Abstract
In peach, the flat phenotype is caused by a partially dominant allele in heterozygosis (Ss), fruits from homozygous trees (SS) abort a few weeks after fruit setting. Previous research has identified a SSR marker (UDP98–412) highly associated with the trait, found suitable for marker assisted selection (MAS). Here we report a ∼10 Kb deletion affecting the gene PRUPE.6G281100, 400 Kb upstream of UDP98-412, co-segregating with the trait. This gene is a leucine-rich repeat receptor-like kinase (LRR-RLK) orthologous to the Brassinosteroid insensitive 1-associated receptor kinase 1 (BAK1) group. PCR markers suitable for MAS confirmed its strong association with the trait in a collection of 246 cultivars. They were used to evaluate the DNA from a round fruit derived from a somatic mutation of the flat variety ‘UFO-4’, revealing that the mutation affected the flat associated allele (S). Protein BLAST alignment identified significant hits with genes involved in different biological processes. Best protein hit occurred with AtRLP12, which may functionally complement CLAVATA2, a key regulator that controls the stem cell population size. RT-PCR analysis revealed the absence of transcription of the partially deleted allele. The data support PRUPE.6G281100 as a candidate gene for flat shape in peach.
Subject terms: Natural variation in plants, Plant breeding, Plant genetics
Introduction
Fruits are the edible part of many cultivated species and their study is one of the major topics in plant research. Peach (Prunus persica (L.) Batsch) is one of the most economically important fruit species in temperate regions. The fruits are drupes which develop from a single carpel. The calyx and the stamen of the flowers fuse into the hypanthium tissue forming a cuplike structure around the ovary. All peach tissues come from the ovary; the outer skin is the exocarp, the mesocarp the edible flesh and the pit the endocarp. Most peach cultivars are round or oval shaped, although commercial interest in flat shape fruits is increasing fast. Nowadays, just in Spain 11,700 ha are cultivated in one year with flat peaches, producing 215,260 tons1.
While little is known about the genetic mechanisms regulating fruit morphogenesis in fruit trees, many genetic studies have aimed to unravel such process in the model species Arabidopsis. In this species leucine-rich receptor like kinases like ERECTA and CLAVATA-1, show functional implications in the maintenance, size and shape meristem2, 3. In particular, ERECTA regulates organ shape and flower architecture, showing the loss-of-function erecta mutants compact inflorescences, short pedicels and round flowers3, 4.
Among cultivated species, fruit shape has been most studied in tomato. The fruits are berries which develop from the ovary after fertilization of the ovules. The wall of the ovary develops into the pericarp and encloses the placenta and seeds. Four genes controlling tomato fruit shape have been cloned: SUN5, OVATE 6, LOCULE NUMBER and FASCIATED 7, 8. In addition several loci which regulate fruit shape have been identified including two suppressor elements of the ovate mutation (Sov1 and Sov2)9. One is the mutant Self1, producing fruit elongation by increasing cell layers in the ovary10, and the other is QTL fs8.1 which also controls fruit elongation11. SUN, OVATE, and fs8.1 act together in additive manner to control fruit shape producing longer fruits. In cucumber, a homolog of the tomato SUN gene (CsSUM) is a candidate for round fruit shape12.
Flat peaches originated in South China, where they are known as “pentao” from the original Chinese “Pan Tao”. In the mid-1800s several Chinese flat varieties were introduced into USA breeding programs as carriers of characters such as low chilling13, but they were popular for a brief period of time. It is believed that the first flat peach variety, bred by Starks Nursery in 1985, was ‘Saturn’ and later, in the 1990s, its cultivation became more widespread14.
The flat shape of the peach fruit is determined in the early stages of flower development by a single gene S/s (for saucer-shaped) mapped in the distal part of chromosome 615. Fruits from individuals with the ss genotype are round, those heterozygous for the flat allele (Ss) are flat, and fruits from homozygous SS plants abort several weeks after anthesis. Although the hypothesis of a single gene explains the phenotypes observed, abortion of homozygous SS plants also suggests two dominant closely linked genes (S/s and Af/af) in repulsion16. Up to now, several markers have been identified around the S locus, by analyzing both mapping progenies and germplasm17–19. One of the markers, the SSR UDP98-412 has been reported to be tightly linked to the S locus and works efficiently in marker assisted selection (MAS)18.
Horn et al.20 mapped ESTs of 3,842 candidate genes for fruit quality in the Prunus reference map, but no candidate genes were identified for fruit shape. Recently, a PpCAD1 gene (Prupe.6G292200, alias ppa003772m peach genome v.1) has been reported19 as candidate for the trait based on a GWAS analysis.
Here we propose a new candidate gene, PRUPE.6G281100 (a LRR-RLK), whose non-functional allele is observed in heterozygosis in flat varieties and in homozygosis in aborting varieties. Its homology with genes involved in organ shape in other species makes this gene a good candidate for this trait. We also analysed a somatic mutant of a flat variety that reverted to round shape. The analysis of PRUPE.6G281100 in the DNA from fruit tissues of the flat variety and its round mutant revealed that the non-functional allele of PRUPE.6G281100 suffered a mutation, reinforcing the hypothesis of its role in determining peach fruit shape.
Results
Search for polymorphisms associated to the flat shape trait
To find DNA polymorphisms associated with the flat trait in peach, we explored a 30.2 kb region flanking the SSR UDP98-412, previously reported to be tightly linked to this trait18. We designed 14 primer pairs to amplify and sequence fragments of 350–680 bp in this region, in a small set of three flat and three round peaches. No polymorphisms (SNPs or INDELs) were observed in the DNA fragments amplified by these primers.
The SNPs closest to UDP98-412 annotated in the peach genome database occur 363.5 kb upstream of this marker, with 20 SNPs in a 26.5 kb region (Pp06: 26,254,140.26,254,809). All these SNPs were located in the coding regions of five annotated transcripts. By sequencing nine amplicons of these transcripts we confirmed these 20, plus 10 additional, SNPs in the same set of six flat and round peaches. Thirteen out of the 30 SNPs were associated with the flat phenotype in the small panel of cultivars. All 13 SNPs occurred in a total of 1,150 bp of two partially overlapping amplicons, nine in Amplicon5 and four in Amplicon6 (Supplementary Table S1). In addition, we detected an insertion/deletion (INDEL) polymorphism in Amplicon5 in heterozygosis in flat varieties. To confirm the association of the SNPs and the INDEL with the phenotype we sequenced Amplicon5 and Amplicon6 in 112 varieties (65 round, 47 flat) and three aborting phenotypes (Aborting02, Aborting08 and Aborting17) from the ‘UFO3’ × ‘SweetCap’ progeny. All round varieties were homozygous for the reference allele in 11 out of the previous 13 SNPs and the flat ones heterozygous, while the aborting seedlings where homozygous for the alternative allele, in agreement with the genetics of the trait (Supplementary Table S2). Alignment of the round and aborting sequences of Amplicon5 against the reference genome gave two INDEL variants in the aborting sequence: an 8 bp insertion and, a few bases downstream, a 13 bp deletion. Forward and reverse sequences of Amplicon 5 in flat varieties revealed that they contained both INDELs in heterozygosis. By cloning and sequencing the PCR product of one flat variety (‘UFO-8’) we confirmed that each of the two alleles were identical to round and aborting, respectively. The two haplotypes observed for Amplicon5 and Amplicon6, in homozygosis or heterozygosis in the small panel of flat and round varieties, are shown in Fig. 1.
Figure 1.
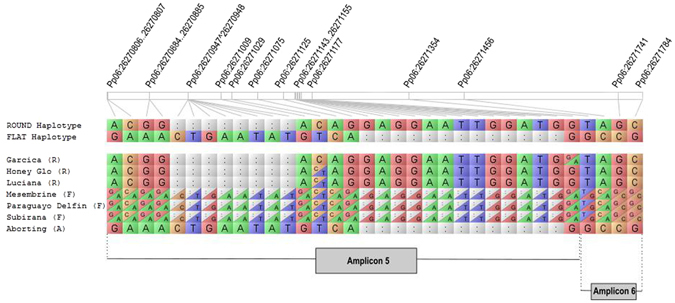
Round and flat associated haplotypes in round (R), flat (F) and aborting (A) peaches. Colon represents the deletion of a nucleotide. The haplotypes consist of 13 SNPs and two INDELS, in Amplicon5 and Amplicon6 (Pp06: 26,270,679.26,271,829) (see Supplementary Table S1).
According to the genome annotation, Amplicon5 and Amplicon6 are part of coding regions of a 2,253 bp long transcript (PRUPE.6G281100). Custom DNA BLAST analysis with PLAZA 3.0 gave significant alignment of PRUPE.6G281100 with seven genes in the peach genome; four (PRUPE.6G281000, PRUPE.6G281200, PRUPE.6G281300 and PRUPE.6G281400) in a region of 36.4 Kb in chromosome 6 containing PRUPE.6G281100; one in chromosome 7 (PRUPE.7G088700) and the two remaining in chromosome 8 (PRUPE.8G054400 and PRUPE.8G054300).
Variant identification and allele cloning
To obtain the whole sequence of the flat and round associated alleles of PRUPE.6G281100 we used primers upstream, downstream and within this gene in two round (‘Garcica’ and ‘Honey Glo’), two flat (‘Paraguayo Delfín’ and ‘Mesembrine’) and two aborting individuals. Amplification with the primer pair flanking the gene (PC1) gave a fragment with the expected size (3.3 Kb) in the round and flat DNAs, but failed with the aborting ones (Fig. 2a). The sequence of the amplified fragment in the flat varieties revealed the presence of the round allele only (lacking the two INDELs). The failed amplification of the flat-associated allele suggested a polymorphism in or near the gene.
Figure 2.

PCR bands reveal the deletion affecting the gene PRUPE.6G281100 in aborting and flat peaches. (a) PC1 failed to amplify the flat-associated allele. (b) Long-range PCR amplification with PC2 produced a fragment about 10 kb shorter in flat and aborting than in round peaches. (c) PCR-amplification with PC3 identified round (941pb), flat (1620/941 bp) and aborting (1620 bp) genotypes. (d) Diagrammatic representation of the position of the primers used to identify the polymorphisms associated with flat shape and the polymorphisms (SNPs and small INDELs) in PRUPE.6G281100, represented as dots and triangles (respectively).
To explore this hypothesis we amplified the samples with forward and reverse primers designed at opposite ends of the gene. Primers 10,072 bp upstream and 558 bp downstream of the gene (PC2) yielded one band of the expected size for the round sample (12,882 kb), and one about 10 kb shorter in the aborting and in the flat peaches (Fig. 2b). The full sequence of the short band revealed a fragment 2,912 nucleotides long, and consequently 9,970 bp less than that expected from the reference genome. The polymorphisms consisted in the loss of a region from 9,324 bp upstream of the start codon (Pp06: 26,260,453) to 693 bp downstream of this codon (Pp06: 26,726,336). Despite amplifying the large band in the round samples, where it occurred in homozygosis, we were not able to obtain this band in the heterozygous flat samples, where the short allele appeared to amplify preferentially. As a result, the presence of this fragment in heterozygosis was validated with a three-primer PCR assay (PC3): two (IndelS_F and IndelS_R) flanking the deletion to amplify a 1,620 bp associated to the flat phenotype and one internal to the deletion (IndelS_2 R) to produce a 941 bp band associated to the round phenotype (Fig. 2c and d).
Variant validation with NGS
The presence of the large deletion in heterozygosis in flat varieties was also validated by resequencing five flat and five round varieties with Illumina NGS technology. The alignments corresponding to Pp06:26,257,000.26,273,000 region (16 kb) of varieties of each fruit type were bulk-analysed. With the CLC-INDELs and Structural Variants tool, the two small INDELs within the gene were identified, but not the large deletion. However this deletion was evident in the visual track of the alignment, where only flat peaches had less reads in the deleted region (Fig. 3).
Figure 3.
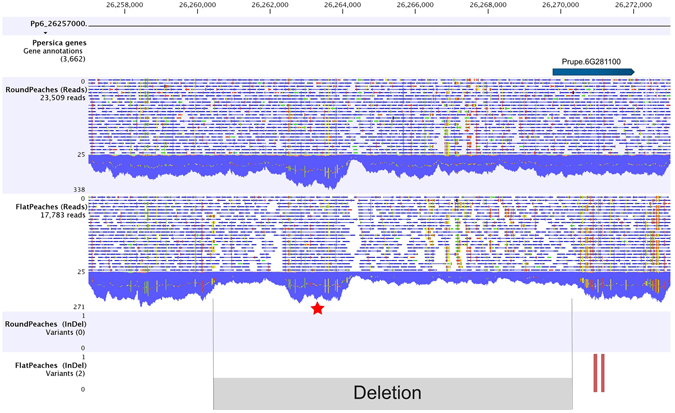
Alignment of round and flat peaches reads against Pp06:26,262,400.26,264,250 region. CLC-Workbench track display including (a) Pp06:26262400.26264250 region Prupe.6G281100, (b) bulked alignment of Illumina reads from five round and (c) five flat peaches. Blue areas represent the sequence depth at each position. The reduction in the number of reads in the flat peaches reveals the deletion in heterozygosis. An increase in the number of reads in the region Pp06:26,262,400.26,264,250 (labeled in the figure with a*) is produced by the spurious alignment (confirmed by Sanger sequencing) of a highly repetitive region. (d) The CLC- InDels and structural variants tool identified the two indels in the flat varieties only.
Polymorphism validation in peach germplasm and markers for seedling selection
The two small INDELs within the gene and the close to 10 kb deletion were tested with PC4 and PC3, respectively, in a panel of 177 flat, round and aborting samples (Supplementary Table S2). All genotypes matched the observed phenotype. For the two small INDELs, the size of the fragments (469 bp for the round and 464 bp for the flat-associated alleles) confirmed that the two INDEL variants were in heterozygosis in flat varieties and seedlings while the respective alleles were homozygous in round and aborting ones. Similarly, the genotype obtained with the primers flanking and within the 10 kb deletion matched the phenotype (941 bp for the round and 1,620 bp for the flat associated alleles).
Expression analysis
RT-PCR amplification of RNA extracted from pistils of round, flat and aborting peaches produced fragments exclusively from the round and flat samples (Fig. 4). The size of the bands and their sequence revealed that, in both cases, the fragment amplified corresponded to the round-associated allele (lacking the two small INDELs), indicating the absence of transcription of the flat-associated allele.
Figure 4.
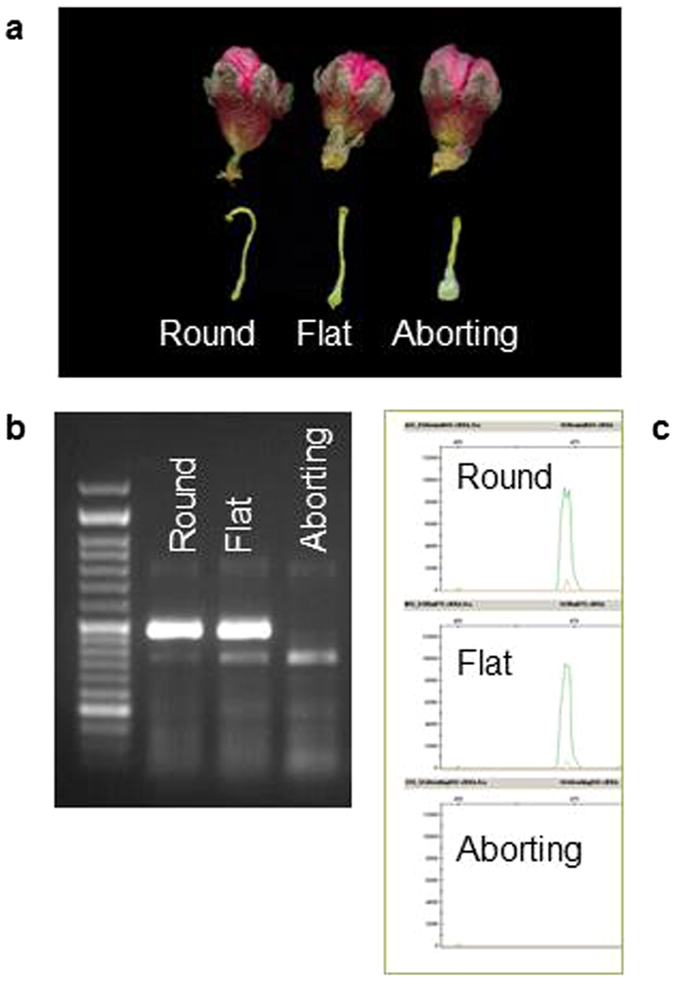
RT-PCR of RNA from round, flat and aborting pistils. (a) Pistil shape observed in flower buds in stage E. On RT-PCR amplification of round, flat and aborting pistils using PC4 no amplification of the flat-associated allele was visible in both (b) agarose and (c) capillary electrophoresis.
Homology and functional prediction of the gene
The gene PRUPE.6G281100 (allias ppa025511m in the peach genome annotation v.1) is a leucine-rich repeat kinase (PLAZA 3.0 gene family HOM03D000009, orthologue group ORTHO03D000261 described as Brassinosteroid insensitive 1-associated receptor kinase 1, BAK1). BLAST analysis of the translated protein against the PLAZA protein sequence database gave best alignments with 250 genes from 55 subfamilies of the gene family HOM03D00009 (Supplementary Table S3). Most of the genes (160 genes, 64%) belonged to four subfamilies (ORTHO03D000261, ORTHO03D000539, ORTHO03D001987, ORTHO03D002896). Thirty-eight of them were annotated as BAK1 (23.8%), 30 (18.8%) as leucine-rich repeat receptor-like kinase protein FLORAL ORGAN NUMBER1 and 81 (50.6%) as leucine-rich repeat receptor–like kinase protein THICK TASSEL DWARF1 in 25 species, including peach and other Rosaceae members such as Fragaria vesca and Malus x domestica. Within peach, these were PRUPE.6G281000, PRUPE.6G281200, PRUPE.6G281300, PRUPE.6G281400, PRUPE.6G281500, PRUPE.6G288800, PRUPE.7G088700, PRUPE.8G054400 and PRUPE.8G054300.
Protein BLAST pairwise alignment against the SwissProt Arabidopsis database gave significant hits with LRR-RLK, involved in different biological processes. Best hit occurred with the AtRLP12 gene. This gene may functionally complement CLAVATA2, a key regulator that acts at the shoot apical meristem (SAM) of plants, controlling the stem cell population size21.
Analysis of the polymorphism in a round somatic mutant
We analyzed the polymorphisms in a round peach generated from a somatic natural mutant of the flat variety ‘UFO-4’ (Fig. 5). The analysis of genomic DNA with PC4 (with formward and reverse priers flanking the two small INDELs inside PRUPE.6G281100) showed a faint amplification of the flat allele in the mutated round cultivar compared to the strong signal observed in the original flat. Amplification of DNA extracted from skin, flesh and stone tissues revealed the absence of the flat associated allele in the flesh mutated DNA while it was present in the skin DNA, indicating that the mutation occurred in the meristematic LII. Faint amplification of the flat allele was observed in the stone DNA of the mutant, which could be due to the invasion of LIII by mutated LII cells.
Figure 5.
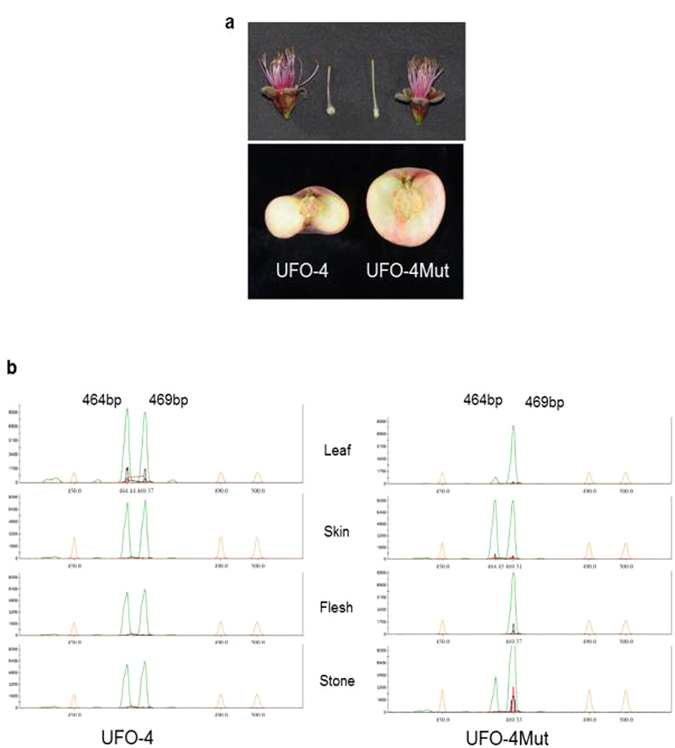
Analysis of a flat variety (‘UFO-4’) and its somatic round mutant (‘UFO-4Mut’). (a) Image of the flat (left) and round (right) pistils and fruits. (b) PCR-amplification products obtained with PC4 to detect the two small INDELs associated with the flat trait. PCR reactions were carried out with DNA extracted from leaf, as well as from skin, flesh and stone fruit tissues. The peaks show that the mutation occurred in flesh tissue (meristematic layer LII) affecting the flat associated allele.
As for ‘UFO-4’, amplification of ‘UFO-4Mut’ flesh DNA with the primers flanking PRUPE.6G281100 (PC1) produced only the round-associated allele, as occurred with those designed to genotype the 10 kb deletion (PC3). These results suggest a mutation affecting the flat associated allele which could have caused the reversion of the phenotype from flat to round.
Discussion
Here we explored the genetic variability in a region associated with the flat shape in peach and identified a candidate gene for this trait. Considering the high level of variability observed genome-wide between round and flat peaches22 the large extension of LD in peach23, 24 and the codominant mode of action of the flat and round alleles, which must be in heterozygosis in flat varieties, we expected to find a substantial level of heterozygosis in the region flanking the SSR marker associated with the trait (UDP98-412). Surprisingly none of the fragments sequenced showed polymorphisms. Thereafter we searched for the closest region to UDP98-412 with annotated SNPs in the databases. This region was 337.5 Kb upstream and contained one SNP every 521 bp, close to the density of 1 SNP every 598 bp found by ref. 25 after sequencing genes in peach varieties, but much higher than the density of 1 SNP every 1076 bp observed by ref. 26 in Chinese edible varieties.
After sequencing nine amplicons of the variable region in a panel of varieties we identified SNPs highly associated with flat, round and aborting phenotypes in two amplicons of the gene PRUPE.6G281100. By amplification, cloning and sequencing part of the gene, 11 SNPs and two INDELs co-segregating with the trait were identified (Fig. 1), which allowed us to design an allelic specific marker diagnostic for this trait. This marker was validated in 177 varieties from different origins, including nineteen where UDP98-412 alleles escaped association with the trait18. In all cases, the genotype obtained was in agreement with fruit shape phenotype, confirming that this region is closer to the S/s locus. In consequence, we provide here a simple marker (FlatIn_F/IndelS_F; PC4) able to amplify two fragments differing in 5 bp, that improves the performance of UDP98-412 and is more efficient for MAS. Additionally to this primer combination, we found several SNPs that can be used for the same purpose. Long-range PCR reactions detected a ~10 Kb deletion of part of the gene (693 bp from the ATG starting codon), affecting the flat-associated allele. Alignment and coverage analysis of NGS reads of five flat and five round varieties allowed visualization of the alignment of the large gap in heterozygosis (Fig. 3). This deletion was validated in the panel of varieties, with all flat varieties sharing the same haplotype and suggesting a unique origin of the flat trait in the panel evaluated. Some of these varieties (18) have been analyzed in ref. 27 with an SNP array of close to 9,000 SNPs. They were distributed along all transects of the variability observed including, the major Oriental and Occidental clusters, indicating that the varieties analyzed here covered a broad range of variability. Unlike peaches and nectarines that are separated in different clusters in Occidental materials23, no specific clusters including only flat peaches occur, which is consistent with the fact that, due to the heterozygous nature of flat peaches, breeding is usually by selecting in round x flat progenies, and that the flat allele, originating from a single source, may have been introgressed in a diverse array of materials.
RT-PCR (Fig. 4) and posterior band sequencing revealed the absence of transcription of the flat associated allele, indicating a loss of function of PRUPE.6G281100. Thereafter PRUPE.6G281100 gene, from the orthologous group ORTHO03D000261, annotated as Brassinosteroid insensitive 1-associated receptor kinase 1 (BAK1) arises here as a candidate for the flat shape of peach fruits. This gene is a leucine-rich repeat receptor-like kinase (LRR-RLK), the proteins constituting ligand-receptor systems that control cell fate specification, and mediate correct cell divisions and cell-to-cell communication, allowing correct generation of tissues and organs through growth and development of both animals and plants28. Plant RLKs can be classified into six classes based on the structural feature of the extracellular domain. The largest class of plant RLKs is the LRR-RLKs class (700 in Arabidopsis and 1,400 in rice)29, proteins that contain leucine-rich repeats, which are tandem repeats of approximately 24 amino acids with conserved leucines involved in protein-protein interactions. Most LRR-RLKs are involved in embryonic pattern formation, which suggests a putative role of this protein in the coordination of cell proliferation during embryogenesis and during morphogenesis of embryonic cells at meristems, shaping the plant30. Two LRR-RLKs, CLAVATA1 and ERECTA, show functional implications in the maintenance, size and shape of meristems2.
The protein showed best homology with BAK1, FLORAL ORGAN NUMBER1 (FON1) and THICK TASSEL DWARF1 (TD1) genes. BAK1 is involved in brassinosteroid (BR) signal transduction, forming heterodimers with BRASSINOSTEORID INSENSITIVE (BRII) modulating growth and development, including cell expansion and reproductive development in species such as Arabidopsis and rice31, 32. The FON1 gene encodes a receptor-like kinase protein (orthologous to Arabidopsis CLAVATA1) that regulates the size of the floral meristem, causing enlargement in Oryza sativa 33. Similarly, TD1 encodes a maize orthologous to CLAVATA1 in Arabidopsis, modulating meristem size during inflorescence and flower development and involved in the regulation of meristem structural organization34. We can therefore hypothesize that the LRR-kinase protein encoded by PRUPE.6G281100 is involved in a cell signaling pathway, during flower development, that ensures a final round shape of the ovary, and consequently of the fruit. While the loss of function of this gene in homozygosis produces unviable fruits, in heterozygosis the allele produces flat fruits. This behavior resembles the mechanism of a haploinsufficient locus. Loss-of-function alleles at haploinsufficient loci are typically dominant because the level of gene function in a heterozygote is below the threshold for producing a wild-type phenotype, and homozygotes typically exhibit more severe phenotypes, including early lethality. The most common explanation is that these loci are involved in cellular processes sensitive to dosage effects and changes in protein concentration35.
We found several homologous genes in the peach genome, which, as suggested for Arabidopsis31 might be functionally redundant. Given that most of its homologues clustered in the same genome region, the large LD in peach and the unusually high variability detected in the haplotype, we need to explore other possible functional polymorphisms in the region acting alone or in combination.
Our candidate gene differs from that suggested by ref. 19, identified through a GWAS approach. These authors found associated SNPs in the fifth intron of a CONSTITUTIVELY ACTIVATED CELL DEATH GENES (CAD1) homologous gene, which negatively controls the salicylic acid (SA) mediated pathway of programmed cell death in plant immunity. This gene is 650 Kb downstream from our candidate gene. Flat varieties contained the polymorphism in either homozygosis (A/A) or heterozygosis (A/T), while round varieties were always homozygous T/T, indicating that these genotypes do not fully correspond with the inheritance of the trait (A/A genotypes should not produce viable fruits). To our knowledge19, study is the first to report a putative role of CAD1 genes in organ shape and development and its high association with the trait could be due to the large LD extension in peach. However we cannot discard possible involvement of both genes in the trait.
Gene function is usually validated by genetic transformation or by the screening of mutants. The main obstacle in validating candidate genes in peach through genetic transformation is the regeneration of transformed plantlets. Although the transformation and regeneration of stable transgenic plantlets in peach has been reported36, 37, this is not yet a well resolved method.
Alternatively, the study of somatic mutants in woody plants, and in particular in peach, has been successfully used to investigate causal genes38, 39. These mutations often occur in only one histogenic layer, so are chimeric and most are not sexually transmitted. In peach, the histogenic layer LI gives rise to epidermal tissues, LII to subepidermal tissues, and the male and female sporogenous tissues, and LIII to the remainder of the shoots. In fruits, LI produces the skin, LII the flesh and LIII the stone.
Here we investigated the PRUPE.6G281100 gene in a chimeric natural mutation occurring in the meristematic LII (producing the fruit flesh tissue), which reverted from the flat to the round phenotype (Fig. 5). Although we did not obtain the sequence of the mutated flat allele, the analysis of flesh DNA with allele specific primers to amplify part or the gene revealed a new structural mutation affecting the flat allele, while the skin DNA shows the intact flat and round-associated alleles. One hypothesis for the gain of function compatible with the haploinsuficiency mechanism is the recombination of the mutant flat allele with others of the LRR-Kinase genes present in the candidate gene region. As demonstrated by ref. 40, chimeric kinase receptors made in the lab can produce new functional receptors. In fact, sequence divergence, genetic recombination, duplication events and selective forces have been proven to be the main forces for the continuous RLK gene expansion41. Alternatively, chromosome replacement of the ‘flat’ region by the homologous ‘round’ region is also a plausible hypothesis. In grape, this type of molecular mechanism produced the mutant Pinot blanc from Pinot gris42. Cloning the new mutated allele will provide information of the gene mechanism.
Methods
Plant material
In total we studied 249 peach individuals, classified as round, flat or aborting (in those cases where either all or most of fruit set stopped within a few weeks after pollination). Of these, 177 corresponded to peach cultivars (110 round and 67 flat; see Supplementary Table S2). Seventy-one were F1 seedlings from the cross between the two flat peaches ‘UFO-3’ × ‘Sweet cap’ (with round (14), flat (40) and aborting (17) phenotypes) and three were round, flat and aborting seedlings (P07F202A065, P07F202A071 and P07F202A056, respectively) from ‘ASF08.81’ open pollination. In addition, we included a flat peach variety (‘UFO-4’) and its round somatic mutant (‘UFO-4Mut’). Buds of the branch containing the mutation were grafted and maintained at the greenhouse facilities of IRTA in Torre Marimon (Barcelona).
DNA and RNA extraction
DNA from all materials used was extracted from young leaves using the Doyle and Doyle method43. For ‘UFO-4’ and ‘UFO-4Mut’ DNA was extracted from leaves, flesh fruit, skin and stone using the DNAsy Qiagen kit (Qiagen, Hilden, Germany). Branches with flowers in Baggiolini stage E (not expanded petals) from round (P07F202A065), flat (P07F202A071) and aborting (P07F202A056) peaches (all progenies from ‘ASF08.81’ open pollination) were cut in the field, and pistils collected and frozen in liquid nitrogen and conserved at −80 °C prior to total RNA extraction using the RNeasy® Plant Mini Kit (Qiagene) following the manufacturer’s protocol. RNA integrity was confirmed by 1% agarose gel electrophoresis.
DNA genotyping to identify new polymorphisms associated with the trait
All samples were genotyped with the SSR marker UDP98-412 (Pp06: 26,617,638.26,618,013) using the PCR and electrophoresis conditions described in Picañol et al.18.
Using the peach genome sequence v.244 we designed 23 primer pairs to amplify fragments of 200–700 bp in a 388.6 kb region (Pp06: 26,254,140.26,642,759) (Supplementary Table S1). Fourteen of them (primers UDP98-412(−17 K) to UDP98-412(+25 K)) were designed covering a 42.8 Kb region (Pp06: 26,599,970.26,642,759) flanking UDP98-412 and the nine remaining (primers Amplicon 1 to Amplicon 9) in a region spanning 26.6 Kb (Pp06: 26,254,140.26,280,026) 363.5 Kb upstream. This region was the closest to UDP98-412, where SNPs of the 9 K peach chip45 were identified. Primers were designed using Primer3 software46 avoiding amplification of SSR regions.
Primers were first tested in six varieties, three with flat (‘Mesembrine’, ‘Paraguayo delfín’ and ‘Subirana’) and three with round fruits (‘Garcica’, ‘HoneyGlo’ and ‘Luciana’). PCR products with a single band were purified with Exosap-it (GE HealthcareLife Science) in a single pipetting step and used as template for sequencing using the BigDyeTMTerminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and forward primers. The sequencing reaction profile included an initial denaturation at 96 °C for 1 m, followed by 25 cycles of 96 °C for 10 s, 50 °C for 6 s, and 60 °C for 4 min; the sequences obtained with an ABI Prism 3130 × l DNA Analyzer (Applied Biosystems, Foster City, California, CA, USA) were visualized and manually edited with Sequencher 5.0 software (Gene Codes Corporation; Ann Arbor, MI, USA). Fragment ends were trimmed to remove low-quality sequence. Haplotypes were graphically represented with Flapjack software47.
Cloning of PCR fragments
For one flat variety (‘UFO-8’) PCR products were cloned into the pGEM T-easy vector (Promega) following the manufacture instructions. Escherichia coli DH5alpha electro competent cells (Invitrogen) were transformed with the ligated plasmid by electroporation in the Gene PulserXcel electroporation system (BIORAD), with a capacitance 25 μF, resistance of 200 ohm and a voltage of 1,8 kv. Transformed cells were shaken horizontally at 250 rpm and 37 °C for 1.5 h in 1 ml liquid Luria-Bertani (LB) medium. Fifty microliters of transformed cell solution was then pipetted onto 10 cm LB agar plates containing 50 µg/ml ampicillin, 80 µg/ml X-gal and 0.5 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG). Positive colonies were picked from the LB plates as template DNA for colony PCR. Colonies were screened by PCR following the conditions described above. Those carrying the desirable allele were grown in 5 mL of LB liquid broth containing 50 µg/ml of carbenicillin with overnight incubation at 37 °C in a shaking oven at 250 rpm. Bacterial culture pellets were obtained by centrifugation at 3000 rpm for 10 min. Plasmids were extracted from bacterial cells using a QIAprep miniprep spin-kit (Qiagen) according to the manufacturer’s protocol, then resuspended in 50 μl of sterile water and 4 µl of each extract were sequenced with the vector specific primers, either T7 or SPS6, following the sequencing protocol previously described.
Sequencing PRUPE.6G281100
Round shape-associated allele
Using the peach genome sequence as reference, we designed seven primer pairs (Supplementary Table S4) flanking and within PRUPE.6G281100 (Pp06:26,269,777.26,272,029) to obtain the full sequence of the gene. Primers were designed to amplify single fragments, avoiding amplification of duplicated regions. Amplification and sequencing reactions were as described above.
Flat shape-associated allele
The forward primer Prupe.6G281100(−10K)_F was designed 10,072 bp upstream PRUPE.6G281100 and was combined with the reverse primer Prupe.6G281100_3PrimF (primer combination 2 (PC2) in Supplementary Table S4), 558 bp downstream of the gene, to amplify fragments with an expected size of 12.9 Kb. For long-range PCR, LongAmp® Taq Polymerase (New England BioLabs ®INC) was used. Each reaction contained 1x LongAmp reaction buffer, 0.3 mM dNTP mix, 0.8 µM each primer, 5% DMSO, 5 units of polymerase, 40 ng of template DNA, and sterile Milli-Q water to a final volume of 25 µl. The following PCR protocol was performed on a S-1000TM Thermal Cycler (Bio-Rad Laboratories, Inc.; Hercules, California, USA): 95 °C for 5 min; 35 cycles of 95 °C (30 sec), 60 °C (30 sec) and 65 °C (17 min); followed by a final step at 65 °C for 10 min. All PCR amplicons were checked on 1% agarose gel in TAE buffer. Ethidium bromide staining was used for band visualization.
The PCR bands were purified with the High Pure PCR product purification kit (Roche Diagnostic, Basel, Switzerland). Thirty nanograms of purified product were used as template to obtain the whole sequence of the amplicons in four sequencing reactions using the primers Prupe.6G281100(−10K)_F, Prupe.6G281100_4 R, Prupe.6G281100_5 R and Prupe.6G281100_3PrimF (Supplementary Table S4).
Variant validation with NGS
To validate the large variant alignment we re-sequenced, with Illumina technology (27×), five flat (‘Flatmoon’, ‘Cakereine’, Blanvio-10’, ‘Subirana’, ‘UFO-4’) and five round (‘Nectalady’, ‘Armking’, ‘Belbinette’, ‘Nectaross’, ‘Tifany’) varieties. High quality DNA of each sample was delivered to the CNAG (Centre Nacional d’Anàlisi Genòmica, Barcelona) for library preparation and 2 × 100 pb paired-end sequencing using illumina HiSeq. 2000 sequencer. Adapter removal and quality-based trimming of the raw resequencing data was with Trimmomatic version 0.3648. FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) was used for read quality control before and after trimming. High quality reads were mapped to the peach genome version 2.0 using BWA49 and the resulting alignment files were sorted and filtered by discarding multi-mapped reads and annotating PCR duplicates. Reads mapping to the Pp06:26,257,000.26,273,000 region were extracted from the alignment files and bulk aligned against the reference peach genome v2 using CLC-genomics workbench 8.5.1 (https://www.qiagenbioinformatics.com/). CLC-InDels and Structural Variants analysis tools were run separately in the flat and round peach alignments. Genes annotated in v.2 were downloaded from the GDR database50 and included in the alignment track for visualization. Reads are available at the European Nucleotide Archive under the accession number ENA: PRJEB21538.
Design of markers for genotyping
To validate the polymorphisms in germplasm and progenies, we designed primers to amplify a small INDEL within the candidate gene as well as the large deletion upstream from the candidate gene. Thus primer pairs FlatIn_F and IndelS_R (both inside the gene; PC4 in Supplementary Table S4) yielded product sizes of 464 bp and 469 bp for the flat and round alleles, respectively. PCR conditions, fragment separation and analysis in the ABI Prism 3130 × l DNA Analyzer were as previously described for the SSR marker.
A three-primer combination (PC3 in Supplementary Table S4), consisting of two primers flanking the deletion (one forward and one reverse) and an inner reverse primer (IndelS_F + IndelS_2 R + IndelS_R), was designed to genotype the large deletion identified in this region; IndelS_F and IndelS_R (flanking the deletion) amplified a 1,620 bp fragment associated to the flat phenotype, while IndelS_2 R (within the deletion) in combination with IndelS_F produced a 941 bp band associated to the round phenotype. PCR was carried out in a 10 µl reaction containing 20 ng of DNA, 1x PCR buffer, 1.5 mM MgCl2, 200 µM of each dNTP, 0.2 µM of each primer and 1 U of BIOTAQ (Biolab). The following PCR protocol was used in a S-1000TM Thermal Cycler (Bio-Rad Laboratories, Inc.; Hercules, California, USA): 95 °C for 2 min; 35 cycles of 94 °C (30 sec), 55 °C (30 sec), 72 °C (60 sec); followed by a final step at 65 °C for 10 min. All PCR amplicons were checked on 1% agarose gel in TAE buffer. Ethidium bromide staining was used for band visualization.
RT-PCR analysis
RNA was reverse-transcribed to cDNA using the reverse primer IndelS_R (see Supplementary Table S4). For this, 1 µl of RNA was hybridized with 2 µl of primer in a total volume of 13 µl. After 10 min incubation at 70 °C and 5 min cooling on ice, cDNA was obtained using PrimeScript RT-PCR kit (Takara). PCR was conducted with PC4 following the protocol described above. The forward primer was florescent labeled to check the size of the fragment in the ABI Prism 3130 × l DNA Analyser.
Gene homology and functional prediction
Functional annotation and orthologues for the PRUPE.6G281100 gene were determined using Dicots PLAZA 3.0. (http://bioinformatics.psb.ugent.be/plaza/versions/plaza_v3_dicots/51). Custom DNA BLAST (blastn program) against PLAZA Transcript Sequences database were used for similarity searches, filtering for low complexity and using the BLOSUM62 score matrix.
For the protein sequence of PRUPE.6G281100, associated with the round allele, the DNA sequence was entered in the Translate tool of the ExPASy Bioinformatics Resource Portal (http://web.expasy.org/translate/). Similarity searches were performed on the NCBI web page (www.ncbi.nlm.nih.gov) against the nr (non-redundant) collection of sequences in GenBank and the UniProtKB/SwissProt databases, using the blastp and the Position-Specific iterated BLAST algorithm52. The quality of the pairwise sequence alignment was evaluated in a BLOSUM62 protein substitution matrix allowing a gap existence value of 11 and an extension value of 1.
Electronic supplementary material
Acknowledgements
We thank Cristian Fontich (IRTA) and Jesús García Brunton (IMIDA) for maintaining and providing peach material. This work received financial support from the Spanish Ministry of Economy and Competitiveness, through the “Severo Ochoa Programme for Centres of Excellence in R&D” 2016–2019 (SEV-2015-0533)” and through the project AGL2015-68329-R, and from the CERCA Programme/Generalitat de Catalunya.
Author Contributions
The experiments were conceived and designed by E.L.-G., Y.Z., M.J.A. Experiments were conducted by E.L.-G., Y.Z., M.J.A. Data was analyzed by E.L.-G., Y.Z., M.J.A. and bioinformatics analysis was performed by E.L.-G., J.R.H.-M., K.G.A., M.J.A. Contributed reagents and materials I.E., P.A., M.J.A. Paper was written by E.L.-G., P.A. and M.J.A. All authors have critically revised the manuscript and approved the final document.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07022-0
References
- 1.Iglesias I. El melocotón plano en España: 15 años de innovación tecnológica y comercial. Revista de Fruticultura. 2014;35:6–31. [Google Scholar]
- 2.Mandel T, et al. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development. 2014;141:830–841. doi: 10.1242/dev.104687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torii KU, et al. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–746. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shpak ED, Lakeman MB, Torii KU. Dominant-Negative Receptor Uncovers Redundancy in the Arabidopsis ERECTA Leucine-Rich Repeat Receptor-Like Kinase Signaling Pathway That Regulates Organ Shape. The Plant Cell. 2003;15:1095–1110. doi: 10.1105/tpc.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao H, Jiang N, Schaffner E, Stockinger EJ, van der Knaap E. A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science. 2008;319:1527–1530. doi: 10.1126/science.1153040. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Van Eck J, Cong B, Tanksley SD. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc Natl Acad Sci USA. 2002;99:13302–13306. doi: 10.1073/pnas.162485999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez GR, et al. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 2011;156:275–285. doi: 10.1104/pp.110.167577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muños, S. et al. Increase in tomato locule number is controlled by two single-nucleotide polymorphisms located near WUSCHEL. Plant Physiol 156, 2244–2254, doi:10./pp.111.173997 (2011). [DOI] [PMC free article] [PubMed]
- 9.Rodriguez GR, Kim HJ, van der Knaap E. Mapping of two suppressors of OVATE (sov) loci in tomato. Heredity. 2013;111:256–264. doi: 10.1038/hdy.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chusreeaeom K, et al. A novel tomato mutant, Solanum lycopersicum elongated fruit1 (Slelf1), exhibits an elongated fruit shape caused by increased cell layers in the proximal region of the ovary. Mol Genet Genomics. 2014;289:399–409. doi: 10.1007/s00438-014-0822-8. [DOI] [PubMed] [Google Scholar]
- 11.Paran I, van der Knaap E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J Exp Bot. 2007;58:3841–3852. doi: 10.1093/jxb/erm257. [DOI] [PubMed] [Google Scholar]
- 12.Pan, Y. et al. Round fruit shape in WI7239 cucumber is controlled by two interacting quantitative trait loci with one putatively encoding a tomato SUN homolog. Theor Appl Genet, 1–14, doi:10.1007/s00122-016-2836-6 (2016). [DOI] [PubMed]
- 13.Cullinan, F. P. In United States Department of Agriculture Yearbook of Agriculture (ed United States Government Printing Office) 665–748 (1937).
- 14.Bassi, D. & Monet, R. In The peach: Botany, Productin and Uses (ed Bassi D. Layne DR) 1-36 (CABI, 2008).
- 15.Dirlewanger E, et al. Genetic linkage map of peach [Prunus persica (L.) Batsch] using morphological and molecular markers. Theor Appl Genet. 1998;97:888–895. doi: 10.1007/s001220050969. [DOI] [Google Scholar]
- 16.Dirlewanger E, et al. Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genet. Genomes. 2006;3:1–13. doi: 10.1007/s11295-006-0053-1. [DOI] [Google Scholar]
- 17.Lambert P, et al. Identifying SNP markers tightly associated with six major genes in peach [Prunus persica (L.) Batsch] using a high-density SNP array with an objective of marker-assisted selection (MAS) Tree Genet. Genomes. 2016;12:121. doi: 10.1007/s11295-016-1080-1. [DOI] [Google Scholar]
- 18.Picañol R, et al. Combining linkage and association mapping to search for markers linked to the flat fruit character in peach. Euphytica. 2013;190:279–288. doi: 10.1007/s10681-012-0844-4. [DOI] [Google Scholar]
- 19.Cao K, et al. Genome-wide association study of 12 agronomic traits in peach. Nat Commun. 2016;7:13246. doi: 10.1038/ncomms13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn R, et al. Candidate gene database and transcript map for peach, a model species for fruit trees. Theor Appl Genet. 2005;110:1419–1428. doi: 10.1007/s00122-005-1968-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, et al. Functional Analyses of the CLAVATA2-Like Proteins and Their Domains That Contribute to CLAVATA2 Specificity. Plant Physiol. 2010;152:320–331. doi: 10.1104/pp.109.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aranzana MJ, Carbó J, Arús P. Microsatellite variability in peach [Prunus persica (L.) Batsch]: cultivar identification, marker mutation, pedigree inferences and population structure. Theor Appl Genet. 2003;106:1341–1352. doi: 10.1007/s00122-002-1128-5. [DOI] [PubMed] [Google Scholar]
- 23.Aranzana MJ, Abbassi E-K, Howad W, Arús P. Genetic variation, population structure and linkage disequilibrium in peach commercial varieties. BMC Genet. 2010;11:69. doi: 10.1186/1471-2156-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X-w, et al. Peach genetic resources: diversity, population structure and linkage disequilibrium. BMC Genet. 2013;14:84. doi: 10.1186/1471-2156-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aranzana M, Illa E, Howad W, Arus P. A first insight into peach [Prunus persica (L.) Batsch] SNP variability. Tree Genet. Genomes. 2012;8:1359–1369. doi: 10.1007/s11295-012-0523-6. [DOI] [Google Scholar]
- 26.Cao K, et al. Comparative population genomics reveals the domestication history of the peach, Prunus persica, and human influences on perennial fruit crops. Genome Biol. 2014;15:415. doi: 10.1186/s13059-014-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micheletti D, et al. Whole-Genome Analysis of Diversity and SNP-Major Gene Association in Peach Germplasm. PLoS ONE. 2015;10:e0136803. doi: 10.1371/journal.pone.0136803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cock JM, Vanoosthuyse V, Gaude T. Receptor kinase signalling in plants and animals: distinct molecular systems with mechanistic similarities. Curr Opin Cell Biol. 2002;14:230–236. doi: 10.1016/S0955-0674(02)00305-8. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima N, Miyashita H. Leucine-Rich Repeat (LRR) Domains Containing Intervening Motifs in Plants. Biomolecules. 2012;2:288–311. doi: 10.3390/biom2020288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Smet I, Vosz U, Jurgens G, Beeckman T. Receptor-like kinases shape the plant. Nat Cell Biol. 2009;11:1166–1173. doi: 10.1038/ncb1009-1166. [DOI] [PubMed] [Google Scholar]
- 31.He K, et al. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Bai MY, Chong K. Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 2014;33:683–696. doi: 10.1007/s00299-014-1578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzaki T, et al. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development. 2004;131:5649–5657. doi: 10.1242/dev.01441. [DOI] [PubMed] [Google Scholar]
- 34.Bommert P, et al. thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development. 2005;132:1235–1245. doi: 10.1242/dev.01671. [DOI] [PubMed] [Google Scholar]
- 35.Birchler JA, Veitia RA. The gene balance hypothesis: implications for gene regulation, quantitative traits and evolution. New Phytol. 2010;186:54–62. doi: 10.1111/j.1469-8137.2009.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padilla IMG, Golis A, Gentile A, Damiano C, Scorza R. Evaluation of transformation in peach Prunus persica explants using green fluorescent protein (GFP) and beta-glucuronidase (GUS) reporter genes. Plant Cell Tissue Organ Cult. 2006;84:309–314. doi: 10.1007/s11240-005-9039-1. [DOI] [Google Scholar]
- 37.Pérez-Clemente RM, Pérez-Sanjuán A, Garcà-Férriz L, Beltrán J-P, Cañas LA. Transgenic peach plants (Prunus persica L.) produced by genetic transformation of embryo sections using the green fluorescent protein (GFP) as an in vivo marker. Mol Breed. 2004;14:419–427. doi: 10.1007/s11032-004-0506-x. [DOI] [Google Scholar]
- 38.Falchi R, et al. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013;76:175–187. doi: 10.1111/tpj.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandi F, et al. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011;11:24. doi: 10.1186/1471-2229-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert M, et al. Arabidopsis thaliana pattern recognition receptors for bacterial elongation factor Tu and flagellin can be combined to form functional chimeric receptors. J Biol Chem. 2010;285:19035–19042. doi: 10.1074/jbc.M110.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers-Melnick E, et al. Contrasting patterns of evolution following whole genome versus tandem duplication events in Populus. Genome Res. 2012;22:95–105. doi: 10.1101/gr.125146.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelsy F, Dumas V, Bévilacqua L, Hocquigny S, Merdinoglu D. Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine. PLOS Genetics. 2015;11:e1005081. doi: 10.1371/journal.pgen.1005081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle J, Doyle J. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 44.Verde I, et al. The Peach v2.0 release: high-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genomics. 2017;18:225. doi: 10.1186/s12864-017-3606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verde I, et al. Development and Evaluation of a 9K SNP Array for Peach by Internationally Coordinated SNP Detection and Validation in Breeding Germplasm. PLoS ONE. 2012;7:e35668. doi: 10.1371/journal.pone.0035668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 47.Milne I, et al. Flapjack-graphical genotype visualization. Bioinformatics. 2010;26:3133–3134. doi: 10.1093/bioinformatics/btq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung S, et al. The Genome Database for Rosaceae (GDR): year 10 update. Nucleic Acids Res. 2014;42:D1237–1244. doi: 10.1093/nar/gkt1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proost S, et al. PLAZA 3.0: an access point for plant comparative genomics. Nucleic Acids Research. 2015;43:D974–D981. doi: 10.1093/nar/gku986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


