier FM and AM detection at different rates in diotic and dichotic conditions across a large cohort of listeners varying in age, potentially providing insight into which tasks utilize temporal coding of TFS, given the number of earlier studies indicating an effect of age on tasks thought to employ temporal coding of TFS (e.g., Grose and Mamo 2010; Grose and Mamo 2012; Ruggles et al. 2012; Füllgrabe 2013; Gallun et al. 2014). The variability in performance on slow dichotic FM was considerably larger in the current sample than in Whiteford and Oxenham’s (2015) cohort of young NH listeners (see Table 1). This could reflect an increase in variability in TFS coding due to our inclusion of older listeners. However, the variability on slow dichotic AM and fast dichotic FM was also much higher in the current sample relative to the younger listeners. Because the increase in variability in the dichotic tasks was not specific to slow dichotic FM, the specific source of this variability is unclear and may not, in fact, be driven by variability in peripheral temporal coding. The implications of increased variability in several binaural tasks with age, even non-TFS binaural tasks, are important and suggest that assuming performance variability on binaural tasks largely reflects differences in peripheral coding may not be appropriate. These findings, in addition to the lack of significant correlation between slow dichotic FM and age once controlling for performance on slow dichotic AM (Fig. 5c), could mean that previous attempts to measure TFS processing using IPDs in older NH or HI listeners may have reflected variability in more central binaural coding (e.g., Grose and Mamo 2010; Hopkins and Moore 2011; Moore et al. 2012a).
TABLE 1.
SD for each modulated task in the current study, with a wide age range (20.1–79.5) of participants, and Whiteford and Oxenham’s (2015) study, with a smaller age range (18–32) of strictly NH participants
| Current study (n = 85) | Whiteford and Oxenham (2015) (n = 100) | |
|---|---|---|
| Slow dichotic FM | 4.43 | 3.38 |
| Fast dichotic FM | 3.83 | 3 |
| Slow diotic FM | 1.84 | 1.84 |
| Fast diotic FM | 1.34 | 1.46 |
| Slow dichotic AM | 6.76 | 5.61 |
| Fast dichotic AM | 4.4 | 4.44 |
| Slow diotic AM | 4.54 | 4.64 |
| Fast diotic AM | 2.38 | 2.95 |
Conclusions
Findings from the present study across a large cohort of listeners varying in age provide mixed evidence for the role of temporally based TFS coding in slow, but not fast, FM detection. Slow but not fast diotic FMDLs correlated with age, but this specific trend was only observed once controlling for sensitivity to diotic AM. Overall, AMDLs were not strongly correlated with age. FMDLs and AMDLs were correlated, even for tasks thought to use different peripheral codes, potentially implicating a role of central processing, including more central sensory coding, as well as non-sensory cognitive factors, such as sustained attention, on FM and AM detection. Therefore, the effects of age on peripheral coding may be outweighed by variability in non-peripheral factors.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 DC005216).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Kelly L. Whiteford, Phone: (612)-626-3258, Email: whit1945@umn.edu
Heather A. Kreft, Email: plumx002@umn.edu
Andrew J. Oxenham, Email: oxenham@umn.edu
References
- Demany L, Semal C. Detection thresholds for sinusoidal frequency modulation. J Acoust Soc Am. 1989;85:1295–1301. doi: 10.1121/1.397460. [DOI] [PubMed] [Google Scholar]
- Füllgrabe C. Age-dependent changes in temporal-fine-structure processing in the absence of peripheral hearing loss. Am J Audiol. 2013;22:313. doi: 10.1044/1059-0889(2013/12-0070). [DOI] [PubMed] [Google Scholar]
- Füllgrabe C, Moore BCJ, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2015;7:1–25. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallun FJ, McMillan GP, Molis MR, et al. Relating age and hearing loss to monaural, bilateral, and binaural temporal sensitivity. Front Neurosci. 2014;8:1–14. doi: 10.3389/fnins.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear. 2010;31:755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Frequency modulation detection as a measure of temporal processing: age-related monaural and binaural effects. Hear Res. 2012;294:49–54. doi: 10.1016/j.heares.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann WM, Hnath GM. Detection of mixed modulation. Acustica. 1982;50:297–312. [Google Scholar]
- He N, Mills JH, Dubno JR. Frequency modulation detection: effects of age, psychophysical method, and modulation waveform. J Acoust Soc Am. 2007;122:467–477. doi: 10.1121/1.2741208. [DOI] [PubMed] [Google Scholar]
- He N, Mills JH, Ahlstrom JB, Dubno JR. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am. 2008;124:3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins K, Moore BCJ. The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. J Acoust Soc Am. 2011;130:334–349. doi: 10.1121/1.3585848. [DOI] [PubMed] [Google Scholar]
- King A, Hopkins K, Plack CJ. The effects of age and hearing loss on interaural phase difference discrimination. J Acoust Soc Am. 2014;135:342–351. doi: 10.1121/1.4838995. [DOI] [PubMed] [Google Scholar]
- Lacher-Fougère S, Demany L. Modulation detection by normal and hearing-impaired listeners. Audiology. 1998;37:109–121. doi: 10.3109/00206099809072965. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. doi: 10.1121/1.1912375. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Are cochlear implant patients suffering from perceptual dissonance? Ear Hear. 2005;26:435–450. doi: 10.1097/01.aud.0000179688.87621.48. [DOI] [PubMed] [Google Scholar]
- Loeb GE, White MW, Merzenich MM. Spatial cross-correlation. Biol Cybern. 1983;47:149–163. doi: 10.1007/BF00337005. [DOI] [PubMed] [Google Scholar]
- Maiwald D. Die Berechnung von Modulationsschwellen mit Hilfe eines Funktionsschemas. Acustica. 1967;18:193–207. [Google Scholar]
- Maiwald D. Ein Funktionsschema des Gehörs zur Beschreibung der Erkennbarkeit kleiner Frequenz-und Amplitudenänderungen. Acustica. 1967;18:81–92. [Google Scholar]
- Micheyl C, Schrater PR, Oxenham AJ. Auditory frequency and intensity discrimination explained using a cortical population rate code. PLoS Comput Biol. 2013;9 doi: 10.1371/journal.pcbi.1003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Ernst SMA. Frequency difference limens at high frequencies: evidence for a transition from a temporal to a place code. J Acoust Soc Am. 2012;132:1542–1547. doi: 10.1121/1.4739444. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Sek A. Detection of combined frequency and amplitude modulation. J Acoust Soc Am. 1992;92:3119–3131. doi: 10.1121/1.404208. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Sek A. Effects of carrier frequency and background noise on the detection of mixed modulation. J Acoust Soc Am. 1994;96:741–751. doi: 10.1121/1.410312. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Sek A. Effects of carrier frequency, modulation rate, and modulation waveform on the detection of modulation and the discrimination of modulation type (amplitude modulation versus frequency modulation) J Acoust Soc Am. 1995;97:2468–2478. doi: 10.1121/1.411967. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Sek A. Detection of frequency modulation at low modulation rates: evidence for a mechanism based on phase locking. J Acoust Soc Am. 1996;100:2320–2331. doi: 10.1121/1.417941. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Skrodzka E. Detection of frequency modulation by hearing-impaired listeners: effects of carrier frequency, modulation rate, and added amplitude modulation. J Acoust Soc Am. 2002;111:327–335. doi: 10.1121/1.1424871. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR, Stoev M, et al. The influence of age and high-frequency hearing loss on sensitivity to temporal fine structure at low frequencies (L) J Acoust Soc Am. 2012;131:1003. doi: 10.1121/1.3672808. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Vickers DA, Mehta A. The effects of age on temporal fine structure sensitivity in monaural and binaural conditions. Int J Audiol. 2012;51:715–721. doi: 10.3109/14992027.2012.690079. [DOI] [PubMed] [Google Scholar]
- Neff DL. Confusion effects with sinusoidal and narrow-band noise forward maskers. J Acoust Soc Am. 1986;79:1519–1529. doi: 10.1121/1.393678. [DOI] [PubMed] [Google Scholar]
- Otsuka S, Furukawa S, Yamagishi S, et al. Relation between cochlear mechanics and performance of temporal fine structure-based tasks. J Assoc Res Otolaryngol. 2016;17:541–557. doi: 10.1007/s10162-016-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ. Predicting the perceptual consequences of hidden hearing loss. Trends Hear. 2016;20:1–6. doi: 10.1177/2331216516686768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ, Micheyl C, Keebler MV. Can temporal fine structure represent the fundamental frequency of unresolved harmonics? J Acoust Soc Am. 2009;125:2189–2199. doi: 10.1121/1.3089220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ, Micheyl C, Keebler MV, et al. Pitch perception beyond the traditional existence region of pitch. Proc Natl Acad Sci U S A. 2011;108:7629–7634. doi: 10.1073/pnas.1015291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraouty N, Lorenzi C. Using individual differences to assess modulation-processing mechanisms and age effects. Hear Res. 2017;344:38–49. doi: 10.1016/j.heares.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Paraouty N, Ewert SD, Wallaert N, Lorenzi C. Interactions between amplitude modulation and frequency modulation processing: effects of age and hearing loss. J Acoust Soc Am. 2016;140:121–131. doi: 10.1121/1.4955078. [DOI] [PubMed] [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27:11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Why middle-aged listeners have trouble hearing in everyday settings. Curr Biol. 2012;22:1417–1422. doi: 10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi K, Hafter ER. A common neural code for frequency- and amplitude-modulated sounds. Nature. 1995;374:537–539. doi: 10.1038/374537a0. [DOI] [PubMed] [Google Scholar]
- Schoof T, Rosen S. The role of auditory and cognitive factors in understanding speech in noise by normal-hearing older listeners. Front Aging Neurosci. 2014;6:1–14. doi: 10.3389/fnagi.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma SA. Speech processing in the auditory system II: lateral inhibition and the central processing of speech evoked activity in the auditory nerve. J Acoust Soc Am. 1985;78:1622–1632. doi: 10.1121/1.392800. [DOI] [PubMed] [Google Scholar]
- Shamma S, Klein D. The case of the missing pitch templates: how harmonic templates emerge in the early auditory system. J Acoust Soc Am. 2000;107:2631–2644. doi: 10.1121/1.428649. [DOI] [PubMed] [Google Scholar]
- Sheft S, Yost WA. Temporal integration in amplitude modulation detection. J Acoust Soc Am. 1990;88:796–805. doi: 10.1121/1.399729. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. doi: 10.1037/0033-2909.87.2.245. [DOI] [Google Scholar]
- Strelcyk O, Dau T. Relations between frequency selectivity, temporal fine-structure processing, and speech reception in impaired hearing. J Acoust Soc Am. 2009;125:3328–3345. doi: 10.1121/1.3097469. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Wallaert N, Moore BCJ, Lorenzi C. Comparing the effects of age on amplitude modulation and frequency modulation detection. J Acoust Soc Am. 2016;139:3088–3096. doi: 10.1121/1.4953019. [DOI] [PubMed] [Google Scholar]
- Whiteford KL, Oxenham AJ. Using individual differences to test the role of temporal and place cues in coding frequency modulation. J Acoust Soc Am. 2015;138:3093–3104. doi: 10.1121/1.4935018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost WA. Discriminations of interaural phase differences. J Acoust Soc Am. 1974;55:1299–1303. doi: 10.1121/1.1914701. [DOI] [PubMed] [Google Scholar]
- Zwicker E. Die elementaren Grundlagen zur Bestimmung der Informationskapazität des Gehörs. Acustica. 1956;6:365–381. [Google Scholar]
- Zwicker E. Masking and psychological excitation as consequences of the ear’s frequency analysis. In: Plomp R, Smoorenburg GF, editors. Frequency analysis and periodicity detection in hearing. Leiden: Sijthoff; 1970. pp. 376–394. [Google Scholar]