Abstract
Immune responses show a high degree of tissue specificity shaped by factors influencing tissue egress and retention of immune cells. The transcription factor Hobit was recently shown to regulate tissue-residency in mice. Whether Hobit acts in a similar capacity in humans remains unknown. Our aim was to assess the expression and contribution of Hobit to tissue-residency of Natural Killer (NK) cells in the human liver. The human liver was enriched for CD56bright NK cells showing increased expression levels of the transcription factor Hobit. Hobitpos CD56bright NK cells in the liver exhibited high levels of CD49a, CXCR6 and CD69. Hobitpos CD56bright NK cells in the liver furthermore expressed a unique set of transcription factors with higher frequencies and levels of T-bet and Blimp-1 when compared to Hobitneg CD56bright NK cells. Taken together, we show that the transcription factor Hobit identifies a subset of NK cells in human livers that express a distinct set of adhesion molecules and chemokine receptors consistent with tissue residency. These data suggest that Hobit is involved in regulating tissue-residency of human intrahepatic CD56bright NK cells in a subset of NK cells in inflamed livers.
Introduction
The quality of immune responses is influenced by a plethora of factors. There is mounting evidence that tissues are shaping immune responses to serve their specific needs through interactions between immune and tissue cells. Tissue homing, retention and egress of immune cells are important in ensuring that the correct immune microenvironment for each tissue is established and maintained. The underlying mechanisms influencing tissue specificity and residency are slowly being unraveled and will improve our understanding of this essential part of immunology.
Natural Killer (NK) cells are part of the innate immune system and play a pivotal role in the early control of infections1 and malignancies2. NK cells can be divided into two main subsets of CD56dim and CD56bright NK cells, based on their expression of CD56 and CD163. In general, CD56bright NK cells act by producing cytokines, while CD56dim NK cells exert their effector functions through secretion of perforin and granzyme2. In the peripheral blood, CD56dim NK cells make up roughly 90% of the NK cells pool, with CD56bright NK cells contributing the remaining 10%. In contrast, CD56bright NK cells represent the dominant population in lymphoid and non-lymphoid tissues4, and are also found in increased frequencies in inflamed and cancer tissues5. Tissue-resident NK cells have now been identified in uterus, liver and lymphoid tissues4, 6, and appear to play important roles not only in the defense against foreign pathogens and cancers4, but also in tissue remodeling and regeneration4, 7. While in mice several markers have been identified to define tissue-resident NK cells and it was shown that these tissue-resident NK cells are not circulating through the periphery8, the factors regulating tissue residency in humans are less well defined9.
Recent studies in humans have shown that some of the markers used to identify tissue-resident NK cells in murine livers, namely CD49a9 and CXCR610, 11, are also expressed on human NK cells within the liver. Additionally, it was shown that CXCR6+ NK cells in human livers exhibited an Eomeshi T-betlo phenotype10, 11, which is in contrast to the Eomeslo T-betint phenotype initially described for CD49a+ NK cells in the liver9. This might suggest that several heterogeneous subsets of liver-resident NK cells exist in human livers. Further evidence for this was provided by a recent study describing CD49e as a marker almost exclusively expressed on NK cells derived from the human blood, whereas more than 50% of NK cells in the liver lack expression of CD49e12. A subset of Eomeshi NK cells in human livers was shown to persist for up to 13 years in a transplantation setting13. However, if and how the turn-over of these liver-resident NK cell subsets is shaped by different transcriptional programs remains unknown. In mice, it was recently described that the transcription factor Hobit (homolog of Blimp1 in T cells or ZNF683), a zinc finger protein, acts in concert with Blimp-1 to serve as a master regulator of tissue-residency for lymphocytes14. Hobit was initially found to regulate NKT cell effector differentiation15 and subsequently also used to identify effector-type lymphocytes in humans16. Hobit acts together with Blimp-1 in regulating expression of genes involved in tissue retention and egress14, thereby shaping the lymphocyte compartment of the tissue, and Hobit knockout mice exhibited less tissue-resident NK cells in their liver14. Whether Hobit is also playing a role in regulating tissue-residency of human NK cells remains unknown. Here we investigated Hobit expression by human NK cells and its role in regulating tissue-residency of intrahepatic NK cells.
Results
CD56bright NK cells are enriched in human liver tissue
Matched liver and blood samples were obtained from individuals undergoing liver transplantation at the Department of Hepatobiliary and Transplant Surgery of the University Medical Center Hamburg. Basic clinical data of the study cohort is summarized in the supplement (Suppl. Table 1). All individuals had advanced liver disease requiring liver transplantation, including alcoholic liver disease, hepatitis C infection, hepatocellular carcinoma and cholangiocarcinoma. Flow cytometric analysis of the samples revealed that the overall frequency of bulk NK cells within the lymphocyte population was slightly higher in human liver samples (Fig. 1a) compared to matched peripheral blood. While this increase was not significant, the distribution of NK cell subsets was notably shifted between liver and blood samples (Fig. 1b), with a marked increase of both CD56bright CD16- and CD56bright CD16+ NK cells and a corresponding decrease of CD56dim CD16+ NK cells (p = 0.016) in liver tissues.
Figure 1.
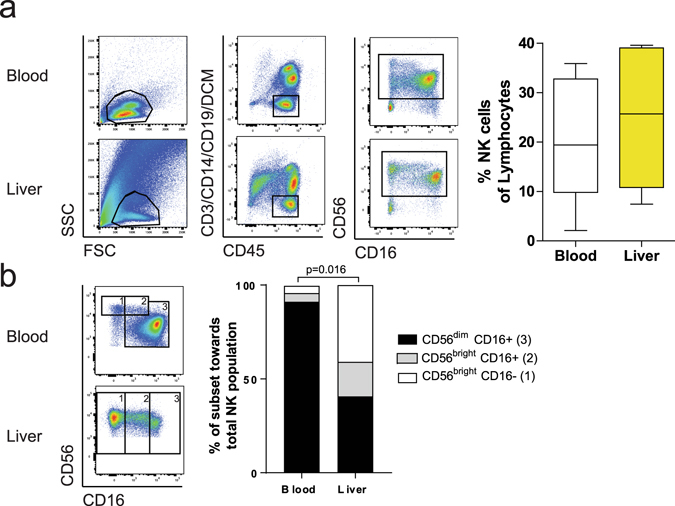
CD56bright NK cells are enriched in human liver tissue. (a) Gating strategy (left panel) for identification of NK cells in peripheral blood (upper row) and liver tissue (lower row). Summarization of the data (right panel) comparing frequency of NK cells in blood and liver from matched patient samples (n = 7). Box and whiskers (Tukey) plots was used. (b) Identification of CD56dim (3), CD56bright CD16+ (2) and CD56bright CD16- (1) NK cells (left panel) in peripheral blood (upper plot) and liver tissue (lower plot). Summarization of the data (right panel) comparing CD56dim,CD56bright CD16+ and CD56bright CD16- NK cells in the blood and liver of matched patient samples (n = 7). Bars show the median for all individuals, Wilcoxon matched pair sign rank test was used to determine statistical differences.
Increased frequency and expression of Hobit on CD56bright liver NK cells
Next, we investigated the expression of the transcription factor Hobit in peripheral blood and liver-derived NK cells. Hobit was expressed on bulk liver-derived NK cells, albeit to a lower level than in matched blood samples (Fig. 2a). While almost all bulk and CD56dim NK cells, independent of the compartment they were derived from, expressed Hobit (Fig. 2b), CD56bright CD16neg liver NK cells contained a significantly higher frequency of Hobitpos cells when compared to CD56bright CD16neg NK cells derived from the blood (Fig. 2b, p = 0.031). Furthermore, these CD56bright NK cells in the liver also expressed significantly higher levels of Hobit compared to their blood counterparts (Fig. 2c, p = 0.0006). CD56bright CD16pos NK cells in the liver showed an intermediate phenotype, both for the %-Hobit+ (Fig. 2b) and expression levels of Hobit (Fig. 2c), when compared to CD56dim and CD56bright CD16- NK cells. Taken together, these data demonstrate that CD56bright NK cells represent a large fraction of the NK cells in the liver, and that these CD56bright NK cells contain a higher frequency of Hobitpos cells expressing higher levels of Hobit compared to CD56bright NK cells in the peripheral blood.
Figure 2.
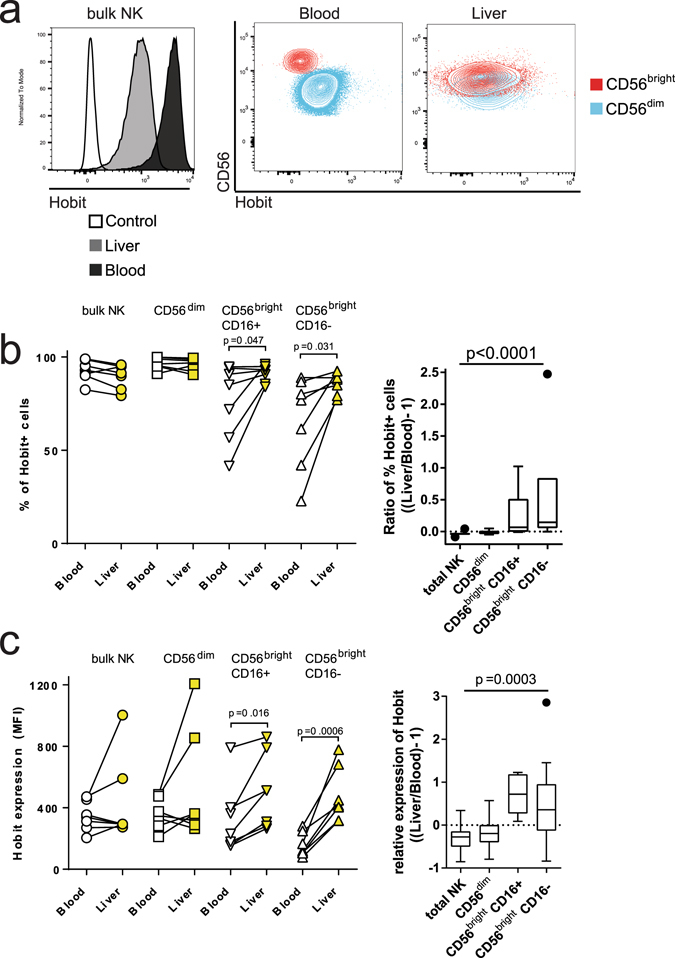
Increased frequencies and expression of Hobit on CD56bright liver NK cells. (a) Histogram (left panel) showing Hobit expression on bulk NK cells in the blood (dark grey), the liver (light grey) and a FMO control (white). Contour plots showing the expression of Hobit on CD56dim (blue) and CD56bright (red) NK cells in the blood and liver. (b) Frequency (left panel) of Hobitpos bulk, CD56dim,CD56bright CD16+ and CD56bright CD16- NK cells in the blood (open symbols) and liver (yellow symbols). Ratio of the frequency (right panel) of Hobitpos cells in each subset between liver and peripheral blood. (c) Expression (left panel) of Hobit on bulk, CD56dim, CD56bright CD16+ and CD56bright CD16- NK cells in the blood (open symbols) and liver (yellow symbols). Ratio of the expression (right panel) of Hobit in each subset between liver and peripheral blood samples. Individual data points are blotted in the graphs on the left side. Box and whiskers (Tukey) was used to depict the expression ratio between liver and blood. Wilcoxon matched pair sign rank test was used to determine statistical differences in the scatter plots and Friedman test was used for box plots.
Hobitpos CD56bright liver NK cells express markers related to tissue residency
To determine whether Hobit expression was associated with the expression of other markers previously linked to tissue-residency of NK cells, we subsequently investigated the expression of the surface markers CD69, CXCR6, and CD49a on Hobitpos NK cells9–11. We observed that CD69, CXCR6 and CD49a were all expressed on liver-derived NK cells, with higher levels on CD56bright NK cells compared to CD56dim NK cells (Fig. 3a). Furthermore, liver Hobitpos CD56bright NK cells contained a significantly higher frequency of CXCR6+ (p = 0.031) and CD49a+ (p = 0.016) cells (Fig. 3b and d), and exhibited a higher expression of CD69, CXCR6 and CD49a (p = 0.016 for all comparisons) compared to Hobitneg CD56bright NK cells (Fig. 3c and e). Overall, Hobitpos CD56bright NK cells in the liver showed a significantly higher expression of adhesion molecules and chemokine receptors related to tissue-residency compared to Hobitneg CD56bright NK cells.
Figure 3.
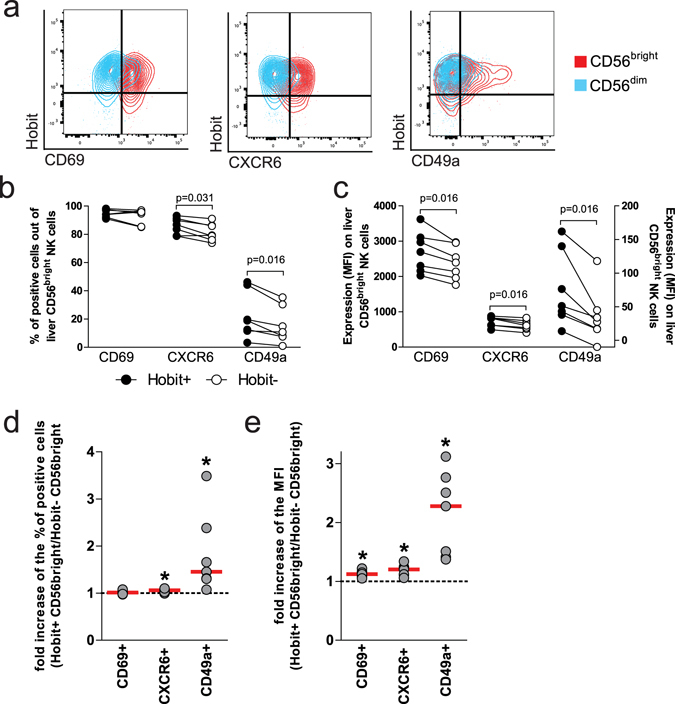
Hobitpos CD56bright liver NK cells express high levels of markers related to tissue residency. (a) Contour plots showing expression CD69 (left plot), CXCR6 (middle plot) and CD49a (right plot) compared to Hobit on CD56bright (red) and CD56dim (blue) NK cells in the liver. Frequency (b) and expression levels (c) of CD69, CXCR6 and CD49a comparing Hobitpos (full symbols) to Hobitneg (open symbols) CD56bright NK cells from the liver. (d) Fold change (left panel) of CD69-, CXCR6- and CD49a-positive CD56bright liver NK cells comparing Hobitpos to Hobitneg cells. (e) Fold increase of the MFI (right panel) of CD69, CXCR6 and CD49a on CD56bright liver NK cells comparing Hobitpos with Hobitneg cells. Wilcoxon matched pair sign rank test was used to determine statistical differences in the scatter plots.
Altered expression of transcription factors in Hobitpos CD56bright liver NK cells
Following the observation that Hobitpos NK cells in the liver expressed markers of tissue-residency, we next assessed whether other transcription factors were also differentially expressed on CD56bright NK cells in human livers. We therefore analyzed the expression of Blimp-1, T-bet and Eomes comparing bulk, CD56dim and CD56bright NK cells (Fig. 4a) derived from either peripheral blood or matched liver tissue. Blimp-1 was decreased in liver-derived bulk and CD56dim NK cells compared to peripheral blood (Fig. 4b, left panel, p = 0.047, respectively). While the expression of Blimp-1 showed a tendency to be lower in liver-derived CD56bright NK cells compared to peripheral blood (Fig. 4b, left panel, p = 0.3), this decrease in Blimp-1 expression was less pronounced compared to the relative decrease for bulk and CD56dim NK cells, as reflected by the ratios of Blimp-1 expression between liver- and blood-derived NK cells (Fig. 4b, right panel, p = 0.031). Eomes expression was lower on liver-derived bulk, CD56dim and CD56bright NK cells, albeit only reaching significance for the CD56dim NK cells from the liver (Fig. 4c, p = 0.016). T-bet was expressed at similar levels among bulk and CD56dim NK cells comparing to blood and liver NK cells, but was significantly increased in liver-derived CD56bright NK cells (Fig. 4d, p = 0.016). We subsequently focused the analysis of the expression of these different transcription factors on the Hobitpos CD56bright NK cell population in the liver, and observed higher frequencies of Blimp-1pos and T-betpos cells compared to to Hobitneg CD56bright NK cells (Fig. 4e, p = 0.031, respectively). Expression levels of Blimp-1 and T-bet followed a similar pattern (Fig. 4f, p = 0.02, respectively), while Eomes expression did not differ significantly, neither for frequency of positive cells (p = 0.4) nor expression levels (p = 0.16). Taken together, liver- and peripheral blood-derived NK cells exhibited distinct expression patterns of transcriptions factors, and this was most apparent for liver-derived Hobitpos CD56bright NK cells.
Figure 4.
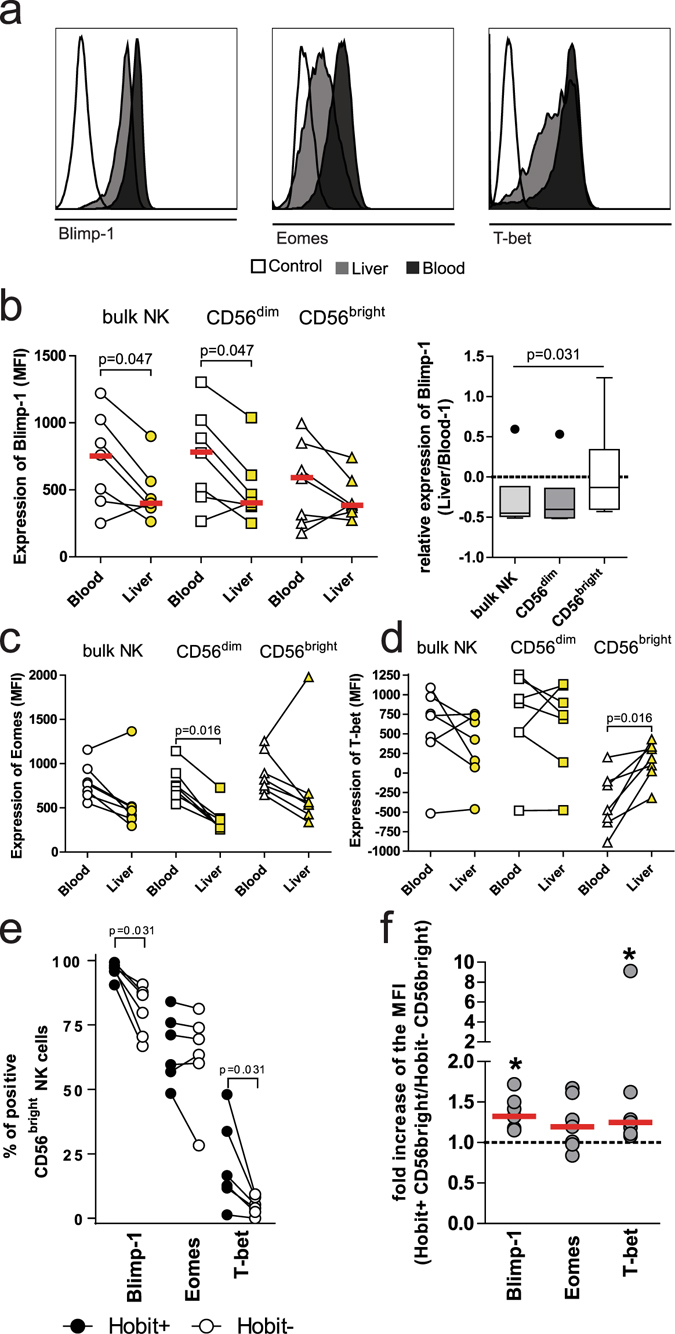
Altered expression of transcriptions factors in Hobitpos CD56bright liver NK cells. (a) Histogram showing expression of Blimp-1 (left panel), Eomes (middle panel) and T-bet (right panel) on bulk NK cells in the blood (dark grey) compared to the liver (light grey) and a FMO control (white). (b) Expression (left panel) of Blimp-1 on bulk, CD56dim and CD56bright NK cells in the peripheral blood (open symbols) and liver (yellow symbols). Ratio of the expression (right panel) of Blimp-1 in each subset between Liver and Blood. (c) Expression of Eomes on bulk, CD56dim and CD56bright NK cells in the blood (open symbols) and liver (yellow symbols). (d) Expression of T-bet on bulk, CD56dim and CD56bright NK cells in the blood (open symbols) and liver (yellow symbols). (e) Frequency of Blimp-1pos, Eomespos and T-betpos cells among CD56bright liver NK cells comparing Hobitpos (black symbol) with Hobitneg (open symbol). (f) Fold change of the expression (MFI) of Blimp-1, Eomes and T-bet on CD56bright liver NK cells comparing Hobitpos with Hobitneg cells. Wilcoxon matched pair sign rank test was used to determine statistical differences in the scatter plots and Friedman test was used for box plots.
Discussion
Dissecting the factors that influence tissue-residency and shape the local immune microenvironment is crucial to improve our understanding of tissue-specific immunity. Several recent studies, mainly in mouse models, have demonstrated that liver-resident NK cells differ from NK cells circulating in the peripheral blood in expression of surface markers and transcription factors that regulate their trafficking behavior. In particular, the transcription factor Hobit has been shown to play a pivotal role in instructing tissue-residency of murine lymphocytes in conjunction with Blimp-114. Hobit was furthermore shown to be expressed at high levels by peripheral blood NK cells16, 17 and iILC1s from human tonsils18. In this study we investigated Hobit expression by human NK cells in matched samples from peripheral blood and explant liver tissues. We demonstrate that CD56bright NK cells in the liver expressed higher levels of Hobit compared to their blood counterparts, and that Hobit-expression was accompanied by an increased expression of tissue residency markers and changes in the expression of transcription factors.
In mice, the transcription factors Hobit and Blimp-1 acted synergistically to regulate the expression of a specific set of genes linked to tissue retention and egress14 of lymphocytes, thereby shaping tissue-specific immunity. Hobit/Blimp-1 double-knockout mice showed a down regulation of Klf2, S1pr1, Tcf7 and CCR7, all genes previously liked to tissue egress or the formation of circulating immunity19–23. While Hobit was shown to be expressed by human NK cells circulating in the peripheral blood, it was almost absent in NK cells derived from human tonsils16, raising the question whether Hobit is also involved in regulating tissue-specific localization of human lymphocytes. A subsequent study showed that Hobit gene expression was high in iILC1 derived from human tonsils relative to other innate and adaptive lymphocyte subsets18. The differences observed in these two studies might be based on differences in the lymphocyte subsets studies, namely CD56+ NK cells16 versus CD56+ NKp44 + CD103+ iILC1s18. In line with the study by Vieira Braga et al.16, we observed Hobit expression by the majority of human NK cells circulating in the peripheral blood. Interestingly, Hobit was also expressed by NK cells derived from livers, in contrast to what had been described for NK cells derived from tonsils16. This demonstrates that expression of transcription factors by human NK cells differs between different tissues, as also suggested by recent studies of innate lymphoid cells in mice24.
While we did not observe differences in the frequency of Hobitpos bulk NK cells or Hobit-expression levels among bulk and CD56dim NK cells derived from peripheral blood or liver, CD56bright NK cells obtained from liver tissues exhibited a significantly increased frequency of Hobitpos cells and Hobit-expression levels compared to CD56bright NK cells in the peripheral blood. These data suggest that CD56bright NK cells, that have been shown to be enriched in tissues, might be required to reduce Hobit-expression in order to egress tissues and enter the peripheral circulation. However, the detection of Hobitpos NK cells in the peripheral blood in humans in our study and the previous study by Vieira-Braga et al.16, while Hobit-expression is almost absent in circulating NK cells in mice, suggests clear differences in the function of Hobit between mice and humans. In humans, high Hobit-expression appears to be compatible with the circulation of CD56dim NK cells in the peripheral blood, as also described for human CD45RA+ effector CD8 T cells, which express Hobit and circulate16, while Hobit-expression was reduce in CD56bright NK cells circulating in the peripheral blood. In line with these differences in Hobit-expression in circulating lymphocytes between mice and humans, a number of Hobit-regulated genes are differentially expressed between mice and humans, including TCF125, CD62L26 and granzyme B27. This indicates that different pathways may be required in mice and humans to facilitate tissue residency, and emphasizes the need to better understand the factors regulating tissue-entry, retention and egress of lymphocytes in humans. In this context it is important to highlight that the tissues used in our study were obtained from patients suffering from end-stage liver diseases of different etiologies, and might therefore not reflect the situation in healthy tissue. Despite the variability in the underlying diseases, we observed consistent differences in Hobit-expression between CD56bright NK cells in livers and the peripheral blood, suggesting that Hobit-expression was involved in the determination of liver-residency of this NK cell subset.
The expression of several surface molecules involved in cell adhesion or chemotaxis have been associated with liver-resident NK cells in humans9–12, 28. Expression of CD49a, the alpha part of the α1β1 integrin complex which facilitates binding to collagen and laminin, is a critical marker for liver-residency in mice8, and was also shown to be almost exclusively expressed on human liver-derived NK cells and absent on peripheral blood NK cells9. CXCR6 has been described to be required to retain NK cells in the liver tissue28, and to be expressed by a subset of T-betlo Eomeshi CD56bright NK cells residing in the liver10, 11. Part of the Eomeshi NK cells have been shown to persist up to 13 years in the liver in a transplantation setting13 and can be replenished by the Eomeslo NK cells from the periphery. Very recently, the lack of CD49e was revealed as another possible marker to discriminate between tissue-resident and circulating NK cells12. We observed that Hobitpos CD56bright NK cells in livers contained an increased frequency of CD49apos and CXCR6pos cells and that Hobitpos CD56bright NK cells in livers expressed higher levels of these markers associated with liver-residency on their surface compared to Hobitneg CD56bright NK cells and CD56bright NK cells in the periphery. In addition, Hobitpos CD56bright NK cells also expressed higher levels of CD69, which has been described to be a hallmark marker of human tissue-resident memory T cells29 and NK cells10. CD49a expression showed the most pronounced difference between Hobitpos and Hobitneg CD56bright NK cells in the liver, thereby being an important contributor to the tissue-resident phenotype. Differences in the expression of CD69 and CXCR6 were less dominant but still significant. These data suggest that Hobitpos CD56bright NK cells represent a tissue-resident population retained in livers by interactions with cell adhesion molecules and chemotaxis, in line with data obtained in Hobit/Blimp-1 knockout mice showing down-regulation of genes related to tissue egress14.
Expression of the transcription factor T-bet together with Eomes has been described for human liver-resident CD56bright NK cells in several recent studies9–11, 13. In line with these studies, we observed that Hobitpos CD56bright NK cells in the liver expressed T-bet, which is, together with IL-15, involved in regulating the expression of Hobit14. Furthermore, while Eomes expression was lower among liver NK cells in general, Eomes expression was higher in Hobitpos CD56bright NK cells compared to Hobitneg CD56bright NK cells within the liver. In addition to expression levels also the frequency of Blimp-1pos and T-betpos cells was increased among Hobitpos CD56bright NK cells. However, Hobitpos NK cells in the liver did not include the T-betlo Eomeshi subset of liver-resident NK cells recently described in two studies10, 11, 13, indicating that Hobit-positivity might define an independent subset of tissue-resident NK cells. Which might be predisposed to be recruited towards inflamed livers, due to the high expression of CD49a. One of the hallmarks of liver inflammation is fibrosis that is accompanied by an increase of collagen production, which acts as a ligand for CD49a30. CD49apos NK cells might therefore have a specific function in the context of liver inflammation, and it has been suggested that NK cells can prevent fibrosis by killing activated stellate cells31, one of the main drivers of liver fibrosis.
In conclusion, we show that the human liver harbors a subset of Hobitpos CD56bright NK cells expressing chemokine receptors and adhesion molecules associated with liver residency. Hobitpos CD56bright NK cells in livers expressed a distinct pattern of transcription factors differing from previously described intrahepatic NK cells. These data suggest that Hobit is involved in regulating tissue-residency of human intrahepatic CD56brigt NK cells and that Hobit-expression can define a distinct subset of liver-resident NK cells in inflamed livers.
Methods
Study Design
The objective of this study was to investigate whether Hobit is expressed by human intrahepatic NK cells and whether it influences tissue-residency of NK cells. Ex vivo phenotyping using flow cytometry was performed on matched liver and blood samples from individuals who underwent liver transplantation. Randomization and blinding were not used in this study.
Patient Cohort
Individuals undergoing liver transplantation at the Department of Hepatobiliary and Transplant Surgery in the University Medical Center Hamburg were included in this study. Reasons for transplantation varied and included alcoholic liver diseases (n = 2), hepatitis C virus infection (n = 2), hepatocellular carcinoma (n = 2) and Cholangiocarcinoma (n = 1). Informed consent was obtained from all patients. This study has been approved by the ethics committee of the Ärtzekammer Hamburg. All experiments were performed in accordance with the relevant guidelines and regulations. Matched blood samples were obtained, either during surgery or directly before. Samples of the explanted livers were collected during surgery, immediately transferred to the Heinrich Pette Institute and processed. Individuals undergoing repeated liver transplantations or individuals in whom no blood samples were obtained were excluded from the analysis.
Sample processing
PBMCs were isolated from blood samples using density centrifugation with Ficoll/Percoll. Liver tissue samples were cut into small pieces using scalpels and subsequently mechanically dissociated using a gentleMACS Octo Dissociator (Miltenyi). No enzymes were used to assist with digestion of the tissue, due to their effects on the surface expression of NK cell markers. Liver tissue samples were subsequently serially filtered using decreasing filter sizes from 100 µm to 70 µm and 40 µm.
Flow Cytometry
The following antibodies were used from Biolegend: anti-Blimp-1-PE, anti-CD16-BV785 (3G8), anti-T-bet-BV711 (4B10), anti-CD69-BV510 (FN50), anti-IgM-BV421 (RMM-1), anti-CD14-AlexaFluor700 (M5E2), anti-CD19-AlexaFluor700 (HIB19), anti-CD3-AlexaFluor700 (UCHT1), Zombie NIR Fixable Viability Kit, Zombie Aqua Fixable Viability Kit, anti-CXCR6-PerCP-Cy5.5 (K041E5), anti-CD56-BV605 (HCD56), anti-CD14-BV510 (M5E2), anti-CD19-BV510 (HIB19), LiveDead-Aqua, anti-CD45-APC-Cy7 (HI30), anti-CD3-BV510 (UCHT1). From BD Bioscience: anti-CD3-PE-CF594 (UCHT1), anti-CD45-BUV395 (HI30) and anti-CD56-BUV737 (NCAM16.2). Additional antibodies used were anti-CD49a-PE-Vio770 (Miltenyi), anti-Eomes-APC (R&D Systems) and anti-CD2-Qdot605 (S5.5, life technologies). The Hobit antibody was kindly provided by Klaas van Gisbergen14.
Stainings of cells (106 PBMCs or 2 × 106 liver-derived lymphocytes) were performed as follows: after washing in PBS with 2% (v/v) FBS and 0.5 M EDTA, cells were stained with an antibody mastermix prepared in the same buffer for 30 min at 4 °C in the dark. After two washing steps, cells were incubated in freshly prepared Fix/Perm solution (eBioscience) for 30 min at 4 °C in the dark. Following one washing steps, with freshly prepared Permwash (eBioscience), cells we stained with the Hobit antibody (1:10 dilution) for 30 min at 4 °C in the dark. Cells were subsequently washed two times and then stained with anti-IgM-BV421 for 30 min at 4 °C in the dark. After washing ones with both Permwash and PBS containing 2% (v/v) FBS and 0.5 M EDTA, cells were fixed in 4% (w/v) PFA. All samples were acquired on a BD LSRFortessa (BD Bioscience). The data was analyzed using FlowJo v10 Software (Treestar).
Statistical Analysis
Statistical analyses were performed using Excel (Microsoft Corp.) and Prism 5 (GraphPad Software Inc.). Due to the small sample size, data were not assumed to be normally distributed and non-parametrical tests were performed. As matched peripheral blood and liver samples were obtained from the same individual, matched analyses were performed. Comparisons between two groups were performed using the Wilcoxon matched pair sign rank test to determine statistical differences. For comparisons between three or more groups, the Friedman test was used. If the p-value is not depicted as number it is abbreviated with *, where * represents p values below 0.05, ** below 0.01 and *** below 0.001.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to thank all donors as well as the surgeons and nurses in the Department of Hepatobiliary Surgery and Transplantation. This work was supported by the DFG through the SFB841.
Author Contributions
S.L., G.M., T.K., H.G., W.S., A.L. performed the experiments. S.L., G.M. and M.A. analyzed the data. S.L., G.M., M.K., M.B., B.N., K.G. and M.A. drafted the paper. All authors reviewed the manuscript. S.L. and G.M. contributed equally to this work.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Sebastian Lunemann and Gloria Martrus contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06011-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16(5):310–320. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 5.Melsen JE, Lugthart G, Lankester AC, Schilham MW. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front Immunol. 2016;7:262. doi: 10.3389/fimmu.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugthart G, et al. Human Lymphoid Tissues Harbor a Distinct CD69+ CXCR6+ NK Cell Population. J Immunol. 2016;197(1):78–84. doi: 10.4049/jimmunol.1502603. [DOI] [PubMed] [Google Scholar]
- 7.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124(5):1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123(4):1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquardt N, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol. 2015;194(6):2467–2471. doi: 10.4049/jimmunol.1402756. [DOI] [PubMed] [Google Scholar]
- 10.Stegmann KA, et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep. 2016;6:26157. doi: 10.1038/srep26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmon, C. et al. Tissue-resident Eomeshi T-betlo CD56bright NK cells with reduced pro-inflammatory potential are enriched in the human adult liver. Eur J Immunol (2016). [DOI] [PubMed]
- 12.Aw Yeang HX, et al. Cutting Edge: Human CD49e- NK Cells Are Tissue Resident in the Liver. J Immunol. 2017;198(4):1417–1422. doi: 10.4049/jimmunol.1601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuff AO, et al. Eomeshi NK Cells in Human Liver Are Long-Lived and Do Not Recirculate but Can Be Replenished from the Circulation. J Immunol. 2016;197(11):4283–4291. doi: 10.4049/jimmunol.1601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352(6284):459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 15.van Gisbergen KP, et al. Mouse Hobit is a homolog of the transcriptional repressor Blimp-1 that regulates NKT cell effector differentiation. Nat Immunol. 2012;13(9):864–871. doi: 10.1038/ni.2393. [DOI] [PubMed] [Google Scholar]
- 16.Vieira Braga FA, et al. Blimp-1 homolog Hobit identifies effector-type lymphocytes in humans. Eur J Immunol. 2015;45(10):2945–2958. doi: 10.1002/eji.201545650. [DOI] [PubMed] [Google Scholar]
- 17.Jamil, K. M. et al. STAT4-associated natural killer cell tolerance following liver transplantation. Gut, (2016). [DOI] [PMC free article] [PubMed]
- 18.Koues OI, et al. Distinct Gene Regulatory Pathways for Human Innate versus Adaptive Lymphoid Cells. Cell. 2016;165(5):1134–1146. doi: 10.1016/j.cell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 20.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature Immunology. 2005;6(9):889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeannet G, et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107(21):9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350(6263):981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toor AA, Lund TC, Miller JS. T-cell factor-1 expression during human natural killer cell development and in circulating CD56(+) bright natural killer cells. Exp Hematol. 2001;29(4):499–506. doi: 10.1016/S0301-472X(00)00680-9. [DOI] [PubMed] [Google Scholar]
- 26.Frey M, et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161(1):400–408. [PubMed] [Google Scholar]
- 27.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26(6):798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Hudspeth K, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun. 2016;66:40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jokinen J, et al. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279(30):31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 31.Gur C, et al. NKp46-mediated killing of human and mouse hepatic stellate cells attenuates liver fibrosis. Gut. 2012;61(6):885–893. doi: 10.1136/gutjnl-2011-301400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.


