Abstract
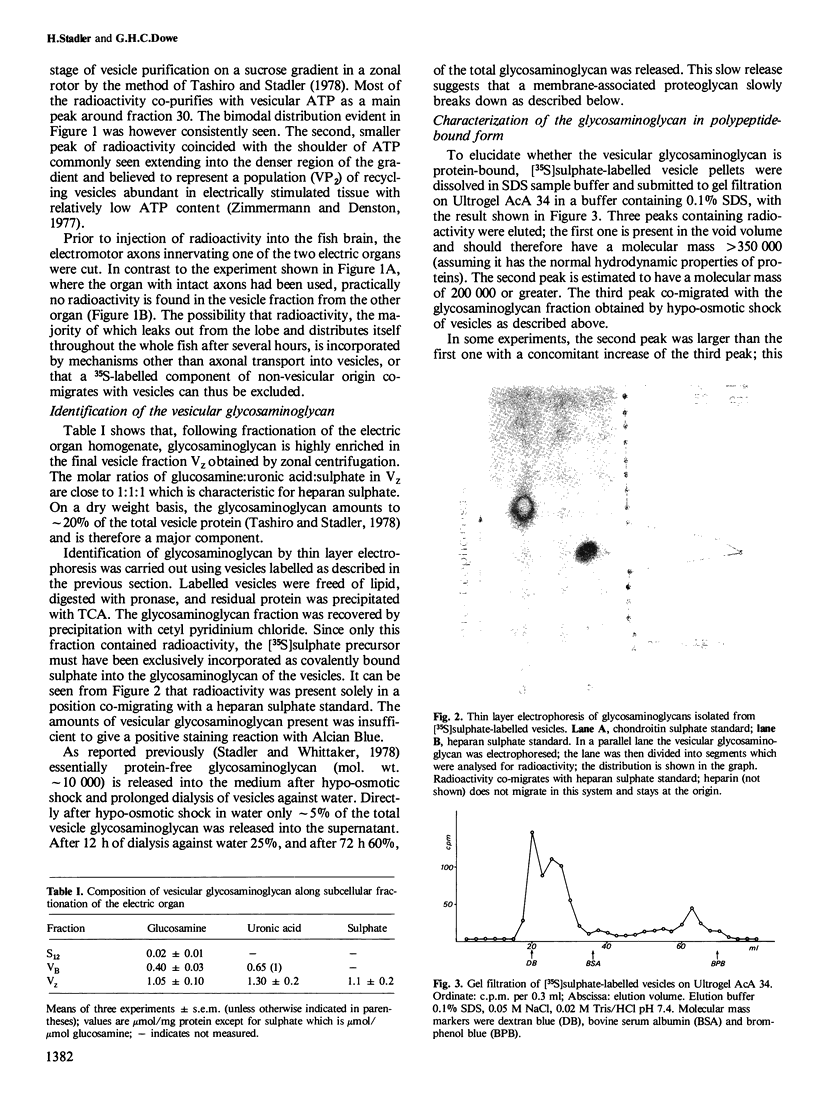
Cholinergic synaptic vesicles isolated from the electric organ of Torpedo marmorata were found to contain a proteoglycan in their core. The glycosaminoglycan part co-migrates upon thin layer electrophoresis with heparan sulphate and shows a chemical composition characteristic for this carbohydrate. [35S]Sulphate injected into the electric lobes of Torpedo, which contain the perikarya of the electromotor neurons innervating the electric organs, appeared 48 h later in covalently bound form in the synaptic vesicle fraction. The radiolabel had been incorporated into the vesicular heparan sulphate. Upon SDS-polyacrylamide gel electrophoresis fluorography of labelled vesicles a major and a minor band are formed both migrating above a protein standard of mol. wt. 200 000. Similarly, a major peak in the void volume and a minor peak in the included volume are seen upon gel filtration in Ultrogel AcA 34 in the presence of SDS. We interpret the minor fraction as being formed by the loss of glycosaminoglycan from the major fraction. The proteoglycan is located inside the vesicle since antibodies directed against it form immunoprecipitates only with vesicles lysed by detergent treatment. The experiments show that it is possible to label a synaptic organelle specifically by axonal transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Boyne A. F., Whittaker V. P. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J. 1974 Apr;140(1):1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füldner H. H., Stadler H. 31P-NMR analysis of synaptic vesicles. Status of ATP and internal pH. Eur J Biochem. 1982 Jan;121(3):519–524. doi: 10.1111/j.1432-1033.1982.tb05817.x. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Walker J. H., Stadler H., Whittaker V. P. Further evidence that glycosaminoglycan specific to cholinergic synaptic vesicles recycles during electrical stimulation of the electric organ of Torpedo marmorata. Cell Tissue Res. 1982;224(3):685–688. doi: 10.1007/BF00213763. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohsawa K., Dowe G. H., Morris S. J., Whittaker V. P. The lipid and protein content of cholinergic synaptic vesicles from the electric organ of Torpedo marmorata purified to constant composition: implications for vesicle structure. Brain Res. 1979 Feb 9;161(3):447–457. doi: 10.1016/0006-8993(79)90674-7. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Stadler H., Füldner H. H. Proton NMR detection of acetylcholine status in synaptic vesicles. Nature. 1980 Jul 17;286(5770):293–294. doi: 10.1038/286293a0. [DOI] [PubMed] [Google Scholar]
- Stadler H., Tashiro T. Isolation of synaptosomal plasma membranes from cholinergic nerve terminals and a comparison of their proteins with those of synaptic vesicles. Eur J Biochem. 1979 Nov 1;101(1):171–178. doi: 10.1111/j.1432-1033.1979.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Stadler H., Whittaker V. P. Identification of vesiculin as a glycosaminoglycan. Brain Res. 1978 Sep 22;153(2):408–413. doi: 10.1016/0006-8993(78)90424-9. [DOI] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs B. An attempt to explain nervous transmitter release as due to nerve impulse-induced cation exchange. Acta Physiol Scand. 1973 Feb;87(2):168–175. doi: 10.1111/j.1748-1716.1973.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Walker J. H., Jones R. T., Obrocki J., Richardson G. P., Stadler H. Presynaptic plasma membranes and synaptic vesicles of cholinergic nerve endings demonstrated by means of specific antisera. Cell Tissue Res. 1982;223(1):101–116. doi: 10.1007/BF00221502. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Essman W. B., Dowe G. H. The isolation of pure cholinergic synaptic vesicles from the electric organs of elasmobranch fish of the family Torpedinidae. Biochem J. 1972 Jul;128(4):833–845. doi: 10.1042/bj1280833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Stadler H. Identification of actin in highly purified synaptic vesicles from the electric organ of Torpedo marmorata. J Neurochem. 1982 Sep;39(3):788–795. doi: 10.1111/j.1471-4159.1982.tb07961.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Denston C. R. Separation of synaptic vesicles of different functional states from the cholinergic synapses of the Torpedo electric organ. Neuroscience. 1977;2(5):715–730. doi: 10.1016/0306-4522(77)90025-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Vesicle recycling and transmitter release. Neuroscience. 1979;4(12):1773–1804. doi: 10.1016/0306-4522(79)90058-7. [DOI] [PubMed] [Google Scholar]




