ABSTRACT
A comparison of the growth, hematological values, fatty acids, and gonadal and growth hormonal changes of river puffer, Takifugu obscurus, tiger puffer, T. rubripes, their hybrids (river puffer × tiger puffer) and hybrid triploids was performed during 3 months of their early growth period. Several features were observed during these 3 months: hybrids showed the highest levels of specific growth rate, 1.48%; hybrid triploids showed the smallest change in viscera fat (P<0.05), but GSI was not significantly different among groups (P>0.05). Considering hematological parameters, hybrid triploids had increased mean corpuscular volume and mean corpuscular hemoglobin (P<0.05), but other parameters were not significantly different between groups (P>0.05). With respect to fatty acids, puffer fish, hybrids and hybrid triploids contained fatty acids such as SFAs, MUFAs, n-3 PUFAs and n-6 PUFAs. There were significantly different amounts of total fatty acids between groups (P<0.05), however, rates of changes in fatty acids did not differ significantly between groups (P>0.05). Gonadal hormone (estradiol and testosterone) changes in the river puffer and tiger puffer were significantly higher than that observed in hybrids and hybrid triploids. The hybrids and tiger puffers had higher amounts of growth hormone (thyroid stimulating hormone and thyroxine) than the hybrid triploids and river puffers (P<0.05).
Keywords: Fatty acid, Hematological parameter, Hormone, Hybrid, Hybrid triploid, River puffer, Tiger puffer
INTRODUCTION
The aquaculture industry has progressed significantly in providing commercial benefits in discoveries of valuable species which have rapid growth and excellent flesh quality (Chevassus, 1979, 1983; Ueno et al., 1986). Hybridization has been long utilized (Chevassus, 1979) to produce traits in hybrids that are superior to those of the parental species, and now it has been successfully applied to many species of fish (Chevassus, 1979; Nam et al., 2004; Rahman et al., 2005). Induction of hybridization in aquaculture is the combining of genomes to obtain a different genome which will have desirable elements of the haploid sets of the parental species (Chevassus et al., 1983; Nam et al., 2004; Rahman et al., 2005). Heterosis combines the beneficial traits from both parental species such as improved resistance to disease and changed aquatic environment (Chevassus et al., 1983; Rahman et al., 2005).
Ploidy manipulation has been extended to exploit polyploidy production methods in aquaculture (Scheerer & Thorgaard, 1983; Thorgaard, 1986; Ueno et al., 1986). The induction of triploids has been used to generate sterility for applications in commercial farming and fishery management (Benfey, 1999; Felip et al., 2001; Feindel et al., 2011). Triploidy focuses on somatic growth instead of sexual maturation, thus impairing gametogenesis, retarding gonad development and restricting reproduction (Thorgaard, 1986; Nam et al., 2004; Feindel et al., 2011; Yoo et al., 2016). Additionally, sterility in triploids may be used to prevent the decline in flesh quality associated with sexual maturation (Wang et al., 2015). However, survival and growth rates in the early life stages are substantially lower in triploids compared with diploids (Thorgaard, 1986; Sutterlin et al., 1987), and triploids appear to be less resilient to stress and less tolerant to poor water quality (Benfey, 1999).
Thus, hybrid triploids produced by hybridization and ploidization have been introduced to aquaculture making it possible to integrate the two features of hybrid and triploids (Nam et al., 2004; Rahi & Shah, 2012; Huang et al., 2016; Yoo et al., 2016). Hybrid triploids are expected to have the combined traits of hybrids and triploids, and be more sterile than hybrid diploids and triploids because of the added retardation of gametogenesis (Rahi & Shah, 2012). Hybrid triploids could be an improved alternative to hybrids with low early viability (Chevassus et al., 1983; Scheerer & Thorgaard, 1983; Shah et al., 1999). Since hybrid triploids do not have viable gametes or gametogenic activity, they have more rapid growth and improved viability (Rahi & Shah, 2012), and often show fewer developmental abnormalities than their diploid counterparts (Sutterlin et al., 1987).
The early growth of fish is determined by feed, aquatic environment and properties of the fish species (Felip et al., 2001; Rahman et al., 2005; Zhang et al., 2008). The increased early growth is important to the commercial aquaculture industry. While there may not be large differences in the early growth of hybrids and triploids in the larval stage, large differences in growth rates were observed after reaching the adult stage (Rahi & Shah, 2012).
Hematological parameters can be indicators of health status and stress (Verdegem et al., 1997). Erythrocyte numbers can indicate the existence of anemia or stress polycythemia, whereas leucocyte counts may reveal leucopenia or leucocytosis, which may confirm possible alterations in immune function (Huffman et al., 1997). Erythrocyte numbers and size will also confirm the diploid and triploid status (Park & Kim, 2000; Goo et al., 2015). The fatty acid composition of fish lipids is influenced by external and internal factors, for example, fish species, trophic aspects and aquatic environment (Farkas et al., 1978).
Most domestic puffer fish are imported from other countries, such as China and Japan (Kang et al., 2007), and puffer fish are regarded as high value fish in Japan and Korea (Kang et al., 2007; Oh & Hwang, 2013). The growth of tiger puffer, Takifugu rubripes, is faster than that of river puffer, T. obscurus, but the price of river puffer is higher than that of tiger puffer (Kang et al., 2007; Kotaro & Takeshi, 2007; Oh & Hwang, 2013). Therefore induction of hybrids between river and tiger puffers will combine the advantages of river and tiger puffers, and their hybrids and hybrid triploids will be spotlighted by faster growth and higher price.
The present study compares the early growth, hematological parameters, fatty acid composition, and changed gonadal (estradiol and testosterone) and growth (thyroid stimulating hormone and thyroxine) hormones among two puffer fish species, and their hybrids and hybrid triploids.
Materials and Methods
1. Production of hybrid and hybrid triploids, and experimental design
Production of river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrids and hybrid triploids was carried out in the Chungnam Fisheries Research and Development Institute, Chungnam, Korea according to the method of Yoo et al. (2016). River puffer and tiger puffer were injected with1,000 IU/g BW human chorionic gonadotropin (HCG, Sigma, USA). Twenty-four hours after injection, the eggs of river puffer and sperm of tiger puffer were extracted and mixed in order to induce the interspecific hybrids (river puffer × tiger puffer). The egg and sperm mixture was added to sufficient seawater. Five minutes after fertilization, a total of 3,000 fertilized eggs were subjected to cold-shock treatment (4℃) for 25 min. to prevent extrusion of the second polar body (Yoo et al., 2016). Induced hybrid triploid juveniles were determined by using flowcytometry (Partec, DE / PA, Germany). Untreated fertilized eggs were used as hybrid groups.
On March 10, 2016, 100 each of experimental river puffer, tiger puffer, hybrid and hybrid triploid specimens from the Fishery Genetics and Breeding Sciences Laboratory of the Korea Maritime and Ocean University, Busan, Korea, were grown for 3 months in an aquarium maintained at a temperature of 24±1.5℃ and 30 ppt, and were given a commercial feed (Table 1, Cheonhajaeil Feed Corporation, Korea) ad libidum twice a day. Total length and body weight of specimens were measured to the nearest 0.1 cm and 0.1 g using digital vernier calipers (CD-20 CP; Mitutoyo, Japan) and an electric balance (AX 200, Shimadzu Corp., Japan), respectively. Average initial measurements and weights were as follows: river puffer- 11.4± 2.56 cm and 32.1±6.88 g; tiger puffer- 13.2±2.33 cm and 44.3±8.54 g; hybrids-12.3±2.16 cm and 34.2±9.17 g; and hybrid triploids-11.9±1.98 cm and 33.8±7.41 g, respectively.
Table 1. Composition of the experimental diets used in this experiment.
| Nutrition | Content |
|---|---|
| Crude protein | 40.0 |
| Crude fat | 4.0 |
| Crude fiber | 5.0 |
| Ash | 15.0 |
| Calcium | 1.0 |
| Phosphorus | 1.0 |
| Mineral premix*2 | 1.0 |
| Vitamin premix*3 | 1.0 |
*1 Ehwa Feed Coporation (Busan, Korea).
*2 Vitamin premix contained the following amount which were diluted in cellulose (g kg-1 mix): L-ascorbic acid, 121.2; DL-α-tocopheryl acetate, 18.8; thiamin hydrochloride, 2.7; riboflavin, 9.1; pyridoxine hydrochloride, 1.8; niacin, 36.4; Ca-D-pantothenate, 12.7; myo-inositol, 181.8; D-biotin, 0.27; folic acid, 0.68; p-aminobenzoic acid, 18.2; menadione, 1.8; retinyl acetate, 0.73; cholecalciferol, 0.003; cyanocobalamin, 0.003.
*3 Mineral premix contained the following ingredients (g kg-1 premix): NaCl, 43.3; MgSO4·7H2O, 136.5; NaH2PO4· 2H2O, 86.9; KH2PO4, 239.0; CaH4(PO4) ·2H2O, 135.3; ferric citrate, 29.6; ZnSO4·7H2O, 21.9; Ca-lactate, 304.0; CuCl2, 0.2; AlCl3·6H2O, 0.15; KI, 0.15; Na2Se2O3, 0.01; MnSO4·H2O, 2.0; CoCl2·6H2O, 1.0.
2. Early growth examination
All experimental samples were 4 months after hatched out, samples were measured from March to July, 2016, respectively. Each 100 specimens of river puffer, tiger puffer, hybrid and hybrid triploid were measured for body weight, viscera fat weight and gonadosomatic index (GSI) during 3 months of growth. Body weight, viscera fat weight and GSI were measured by electric balance, and GSI was calculated with the following equation: (gonad weight/ body weight) × 100.
3. Analysis of hematological parameters
Blood was drawn from the caudal blood vessel complex of anesthetized fishes (clove oil and methanol, Sigma, USA) using heparinized syringes (3 mL, Sung Shim Medical Co., Ltd, Bucheon, Korea). Hematological analysis was carried out with an auto hematology analyzer (PE-6800, Prokan, China). The red blood cells were analyzed immediately with an automatic blood analyzer (Excel 500, USA). Hematocrit values were determined using a microhaematocrit centrifuge at 15,500 g for 3 min. Hemoglobin concentrations were estimated by spectrophotometry at 540 nm using the cyanomethahaemoglobin method as described by Seol et al. (2008). Erythrocyte indices include a mean cell volume (MCV), mean cellular hemoglobin content (MCH), and mean cell hemoglobin concentration (MCHC) determined according to the following formulas (Seol et al. 2008):
MCV (fL) = HCT/EC (106 uL-1), MCH (pg) = [Hb (g L-1)]/ EC(106 uL-1) and MCHC (g dL-1) = [Hb (g L-1)]/HCT.
4. Investigation of fatty acids
Fatty acid methyl ester was prepared with 14% BF3/ methanol and analyzed with a gas chromatograph (CP-3380, Varian Inc., Palo Alto, CA, USA) using a flame-ionization detector, as described previously (Pawlosky et al. 1996). The gas chromatograph utilized a CP-SIL 5 CB fused silica capillary column (60 m length × 0.32 mm i. d., film thickness 0.01 μm, Varian Inc., Palo Alto, CA, USA). Peaks were identified by comparison with fatty acid standards (GLC-462, Nu-Check-Prep, Elysian, MN, USA), and the area and percentage for each resolved peak were analyzed using Galaxie chromatography software.
5. Measurement of gonadal and growth hormone
Blood was collected from the caudal fin of anesthetized fishes using heparinized syringes. Blood was extracted within 1 min to minimize the handling stress imposed on the fish and centrifuged for 5 min at 12,000 g (Centrifuge Micro 17R, Hanil Science Industrial Co., Ltd, Korea). Estradiol, testosterone, thyroid stimulating hormone and thyroxine were measured by a radioimmunoassay method using fluorophotometry (i-Chroma, Sun Kyung Medical, Korea).
One-and two-way analysis of variance (ANOVA) were performed on the data collected from different treatments, and Duncan’s multiple range test (Duncan, 1955) was performed when significant differences were found (P<0.05; SPSS statistics package SPSS 9.0; SPSS Inc., USA). All experiments were performed in triplicate. Unless otherwise stated, differences were considered to be statistically significant at P<0.05.
Results
The results of early growth performance in terms of initial and final individual weights, viscera fat weights and GSIs of river puffer, Takifugu obscurus, tiger puffer, T. rupbripes, hybrid and hybrid triploid specimens fed experimental diets are shown in Table 2. The specific growth rate (SGR) increased with increasing viscera fat weight. The final body weights (FBW) and the SGRs of the hybrid triploids (10.3%) were lower than those of diploids (Table 2). The SGR was significantly higher in hybrid diploids (17.1%) (P<0.05). The change in viscera fat weight was relatively small compared with the SGR. No significant difference in early growth was observed among parental fish, hybrids and hybrid triploids (P>0.05). No significant differences were seen in gonadosomatic index (GSI), where hybrid and hybrid triploids had relatively low values compared to their increased weight (P>0.05).
Table 2. Comparative analysis of gonadosomatic index (GSI), sex hormone and thyroid hormone in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀)×tiger puffer (♂) between their parents and hybrid triploid*1.
| Initial group*2 | 3 months after initial measurement*2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Riverpuffer | Hybrid | Hybrid triploid | Tigerpuffer | Riverpuffer | Hybrid | Hybridtriploid | Tiger puffer | |
| Body weight (BW, g) | 32.1±6.88a | 34.2±9.17a | 33.8±7.41a | 44.3±8.54b | 44.1±5.3a | 50.8±3.14b | 43.8±4.18a | 54.3±6.89b |
| Viscera fat weight/BW (%) | 7.7±0.54a | 10.5±1.02b | 8.9±0.77a | 11.6±1.06b | 9.7±1.81a | 13.5±1.72b | 9.5±1.81a | 13.7±1.84b |
| GSI (%)*3 | 0.1±0.05a | 0.1±0.02a | 0.1±0.02a | 0.4±0.02b | 0.1±0.03a | 0.1±0.04a | 0.1±0.04a | 0.4±0.03b |
*1 The values are means ± SD (n=10) of triplicate groups. Values in the same column not sharing common superscripts are significantly different among ploidy and season (P<0.05).
*2 Allparameters of each group were measured in March to July, 2016, respectively.
*3 Gonadosomatic index (GSI)=(Gonad weight/Body weight) ×100.
Table 3. Comparative analysis of hematological parameters in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀)×tiger puffer (♂) between their parents and hybrid triploid*1
Table 3 summarizes the values for six hematological parameters: erythrocyte (EC), hematocrit (HCT), total hemocyte count (THC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). The EC and HCT values of species did not change significantly during the three months of observation (P > 0.05). THC and MCHC values were also not significantly different among samples (P<0.05), but MCH and MCV variation of hybrid triploids was 10% higher than those of parental fish and hybrid diploids (P<0.05).
Table 3. Comparative analysis of hematological parameters in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀)×tiger puffer (♂) between their parents and hybrid triploid*1.
| Hematologicalparameters | Initial group*2 | 3 months after initial measurement*2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Riverpuffer | Hybrid | Hybrid triploid | Tiger puffer | Riverpuffer | Hybrid | Hybrid triploid | Tigerpuffer | ||
| Erythrocyte count(cells/μL) | 2.5±0.24b | 2.7±0.51b | 1.5±0.66a | 2.3±0.71b | 2.5±0.11b | 2.6±0.74b | 1.4±0.57a | 2.4±0.22b | |
| Hematocrit (%) | 35.7±2.18c | 33.7±1.91b | 32.8±1.88a | 31.7±2.64a | 35.1±2.93b | 33.1±1.97a | 31.9±2.87a | 32.9±1.81a | |
| Mean corpuscularvolume (μm3) | 137.4±5.42a | 149.3±4.18b | 171.5±3.14c | 152.0±4.46b | 134.0±4.11a | 154.8±6.91b | 194.2±5.73c | 157.8±1.92b | |
| Total hemoglobincontent (g/100 mL) | 8.1±0.95a | 8.9±0.83a | 8.8±1.04a | 9.9±0.35b | 8.3±1.14a | 8.8±0.99a | 8.9±0.85a | 9.9±0.94b | |
| Mean corpuscularHemoglobin (pg) | 31.5±2.98a | 35.4±2.76a | 46.5±3.84b | 37.1±2.67a | 33.2±2.56a | 36.1±2.69a | 54.9±2.87c | 41.5±3.71b | |
| Mean corpuscularhemoglobin concentration (%) | 26.6±1.83a | 28.1±0.97b | 27.9±1.07b | 30.1±1.44c | 26.1±0.99a | 28.4±1.24b | 28.0±1.42b | 30.3±2.89c | |
*1The values are means ± SD (n=10) of triplicate groups. Values in the same column not sharing common superscripts are significantly different among ploidy and season (P<0.05).
*2 All parameters of each group were measured in March to July, 2016, respectively.
Table 4. Comparative analysis of fatty acids in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀) × tiger puffer (♂) between their parents and hybrid triploid*1
Whole body fatty acid compositions shown as percentages of total fatty acids are given in Table 4. The most abundant fatty acids were palmitic acid (SFAs, C16:0),
Table 4. Comparative analysis of fatty acids in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀) × tiger puffer (♂) between their parents and hybrid triploid*1.
| Fatty acid | Initial group*2 | 3 months after initial measurement*2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Riverpuffer | Hybrid | Hybrid triploid | Tiger puffer | Riverpuffer | Hybrid | Hybrid triploid | Tiger puffer | ||||||
| C10:0 | 0 0 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| C12:0 | 0 0 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| C14:0 | 02.8±0.35a | 03.8±0.35b | 3.8±0.47b | 3.1±0.32a | 01.7±0.25a | 02.6±0.38b | 2.8±0.18b | 2.1±0.12a | |||||
| C16:0 | 21.2±0.47c | 17.1±0.63a | 20.7±0.62c | 18.6±0.23b | 19.8±0.67b | 17.8±0.60a | 18.3±0.65a | 16.6±0.83a | |||||
| C18:0 | 06.3±0.36b | 06.8±0.32b | 6.5±0.25b | 5.9±0.15a | 05.8±0.19a | 06.5±0.08b | 5.4±0.25a | 5.6±0.14a | |||||
| C20:0 | 00.5±0.26b | 00.7±0.21b | 1.2±0.21c | 00.1±0.01a | 00.1±0.01a | 00.7±0.01b | 0.6±0.12b | 00.1±0.01a | |||||
| C22:0 | 00.9±0.41b | 01.1±0.31b | 0 | 0.1±0.01a | 00.3±0.11a | 00 | 0.4±0.09a | 0.2±0.01a | |||||
| C24:0 | 00.5±0.02a | 00.6±0.03a | 0.7±0.13b | 0.8±0.11b | 00.5±0.08b | 00.7±0.11c | 0.2±0.10a | 0.8±0.22c | |||||
| Saturates*3 | 31.3±1.96b | 30.8±1.83b | 31.7±1.05b | 26.3±1.84a | 27.9±1.12b | 26.2±1.58b | 25.9±1.33b | 23.3±1.21a | |||||
| C12:1n | 0 0 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| C14:1n-9 | 0 0 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| C16:1n-7 | 08.4±0.25c | 06.9±0.17a | 7.7±0.33b | 7.1±0.02a | 07.4±0.75b | 06.5±0.47a | 6.5±0.14a | 6.7±0.22a | |||||
| C18:1n-7 | 15.2±0.35c | 10.3±0.22a | 15.5±0.18c | 13.1±0.02b | 14.7±0.75b | 12.6±0.75a | 12.9±0.16a | 13.1±0.22a | |||||
| C18:1n-9 | 11.1±0.07a | 15.8±0.31b | 15.4±0.31b | 11.6±0.03a | 12.6±0.07a | 12.8±0.75a | 15.9±0.68b | 13.8±0.03a | |||||
| C20:1n-9 | 03.3±0.03b | 03.4±0.35b | 2.3±0.11a | 01.9±0.45a | 02.3±0.06b | 01.8±0.10a | 2.6±0.22b | 002.5±0.05b | |||||
| C22:1n-9 | 00.2±0.04a | 00.8±0.12b | 0.2±0.09a | 0.6±0.02b | 00.1±0.08a | 00.17±0.09a | 0 | 0.1±0.02a | |||||
| C24:1n-9 | 00.2±0.06a | 00.3±0.04a | 0 | 0.3±0.01a | 00.3±0.01a | 0 | 0.3±0.01a | 0.3±0.01a | |||||
| Monoenes*4 | 37.9±0.73b | 36.3±0.92a | 40.7±0.88c | 35.3±0.69a | 36.9±1.51b | 32.4±1.71a | 38.0±1.17b | 31.7±1.44a | |||||
| C18:2n-6 | 09.1±0.88c | 07.8±0.18b | 5.0±0.27a | 04.5±0.54a | 11.1±1.04c | 08.3±0.33a | 09.3±0.30b | 15.3±1.54d | |||||
| C18:3n-6 | 04.7±0.54c | 02.3±0.26a | 3.2±0.01b | 03.6±0.25b | 04.7±0.54d | 02.2±0.15a | 2.1±0.01b | 03.6±0.36c | |||||
| C20:2n-6 | 00.4±0.05c | 00.2±0.01b | 0.3±0.01b | 0.1±0.02a | 00.4±0.15b | 00.9±0.11c | 0.6±0.01b | 0.1±0.02a | |||||
| C20:3n-6 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| C20:4n-6 | 00.3±0.06a | 00.5±0.12b | 0.3±0.02a | 0.9±0.15c | 00.3±0.04a | 00.7±0.21b | 0.6±0.01b | 0.5±0.14b | |||||
| C22:2n-6 | 00.5±0.15b | 00 | 0.2±0.04a | 0.8±0.22c | 00.5±0.18b | 00.1±0.12a | 0.1±0.07a | 0.7±0.21b | |||||
| C22:4n-6 | 0 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Total n-6*5 | 13.4±1.28c | 09.8±0.47b | 08.7±0.16a | 8.3±0.74a | 16.1±1.48b | 11.7±0.29a | 11.4±0.54a | 18.3±1.74b | |||||
| C18:3n-3 | 00.7±0.14b | 00.2±0.04a | 0.3±0.11a | 00.6±0.06b | 00.7±0.12b | 00.1±0.01a | 0.5±0.10b | 00.6±0.18b | |||||
| C20:3n-3 | 00.4±0.05b | 00.2±0.06a | 0 | 0.1±0.02a | 00.4±0.11b | 00.1±0.06a | 0.4±0.02b | 0.1±0.02a | |||||
| C20:5n-3 | 03.1±0.17b | 03.8±0.11c | 2.2±0.02a | 03.6±0.13c | 02.3±0.20a | 02.1±0.15a | 2.6±0.01b | 02.7±0.12b | |||||
| C22:5n-3 | 04.3±0.31c | 03.9±0.11b | 2.7±0.01a | 2.9±0.15a | 03.4±0.26b | 02.4±0.02a | 2.4±0.13a | 2.2±0.19a | |||||
| C22:6n-3 | 02.5±0.38a | 06.4±0.42d | 5.1±0.47c | 3.8±0.92b | 02.5±0.38a | 4.5±0.43b | 4.7±0.64b | 3.8±0.40b | |||||
| Total n-3*6 | 09.6±0.76a | 14.4±0.81b | 9.9±0.41a | 10.5±1.71a | 08.7±0.82a | 9.1±0.58a | 9.8±0.62a | 9.6±1.71a | |||||
*1 The values are means ± SD (n=100) of triplicate groups. Values in the same column not sharing common superscripts are signifi cantly different among ploidy and season (P<0.05).
*2 All parameters of each group were measured in March to July, 2016, respectively.
*3 Total saturated fatty acids, *4 Total monounsaturated fatty acids.
*5 Total n-6 polyunsaturated fatty acids.
*6 Total n-3 polyunsaturated fatty acids.
palmitoleic acid (MUFAs, C16:1n-7), oleic acid (MUFAs, C18:1n-9) and linoleic acid (PUFAs, C18:2n-6), and relatively low contents, but puffer fish also contain omega-3 fatty acids, EPA (PUFAs; C20:5n-3; eicosapntemacnioc acid), DPA (PUFAs; C22:5n-3; docosapentaenoic acid) and DHA (PUFAs; C22:6n-3; docosahexaenoic acid). Fatty acid (14:0), palmitic acid (16:0), stearic acid (18:0) and total saturated fatty acids (total SFA) were significantly different among puffer fish, hybrids and hybrid triploids (P<0.05), but other fatty acids were not significantly different (P>0.05). The 16:1n-7, 18:1n-7, 18:1n-9, 20:1n-9 and total mono unsaturated fatty acids (total MUFA) showed significant differences among puffer fish, hybrid and hybrid triploid (P<0.05).Other mono unsaturated fatty acids were not significantly different (P>0.05). In 18:2n-6, 18:3n-6 and polyunsaturated fatty acids (total n-6 PUFA), 20:5n-3, 22:5n-3, 22:6n-3 and n-3 polyunsaturated fatty acids (total n-3 PUFA) showed significant differences among hybrids and hybrid triploids (P<0.05).Other PUFA and MUFA values were not significantly different (P>0.05).
Estradiol and testosterone variations are shown in Fig. 1. There were no noticeable trends observed between parental and hybrid groups. From beginning to end of the experiment, river puffer and tiger puffer showed more than four times higher values of these hormones than hybrids and hybrid triploids (P<0.05). Hybrid groups had relatively low initial and final levels of testosterone, whereas parental fish had relatively higher values than hybrid groups (P<0.05). Fig. 2 shows the total plasma thyroid hormone concentrations in parental fish, hybrids and hybrid triploids fed the same diets. Plasma triiodothyronine (T3) levels were significantly higher (P<0.05) in the hybrid and tiger puffer samples throughout the 3 months, but no significant differences were observed in river puffer and hybrid triploids (P>0.05). The highest measured values were found in the initial analysis. The levels of thyroxine (T4) among the samples during the 3 months showed a similar tendency to the plasma T3 levels. Hybrids and tiger puffers had the highest values, and river puffers and hybrid triploids had relatively low values (P<0.05).
Fig. 1. Comparative analysis of estradiol (Bar graph) and testosterone (Line graph) in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀) × tiger puffer (♂) and hybrid triploid in this experiment.
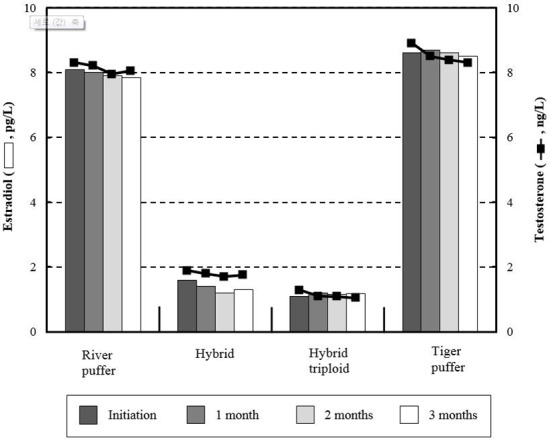
Values are means ± SE of triplicate experiments (n=10).
Fig. 2. Comparative analysis of thyroid stimulating hormone (Bar graph) and thyroxine (Line graph) in river puffer, Takifugu obscurus, tiger puffer, T. rubripes, hybrid river puffer (♀) × tiger puffer (♂) and hybrid triploid in this experiment.
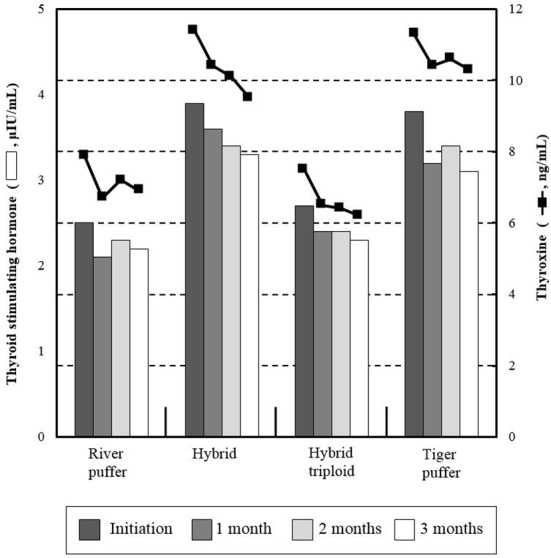
Values are means ± SE of triplicate experiments (n=10).
Discussion
The growth performance of hybrid and triploid groups depended on the species, age and breeding conditions (Chevassus, 1979; Thorgaard, 1986). Induced hybridization by inseminating the genes of both parents by artificial methods has been tried many times (Chevassus, 1979), (Chevassus, 1983; Rahman et al., 2005). Induced hybrids of puffer fish have 2 superior traits: first is a rapid growth of tiger puffer, Takifugu rubripes, and second is abundant flavor of river puffer, T. obscurus (Kang et al., 2007; Kotaro & Takeshi, 2007). Particularly, growth conditions of river puffer are very difficult and sensitive to contamination so that river puffer is designated as a high value and endangered species. Triploid fish have been produced many times. Triploids cease to develop maturation and focus on growth (Thorgaard, 1986; Ueno et al., 1986).
Because of the apparent advantages of hybrids and triploids, we analyzed the characteristics of hybrid triploids. Rahi & Shah (2012) compared the growth performance of rohu (Labeo rohita), mrigal (Cirrhinus cirrhosis), hybrids (rohu × mrigal) and hybrid triploids. The highest growth rate was observed in hybrid triploids. Hybrid diploids also showed a considerably higher growth rate than single species of rohu and mrigal during the experimental period. Felip et al. (2001) observed a similar growth rate in diploid and triploid fish over a 2 year period. Triploid fish had a better growth rate and food conversion rate than diploids because of their sterility (Benfey, 1999; Shah et al., 1999; Rahi & Shah, 2012). Hybrid groups in the present study did not have an outstanding growth rate in their early growth period; however as time progressed, hybrids and hybrid triploids exhibited significantly higher growth than diploids. Ueno et al. (1986) described that the edible portions of female and male triploids were about 30% and 15% larger than those of diploids, respectively.
Triploid fish usually grow faster than their diploid counterparts because of larger cell size, and the larger cell size is offset by a decrease in cell number (Thorgaard, 1986; Park & Kim, 2000; Goo et al., 2015). Among hematological parameters, the hemoglobin concentration and the hematocrit value are related to respiratory function of fish (Ikeda et al. 1986). Hybrid triploids had an 11% increase in MCV and a 14 % increase in MCH compared to that of diploid fish. Therefore, a lower EC and a higher MCV of triploids will result in a lower oxygen exchange capacity than that of diploid fish. In addition to the above mentioned triploid drawback, there are other disadvantages. Zhang et al. (2008) compared triploidization in Chinese shrimp (Fenneropenaeus chinensis) and showed that triploids do not change their adaptability to abrupt variations in salinity. Some reports showed that triploids do not cope well with chronic stresses such as chronic high temperature and long-term starvation (Ojolick et al., 1995).
In general, the fatty acid composition of whole body fish reflected the composition of their diets and body components (Farkas et al., 1978; Pawlosky et al., 1996). We observed palmitic acids and oleic acids in puffer fish, hybrids and hybrid triploids. Palmitic acids decrypt liver toxins, and oleic acids lower bad cholesterol in the blood, prevent arteriosclerosis and cardiovascular disease, and have anticancer effects (Pawlosky et al., 1996). According to Wang et al. (2015), palmitic and oleic acid content of diploids and triploids are not significantly different. The present study also showed that there were no significant differences in palmitic and oleic acids in puffer fish, hybrids and hybrid triploids (P>0.05). Puffer fish contain essential fatty acids and omega-3 fatty acids (EFA, DHA and EPA) (Kang et al., 2007; Oh & Hwang, 2013). The high DHA content of larvae is a reflection of its importance to larval development, and suggests that the higher content of both DHA and EPA is essential to the larval stage (Farkas et al., 1978; Watanabe & Kiron, 1994). The fatty acid content influences flavor and nutrition so that larvae are able to utilize commercially valuable species (Farkas et al., 1978; Watanabe & Kiron, 1994).
Our results from river puffer, tiger puffer, hybrids and hybrid triploids showed that parental fish have relatively higher gonadal hormone values than hybrids and hybrid triploids (P<0.05). Nam et al. (2004) described transgenic hybrid triploids (cyprinid loach (Misgurnus anguillicaudatus) × mud loach (M. mizolepis)) in which a few sperm were still observed in their testes. Differences in gonadal hormone values retard gonad development and restrict reproduction (Nam et al., 2004; Feindel et al., 2011; Huang et al., 2016; Yoo et al., 2016). Unlike the gonadal hormones, differences in growth hormones have been observed between groups. Hybrids and tiger puffer had relatively higher values than river puffer and hybrid triploids (P<0.05). Huang et al. (2016) found that hybrid diploids and hybrid triploids (orange-spotted grouper (Epinephelus coioides) × the giant grouper (Epinephelus lanceolatus)) showed a more rapid growth rate than parental fish, and among them, hybrid triploids had the highest growth rate. On the other hand, our hybrid triploids had a relatively lower growth rate than hybrid diploids and tiger puffers.
In this experiment, the characteristics of river puffer, tiger puffer, hybrids and hybrid triploids were successfully compared by various methods. The hybrids and hybrid triploids had superior somatic growth compared to river puffer and tiger puffer. Hybrid triploids had inferior hematological parameters of MCV and MCH, and contained a large amount of crude fat. Puffer fish, hybrids and hybrid triploids contained a high DHA and EPA content. We hope that these results will benefit the future research of the hybrid and hybrid triploid-related fish.
ACKNOWLEDGEMENTS
This research was supported by a research grant (Project No. 311045-03-1-SB010). The method for producing hybrids between river puffer and tiger puffer was obtained from a Korean patent (Title: Production method of hybrid between river puffer, Takifugu obscurus and tiger puffer, T. rubripes; Patent No.: 042615012) in June 2016. We are gratefully thanks to the staff of the Fishery, Genetics Breeding Sciences Laboratory of the Korea Maritime and Ocean University, Korea. This manuscript was improved by comments from anonymous reviewers. We declare that all the experiments performed in this study complied with the current laws of Korea (Ordinance of Agriculture, Food and Fisheries, No. 1- the Law Regarding Experimental Animals, No. 9932) and the Ethical Guidelines of Korea Maritime and Ocean University, Korea.
REFERENCES
- 1.Benfey TJ. The physiology and behavior of triploid fishes. Fish Sci. 1999;7:39–67. doi: 10.1080/10641269991319162. [DOI] [Google Scholar]
- 2.Chevassus B. Hybridization in Salmonids: results and perspectives. Aquaculture. 1979;17:113–128. doi: 10.1016/0044-8486(79)90047-4. [DOI] [Google Scholar]
- 3.Chevassus B, Guyomard R, Chourrout D, Quillet E. Production of viable hybrids in Salmonids by triploidization. Gene Select Evol. 1983;15:519–532. doi: 10.1186/1297-9686-15-4-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 5.Farkas T, Csengeri I, Majoros F, Olah J. Metabolism of fatty acids in fish. II. Biosysnthesis of fatty acids in relation to diet in the carp, Cyprinus carpio Linnaeus 1758. Aquaculture. 1978;14:57–65. doi: 10.1016/0044-8486(78)90140-0. [DOI] [Google Scholar]
- 6.Feindel NJ, Benfey TJ, Trippel EA. Gonadal development of triploid Atlantic cod Gadus morhua. J Fish Biol. 2011;78:1900–1912. doi: 10.1111/j.1095-8649.2011.02955.x. [DOI] [PubMed] [Google Scholar]
- 7.Felip A, Piferrer F, Zanuy S, Carrillo M. Comparative growth performance of diploid and triploid European sea bass over four spawning seasons. J Fish Biol. 2001;58:76–88. doi: 10.1111/j.1095-8649.2001.tb00500.x. [DOI] [Google Scholar]
- 8.Goo IB, Im JH, Gil HW, Lim SG, Park I-S. Comparison of cell and nuclear size difference between diploid and induced triploid in marine medaka, Oryzias dancena. Dev Reprod. 2015;3:127–134. doi: 10.12717/DR.2015.19.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Liu Q, Xie J, Wang W, Xiao J, Li S, Zhang H, Zhang Y, Liu S, Lin H. Characterization of triploid hybrid groupers from interspecies hybridization (Epinephelus coioides×Epinephelus lanceolatus) Aquacult Res. 2016;47:2195–2204. doi: 10.1111/are.12672. [DOI] [Google Scholar]
- 10.Huffman PA, Arkoosh MR, Casillas E. Characteristics of peripheral blood cells from rainbow trout evaluated by particle counter, image analysis, and hemocytometric techniques. J Aquat Anim Health. 1997;9:239–248. doi: 10.1577/1548-8667(1997)0092.3.CO;2. [DOI] [Google Scholar]
- 11.Ikeda Y, Ozaki H, Sezaki K. In: Blood Atlas of Fishes. Ikeda Y, Ozaki H, Sezaki K, editors. Tokyo, Japan: MidoriShobo; 1986. p. 361. [Google Scholar]
- 12.Kang HW, Shim KB, Kang DY, Jo KC, Song KC, Lee JH, Song HI, Son SG, Cho YJ. Sitological quality evaluation of cultured and wild river puffer, Takifugu obscurus. Kor J Fish Aquat Sci. 2007;20:147–153. [Google Scholar]
- 13.Kotaro K, Takeshi F. Growth of tiger puffer, Takifugu rubripes at different salinities. J World Aquacult Soc. 2007;38:427–434. doi: 10.1111/j.1749-7345.2007.00114.x. [DOI] [Google Scholar]
- 14.Nam YK, Park I-S, Kim DS. Triploid hybridization of fast-growing transgenic mud loach, Misgurnus mizolepis male to cyprinid loach, Misgurnus anguillicaudatus female: the first performance study on growth and reproduction of transgenic polyploid hybrid fish. Aquaculture. 2004;231:559–672. doi: 10.1016/j.aquaculture.2003.09.046. [DOI] [Google Scholar]
- 15.Oh K-W, Hwang S-M. Comparisons of food component characteristics of wild and cultured edible puffer fishes in Korea. Kor J Fish Aquat Sci. 2013;46:725–732. [Google Scholar]
- 16.Ojolick EJ, Cusack R, Kerr SR. Survival and growth of all-female diploid and triploid rainbow trout (Oncorhynchus mykiss) reared at chronic high temperature. Aquaculture. 1995;131:177–187. doi: 10.1016/0044-8486(94)00338-O. [DOI] [Google Scholar]
- 17.Park I-S, Kim DS. Comparison of some tissues in diploid and induced triploid hybrid between mud loach, Misgurnus mizolepis and cyprinid loach, M. anguillicaudatus. Dev Reprod. 2000;4:19–28. [Google Scholar]
- 18.Pawlosky RJ, Ward G, Salem N Jr. Essential fatty acid uptake and metabolism in the developing rodent brain. Lipids. 1996;31:103–107. doi: 10.1007/BF02637060. [DOI] [PubMed] [Google Scholar]
- 19.Rahi ML, Shah MS. Triploidization in rohu × mrigal hybrid and comparison of growth performance of triploid hybrid. Aquacult Res. 2012;47:2195–2204. doi: 10.1111/j.1365-2109.2011.02996.x. [DOI] [Google Scholar]
- 20.Rahman AM, Uehara T, Lawrence JM. Growth and heterosis of hybrids of two closely related species of Pacific sea urchins (Genus echinometra) in Okinawa. Aquaculture. 2005;245:121–133. doi: 10.1016/j.aquaculture.2004.11.049. [DOI] [Google Scholar]
- 21.Scheerer PD, Thorgaard GH. Increased survival in Salmonid hybrids by induced triploidy. Can J Fish Aquat Sci. 1983;40:2040–2044. doi: 10.1139/f83-235. [DOI] [Google Scholar]
- 22.Seol D-W, Im S-Y, Hur WJ, Park MO, Kim DS, Jo J-Y, Park I-S. Haematological parameters and respiratory function in diploid and triploid far eastern catfish, Silurus asotus. Genes & Genomics. 2008;30:205–213. [Google Scholar]
- 23.Shah MS, Thorgaard GH, Wheeler P. Viability of diploid and triploid hybrids between a few salmon and trout species. Asian Fish Soc; 1999. pp. 259–262. [Google Scholar]
- 24.Sutterlin AM, Holder J, Benfey TJ. Early survival rates and subsequent morphological abnormalities in landlocked anadromous and hybrid (landlocked × anadromous) diploid and triploid Atlantic salmon. Aquaculture. 1987;64:157–164. doi: 10.1016/0044-8486(87)90351-6. [DOI] [Google Scholar]
- 25.Thorgaard GH. Ploidy manipulation and performance. Aquaculture. 1986;57:57–64. doi: 10.1016/0044-8486(86)90180-8. [DOI] [Google Scholar]
- 26.Ueno K, Ikenaga Y, Kariya H. Potentiality of application of triploidy to the culture of ayu. Japan J Genetics. 1986;61:71–77. doi: 10.1266/jjg.61.71. [DOI] [Google Scholar]
- 27.Verdegem MCJ, Hilbrands AD, Boon JH. Influence of salinity and dietary composition on blood parameter values of hybrid red tilapia, Oreochromis niloticus (Linnaeus) × O. mossambicus (Peters) Aquacult Res. 1997;28:453–459. doi: 10.1111/j.1365-2109.1997.tb01063.x. [DOI] [Google Scholar]
- 28.Wang CA, Xu QY, Bai QL, Yin JS, Jia ZH. Comparison of growth performances, nutritional composition in muscle of diploid and triploid masu salmon, Oncorhynchus masou. Turk J Fish Aquat Sci. 2015;15:127–135. doi: 10.4194/1303-2712-v15_1_14. [DOI] [Google Scholar]
- 29.Watanabe T, Kiron V. Prospects in larval fish dietetics. Aquaculture. 1994;124:223–251. doi: 10.1016/0044-8486(94)90386-7. [DOI] [Google Scholar]
- 30.Yoo GY, Seong NC, Park I-S. Cytogenetic analysis of hybrid and hybrid triploid between river puffer, Takifugu obscurus and tiger puffer, T. rubripes. Aquacult Res (Submitted) 2016 [Google Scholar]
- 31.Zhang C, Li F, Yu K, Xiang J. Comparative growth performances of diploid and triploid Chinese shrimp Fenneropenaeus chinensis (Osbeck, 1765) under different salinities. Aquacult Res. 2008;32:962–969. [Google Scholar]


