Abstract
Background and Purpose
The quality of anticoagulation is critical for ensuring the benefit of warfarin, but this has been less well studied in Korean ischemic stroke patients with atrial fibrillation (AF).
Methods
This study retrospectively analyzed the data of patients who had an AF-related ischemic stroke and were treated with long-term warfarin therapy in 16 Korean centers. The quality of warfarin therapy was primarily assessed by the time in therapeutic range [TTR; international normalized ratio (INR), 2.0–3.0] and additionally by the proportion of INR values within the therapeutic range.
Results
The long-term warfarin-treated cohort comprised 1,230 patients. They were aged 70.1±9.7 years (mean±SD), 42.5% were female, and their CHA2DS2-VASc score was 4.75±1.41. The TTR analysis included 33,941 INR measurements for 27,487 months: per patients, 27.6 (SD, 22.4) INR measurements for 22.4 (SD, 12.9) months. The mean TTR of individual patients was 49.1% (95% confidence interval, 47.9–50.3%), and the TTR quartiles were <34.5, 34.5–49.1, 49.1–64.5%, and >64.5%. None of the 16 centers achieved a mean TTR of >60%. Of all INR measurements, 44.6% were within the therapeutic range, 41.7% were <2.0, and 13.7% were >3.0.
Conclusions
In Korean ischemic stroke patients who had AF, the quality of warfarin therapy was low and might be inadequate to effectively prevent recurrent stroke or systemic embolism.
Keywords: warfarin, quality, time in therapeutic range, atrial fibrillation, seconadry stroke prevention
INTRODUCTION
Cardioembolic stroke has increased and now accounts for one in five ischemic strokes in Korea.1,2 Among Korean patients with acute ischemic stroke, 19% had atrial fibrillation (AF), who require long-term anticoagulation for secondary stroke prevention.2
Before the introduction of non-vitamin K antagonist oral antagonists (NOACs), warfarin was the only available oral anticoagulant and is still widely used in clinical practice. The efficacy and safety of warfarin are critically dependent on maintaining the international normalized ratio (INR) within the therapeutic range.3,4 However, it is widely perceived that adequate anticoagulation with warfarin is not well achieved in Koreans, which is partially attributable to their high intake of vitamin-K-rich foods and high frequency of genetic polymorphisms that are unfavorable for stable anticoagulation with warfarin.5,6,7 In pivotal NOAC trials, Korean patients randomized to warfarin achieved a mean time in therapeutic range (TTR) of less than 60%, which was lower than the average TTR of overall patients.8,9,10,11 TTR in real-world practice is expected to be lower than in a clinical trial setting. A single-center study in which warfarin therapy was managed by a formal anticoagulation clinic showed that the TTR in AF-related stroke patients was 57.5%.12 However, a formal anticoagulation clinic is not widely available in Korean centers, and the quality of anticoagulation with warfarin in more-representative Korean AF-related stroke patients has not been systematically investigated.
The current multicenter study aimed to assess the quality of anticoagulation with warfarin in patients with AF-related stroke who received long-term warfarin therapy. Additionally, we explored demographic and clinical factors associated with poor INR control.
METHOD
Study subjects
This was a retrospective observational study designed to analyze data of patients who had AF-related ischemic stroke and were treated with long-term warfarin therapy (long-term warfarin-treated cohort) in the neurology departments of 16 participating centers between January 1, 2011 and December 31, 2012. We collected INR data for this study until December 31, 2014.
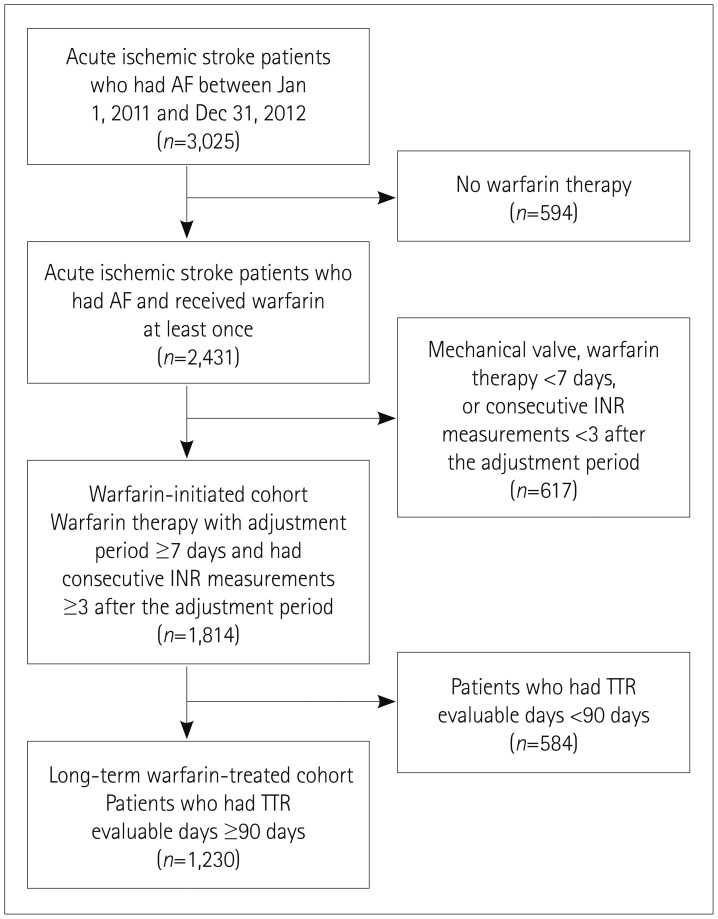
Inclusion criteria for the long-term warfarin-treated cohort were 1) admission due to AF-related ischemic stroke (known AF or newly detected AF), 2) receiving long-term warfarin therapy for at least 90 days after the 7-day warfarin adjustment period, 3) for TTR calculation, the number of consecutive INR measurements of ≥3 after the 7-day warfarin adjustment period, and 4) TTR evaluable for ≥90 days. Exclusion criteria were 1) AF in the presence of a mechanical valve, 2) enrollment in a randomized clinical trial testing anticoagulation, and 3) enrollment in another study affecting the INR target range. Patients who met inclusion criteria of 1) and 3) were included in the warfarin-initiated cohort (Fig. 1).
Fig. 1. Selection of patients. AF: atrial fibrillation, INR: international normalized ratio, TTR: time in therapeutic range.
For TTR analysis, the INR values measured during the following periods were excluded: 1) first 7 days after warfarin initiation, 2) interval between two consecutive INR measurements of >9 weeks, and 3) 7 days before and 21 days after intentional temporary warfarin interruption due to bleeding, surgery, or other invasive procedures. In addition, for patients who received NOAC, the period of NOAC treatment was also excluded.
For each patient, we collected data on demographics, individual components of CHADS2 and CHA2DS2-VASc, dates of first and last warfarin treatments, INR values with dates, and warfarin interruptions and their reasons. This study was approved by the Institutional Review Board (IRB) of each participating center. The informed consent from individual patients or their legally authorized representatives was waived by the relevant IRBs because this was a retrospective study and data were collected while ensuring the anonymity of individual patients.
Outcomes
The primary outcome was the TTR as measured by the proportion of time that INR was within the therapeutic range of 2.0–3.0, using the Rosendaal linear interpolation method.13 We assessed the TTR of each patient and calculated the mean TTR. The secondary outcome was the proportion of INR values within the therapeutic range of 2.0–3.0 as indicated by the numbers of INR values within the therapeutic range divided by the total number of INR measurements.
Statistical analysis
Categorical variables are summarized as frequencies and percentages, while continuous variables are presented as mean±SD or median (interquartile range) values. To assess the quality of warfarin treatment, we calculated the individual TTR for each patient using the Rosendaal linear interpolation method, which assumes a linear relationship between two consecutive INR values and determines the proportion of time that the INR is within the therapeutic range of 2.0–3.0.13 To delineate the overall quality of warfarin treatment, we analyzed the mean TTR with 95% confidence interval (CI) and the overall distribution of TTRs of individual patients. We also analyzed the proportion of INR values within the therapeutic range of 2.0–3.0 and the overall distribution of INR values. In addition, the mean TTR and the proportion of INR values within the therapeutic range were analyzed for each participating center with anonymity.
To explore the factors associated with poor anticoagulation quality, patients were dichotomized into groups with TTR ≥60% and <60%. For univariable analysis, categorical variables were compared using the chi-square test or Fisher's exact test as appropriate, while continuous variables were compared using Student's t-test or the Wilcoxon rank-sum test. For multiple logistic regression analyses to explore the independent factors for TTR <60%, we used the generalizedestimating-equation model to adjust for the within-hospital clustering of the collected data. Covariates with p<0.1 in the comparisons of baseline characteristics between the two groups were adjusted. We analyzed the tolerance limit, variance inflation factor, eigenvalue, and condition index in order to evaluate the presence of multicollinearity in the multivariable models, if indicated.
Sample size calculation
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study, the mean TTR of the enrolled Korean patients was 55%.8 In clinical practice, TTR would be lower than that found in the RE-LY trial, and so we assumed that the mean TTR would be 45%. Including 1,057 subjects would ensure that TTR would be within 3% of the two-sided 95% CI for the expected TTR (namely, 42 to 48%) using the large-sample approximation.14 Noting that no dropout is expected due to a retrospective nature of this study, we aimed to enroll at least 1,057 subjects who met the criteria for the longterm warfarin-treated cohort.
Funding
This study was an investigator-initiated study supported by Boehringer-Ingelheim. The representatives of the sponsor participated in the study design and center selection, but they were not involved in the collection, monitoring, or analysis of data, or in writing this manuscript. Data were collected by the site investigators, and monitoring and management of data were independently conducted. The authors had unrestricted access to the data, performed the data analysis with the study statisticians, and vouch for the completeness and accuracy of the reported data.
RESULTS
Characteristics of the patients
Between January 1, 2011 and December 31, 2012 there were 3,025 patients who had an acute ischemic stroke or transient ischemic attack and AF and were admitted to 16 participating centers. In three centers the warfarin therapy was managed by a formal anticoagulation clinic. We excluded 1,211 patients due to no warfarin therapy being administered, presence of a mechanical valve, duration of warfarin therapy of <7 days, or less than 3 consecutive INR measurements after the warfarin adjustment period. Therefore, the warfarin-initiated cohort comprised 1,814 patients. From the warfarin-initiated cohort, 1,230 patients were finally included in the long-term warfarin-treated cohort after excluding 584 patients for whom TTR was evaluable for <90 days (Fig. 1). During the study period, only three patients were enrolled in clinical trials that potentially affected the target INR range. For these patients, INR data after the trials were included in this study. The long-term warfarin-treated cohort was the main population of the current analysis. Data for the warfarin-initiated cohort were additionally analyzed and are provided in the Supplementary Tables 1, 2, and 3 (in the online-only Data Supplement).
The demographic and clinical characteristics of the longterm warfarin-treated cohort are provided in Table 1. In brief, the subjects were aged 70.1±9.7 years, 42.5% were female, their National Institutes of Health Stroke Scale (NIHSS) score at admission was 6.0±5.9, their CHADS2 score was 3.39±0.96, and their CHA2DS2-VASc score was 4.75±1.41.
Table 1. Demographics and baseline characteristics.
| Variable | Long-term warfarin-treated patients (n=1,230) |
|---|---|
| Age, years | 70.1±9.7 |
| <65 | 300 (24.4) |
| 65–74 | 515 (41.9) |
| ≥75 | 415 (33.7) |
| Female | 523 (42.5) |
| Height, cm | 162.7±8.7 |
| Weight, kg | 63.1±11.4 |
| NIHSS score* | |
| Mean±SD | 6.0±5.9 |
| Median (IQR) | 4.0 (2.0–9.0) |
| <8 | 865 (70.7) |
| 8–16 | 261 (21.2) |
| >16 | 97 (7.9) |
| Creatinine level, mg/dL | 1.01±0.54 |
| CHADS2 score | 3.39±0.96 |
| CHA2DS2-VASc score | 4.75±1.41 |
| Congestive heart failure | 142 (11.5) |
| Hypertension | 842 (68.5) |
| Diabetes mellitus | 315 (25.6) |
| Stroke/transient ischemic attack | 1,230 (100.0) |
| Vascular disease | 210 (17.1) |
| Total INR measurements included in TTR analysis | 33941 |
| Number of INR measurements per patient included in TTR analysis | 27.6±22.4 |
| Total duration in TTR analysis, months | 27487 |
| Duration per patient in TTR analysis, months | 22.4±12.9 |
| Interval between consecutive INR measurements included in TTR analysis, days | 21.7±11.9 |
Data are n (%), mean±SD, or mean (IQR) values.
*NIHSS score was not available in seven patients.
INR: international normalized ratio, IQR: interquartile range, NIHSS: National Institutes of Health Stroke Scale, TTR: time in therapeutic range.
During the study period there was a total of 46,483 INR measurements, corresponding to 37.8±22.9 per patient. The total duration of INR measurements was 34,735 months, corresponding to 28.2±13.6 months per patient, and the interval between consecutive INR measurements was 28.3±16.1 days. After excluding INR data obtained ≤7 days after initiation warfarin, during an intentional temporary warfarin interruption, and with a consecutive INR measurement interval of >9 weeks, the current TTR analysis included 33,941 INR measurements over 27,487 months, constituting 27.6±22.4 INR measurements over 22.4±12.9 months per patient. The interval between two consecutive INR measurements was 21.7±11.9 days; the average interval was 0–15 days in 34.6%, 16–30 days in 44.2%, 31–45 days in 18.3%, 46–60 days in 2.7%, and >60 days in 0.2% of the patients.
Time in therapeutic range
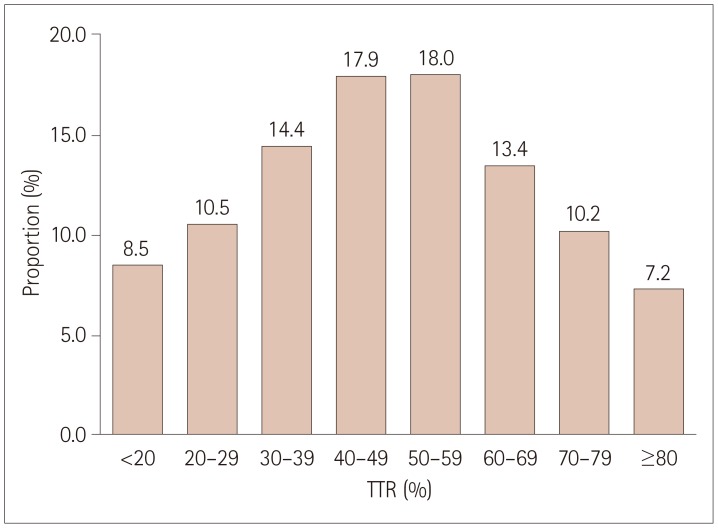
The mean TTR in the long-term warfarin-treated cohort was 49.1% (95% CI, 47.9–50.3%). The quartiles of TTR were <34.5, 34.5–49.1, 49.1–64.5, and >64.5% (Table 2). Of the patients, 30.8% had TTR ≥60% and 17.4% had TTR ≥70% (Fig. 2). Across the 16 centers, the mean TTR ranged from 37.3 to 58.4%, and no center achieved a mean TTR of ≥60%. The mean (95% CI) TTR values of the three centers with formal anticoagulation clinics were 58.4% (54.9–61.9%), 57.5% (53.7–61.3%), and 50.4% (46.0–54.8%), which were generally higher than those of the 13 centers without formal anticoagulation clinics (Table 3).
Table 2. TTR and INR included in TTR analysis.
| Long-term warfarin-treated patients (n=1,230) | |
|---|---|
| Mean TTR (95% CI) | 49.1 (47.9–50.3) |
| TTR quartiles, % | |
| 1st quartile | <34.5 |
| 2nd quartile | 34.5–49.1 |
| 3rd quartile | 49.1–64.5 |
| 4th quartile | >64.5 |
| INR, mean | 2.25±0.89 |
| INR 2.0–3.0, % | 44.6 |
| INR range, % | |
| <1.50 | 16.5 |
| 1.50–1.99 | 25.2 |
| 2.00–2.49 | 27.8 |
| 2.50–3.00 | 16.7 |
| 3.01–3.50 | 7.3 |
| 3.51–4.00 | 3.1 |
| >4.00 | 3.3 |
Data are mean (95% CI), mean±SD, or % values.
INR: international normalized ratio, TTR: time in therapeutic range.
Fig. 2. Proportion of patients according to TTRs in the long-term warfarin-treated cohort. TTR: time in therapeutic range.
Table 3. TTR and proportion of INR 2.0–3.0 included in TTR analysis in each center.
| Center | No. | Mean TTR (95% CI) | INR 2.0–3.0 (%) |
|---|---|---|---|
| 1 | 88 | 55.9 (52.3–59.5) | 46.3 |
| 2 | 95 | 47.4 (43.1–51.7) | 42.9 |
| 3 | 63 | 51.7 (47.5–56.0) | 40.9 |
| 4 | 56 | 42.7 (38.0–47.4) | 36.1 |
| 5* | 121 | 58.4 (54.9–61.9) | 51.7 |
| 6 | 64 | 45.6 (40.8–50.4) | 46.1 |
| 7 | 31 | 45.6 (39.3–51.8) | 40.4 |
| 8 | 60 | 37.1 (32.2–42.0) | 37.0 |
| 9 | 48 | 50.0 (45.0–55.0) | 44.2 |
| 10 | 38 | 49.2 (41.7–56.7) | 44.9 |
| 11* | 107 | 57.5 (53.7–61.3) | 53.4 |
| 12 | 39 | 50.6 (44.0–57.2) | 47.9 |
| 13 | 129 | 38.6 (35.1–42.0) | 36.4 |
| 14 | 131 | 53.6 (49.9–57.2) | 50.1 |
| 15 | 59 | 37.3 (32.0–42.6) | 37.7 |
| 16* | 101 | 50.4 (46.0–54.8) | 43.6 |
Data are mean (95% CI) or % values.
*Centers in which warfarin therapy was managed by a formal anticoagulation clinic during the study period.
INR: international normalized ratio, TTR: time in therapeutic range.
Proportion of INR values within the therapeutic range
Of 33,941 INR measurements included in the TTR analysis, 44.6% were within the therapeutic range of 2.0–3.0, 41.7% were <2.0, and 13.7% were >3.0 (Table 2). Across the 16 centers, the proportion of INR values of 2.0–3.0 ranged from 36.1 to 53.4% (Table 3).
Predictors of poor quality of anticoagulation with warfarin
Table 4 presents the results of unadjusted and adjusted analyses for predictors of poor INR control as determined by TTR <60%. On unadjusted analysis, baseline variables of old age, female sex, small height, low weight, high NIHSS score, high creatinine level, high CHADS2 score, high CHA2DS2-VASc score, and diabetes were associated with TTR <60%. However, when three multivariable models were applied [model 1 was adjusted for age, sex, height, weight, NIHSS score category, creatinine level, and diabetes (variables with p<0.1 based on the protocol); model 2 was the same as model 1 plus adjustment for CHADS2 score; and model 3 was the same as model 1 plus adjustment for CHA2DS2-VASc score], only the highest baseline NIHSS score category (NIHSS score >16) was consistently associated with poor INR control. For models 2 and 3, no significant multicollinearity among variables was found.
Table 4. Predictors of poor anticoagulation quality (TTR <60%).
| Variable | TTR ≥60% (n=379) |
TTR <60% (n=851) |
Unadjusted analysis | Adjusted analysis | |||
|---|---|---|---|---|---|---|---|
| p* | OR (95% CI)† | Model 1 OR (95% CI)† |
Model 2 OR (95% CI)† |
Model 3 OR (95% CI)† |
|||
| Age, years | 69.2±10.0 | 70.5±9.6 | 0.0275 | 1.01 (1.00–1.03) | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) | 1.01 (0.99–1.03) |
| Age category for CHADS2 | 0.1209 | ||||||
| <75 years | 263 (69.4) | 552 (64.9) | Reference | ||||
| ≥75 years | 116 (30.6) | 299 (35.1) | 1.24 (0.95–1.61) | ||||
| Age category for CHA2DS2-VASc | 0.2831 | ||||||
| <65 years | 99 (26.1) | 201 (23.6) | Reference | ||||
| 65–74 years | 164 (43.3) | 351 (41.3) | 1.06 (0.78–1.43) | ||||
| ≥75 years | 116 (30.6) | 299 (35.1) | 1.28 (0.93–1.77) | ||||
| Female | 45 (38.3) | 378 (44.4) | 0.0436 | 1.31 (1.02–1.67) | 0.82 (0.56–1.22) | 0.82 (0.56–1.23) | 0.85 (0.55–1.30) |
| Height, cm | 164.0±8.4 | 162.1±8.8 | 0.0003 | 0.97 (0.96–0.99) | 0.97 (0.94–0.99) | 0.97 (0.94–0.99) | 0.97 (0.94–0.99) |
| Weight, kg | 64.3±11.1 | 62.5±11.6 | 0.0117 | 0.99 (0.98–1.00) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) |
| NIHSS score | 5.29±5.3 | 6.24±6.2 | 0.0063 | 1.03 (1.01–1.05) | |||
| NIHSS score category | 0.0056 | ||||||
| <8 | 287 (76.1) | 578 (68.3) | Reference | ||||
| 8–16 | 72 (19.1) | 189 (22.3) | 1.30 (0.96–1.77) | 1.22 (0.88–1.69) | 1.22 (0.88–1.69) | 1.22 (0.88–1.69) | |
| >16 | 18 (4.8) | 79 (9.3) | 2.18 (1.28–3.71) | 2.51 (1.44–4.37) | 2.50 (1.43–4.36) | 2.50 (1.44–4.35) | |
| Creatinine level, mg/dL | 0.97±0.34 | 1.03±0.61 | 0.0367 | 1.26 (0.96–1.65) | 1.27 (0.96–1.69) | 1.27 (0.96–1.69) | 1.28 (0.96–1.71) |
| CHADS2 score | 3.28±0.93 | 3.44±0.96 | 0.0065 | 1.20 (1.06–1.37) | 1.04 (0.85–1.27) | ||
| CHA2DS2-VASc score | 4.58±1.38 | 4.82±1.42 | 0.0061 | 1.13 (1.04–1.24) | 0.98 (0.82–1.16) | ||
| Congestive heart failure | 38 (10.0) | 104 (12.2) | 0.2661 | 1.29 (0.87–1.91) | |||
| Hypertension | 251 (66.2) | 591 (69.5) | 0.2617 | 1.16 (0.90–1.51) | |||
| Diabetes mellitus | 81 (21.4) | 234 (27.5) | 0.0231 | 1.39 (1.04–1.85) | 1.42 (1.05–1.93) | 1.36 (0.93–2.01) | 1.46 (1.01–2.10) |
| Vascular disease | 67 (17.7) | 143 (16.8) | 0.7067 | 0.93 (0.67–1.27) | |||
Data are n (%) or mean±SD values except where indicated otherwise. Model 1: adjusted for age, sex, height, weight, NIHSS score category, creatinine level, and diabetes (variables with p<0.1 based on protocol), Model 2: adjusted for age, sex, height, weight, NIHSS score category, creatinine level, diabetes, and CHADS2 score, Model 3: adjusted for age, sex, height, weight, NIHSS score category, creatinine level, diabetes, and CHA2DS2-VASc score.
*p value for unadjusted analysis: for categorical variables, chi-square test or Fisher's exact test; for continuous variables, t-test or Wilcoxon rank-sum test, †By logistic regression model; modeling the probability of TTR <60%.
NIHSS: National Institutes of Health Stroke Scale, OR: odds ratio, TTR: time in therapeutic range.
Warfarin-initiated cohort
The warfarin-initiated cohort comprised 1,814 patients, and their demographic and clinical characteristics are provided in Supplementary Table 1 (in the online-only Data Supplement). In this cohort, the mean TTR was 47.3% (95% CI, 46.2–48.3%). Of 42,634 INR measurements included in the TTR analysis, 44.6% were within the therapeutic range of 2.0–3.0 (Supplementary Table 2 in the online-only Data Supplement). Across the 16 centers, the mean TTR ranged from 35.2 to 57.3%, and the proportion of INR values of 2.0–3.0 ranged from 35.4 to 53.8% (Supplementary Table 3 in the online-only Data Supplement).
Discussion
This study shows that the quality of long-term oral anticoagulation with warfarin therapy is inadequate for secondary stroke prevention in Korean AF patients who have experienced stroke. The mean TTR was 49.1%, and only 31% of patients achieved TTR >60%, and 17% had TTR >70%. Given that no single center achieved the mean TTR of >60%, inadequate INR control is likely to be widespread. The proportion of INR values within the therapeutic range of 44.6% also indicates that anticoagulation with warfarin is inadequate in Korean stroke patients with AF.
Dietary and genetic factors in Koreans might contribute to the current low TTR values. According to the Korean National Health and Nutrition Examination Survey, the estimated daily intake of vitamin K was 322.40±6.33 µg for men and 271.20±4.92 µg for women, and it increased significantly with age.5 This daily intake of Koreans was much greater than those estimated in the United Kingdom and the United States,15,16 and higher than those observed in Japanese subjects.17 CYP2C9 and VKORC1 polymorphisms are well known to influence the anticoagulation quality with warfarin. More than 90% of Koreans carry VKORC1 variants that lead to enhanced sensitivity to warfarin.7 In a previous study, the VKORC1 TT variant was an independent predictor for low TTR in Korean stroke patients.12 In addition, the frequency of the CYP2C9*3 allele, which is related to decreased warfarin metabolism, was higher in the Korean population than in other populations.6
The physician factor might also account for the current low TTR rate. Across all INR measurements, 41.7% were INR <2.0 but only 13.7% were >3.0. Therefore, Korean physicians might tend to avoid a high INR level because of bleeding concerns due to Asian populations being at a greater risk of major bleeding with anticoagulation and having a higher incidence rate of primary intracerebral hemorrhage compared to non-Asian populations. This finding is in accordance with data from NOAC trials showing that Asians had higher proportions of INR <2.0 and lower proportions of INR >3.0 compared to non-Asians.18 Since the target INR of 2.0–3.0 has been established in Caucasian populations, some physicians might consider applying the Japanese guidelines that recommend a target INR of 1.6–2.6 for elderly patients aged >70 years.19 However, this recommendation was based on a small randomized trial (Japanese Nonvalvular Atrial Fibrillation–Embolism Secondary Prevention Cooperative Study) involving only 115 patients and another small retrospective study of 203 patients,20,21 and 115 of the 203 patients in the latter study were those included in the former study. Recent data from a large registry in Japan support the lower target INR of 1.6–2.6 for efficacy and safety, but that study only used the baseline INR level and did not analyze follow-up INR or TTR values.22 Because there is no strong evidence for recommending the lower target INR of 1.6–2.6 in Asian populations, our study used the target INR of 2.0–3.0 for the current TTR analysis.
The low quality of warfarin therapy observed in this study suggests that warfarin treatment does not effectively prevent recurrent stroke or systemic embolism in Korean stroke patients. In a post-hoc analysis of Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE-W), patients who were treated at centers with TTR <65% did not benefit from warfarin over clopidogrel plus aspirin, and the minimum TTR threshold driven from a population-average model was about 58%.23 In the present study, only one center exceeded the minimum TTR threshold of 58%. Furthermore, in a large UK population-based study, compared to patients with no antithrombotic therapy, those with TTR >60% had a significant reduction of stroke risk, but those with TTR <50% had a higher risk of stroke.4 Applied to our results, only 31% of our patients were likely to benefit from warfarin therapy, whereas 51.2% of our patients who achieved TTR <50% might be exposed to a higher risk with warfarin therapy compared to no antithrombotic treatment. Therefore, the current findings indicate that the proportion of patients endangered by warfarin might be greater than the proportion of patients who benefit from warfarin.
To improve the target INR achievement, 1) anticoagulation clinics, 2) patient self-testing and self-management using a point-of-care monitor, or 3) computer-software-assisted dose adjustment are recommended.24 However, the accessibility to such approaches is very limited in real-world practice in Korea. Even in the present study in which academic and teaching hospitals participated, only 3 out of 16 centers were able to provide an anticoagulation clinic service. Although the centers with an anticoagulation clinic service had numerically higher TTRs compared to the centers without such a service, their mean TTRs were still less than 60%. Therefore, in Korea, the provision of an anticoagulation clinic service alone would not ensure an improvement in the quality of anticoagulation with warfarin. Moreover, the poor INR control in Korea is not limited to stroke patients. In Korean AF patients who took warfarin for more than 1 year and were largely managed by cardiologists, the proportion of INR values within the target range of 2.0–3.0 was 42.4%, which was slightly lower than observed in the present study; however, that study did not analyze TTR.25
Given the poor INR control observed in the present study, warfarin would not be an ideal oral anticoagulant in Korean AF patients. In the pivotal NOAC trials, for most of the efficacy and safety endpoints, the absolute risk reductions with NOACs compared to warfarin were numerically greater in Asians than in non-Asians. In analyses restricted to Asian populations, compared to warfarin, all NOACs had significantly lower risks of intracranial hemorrhage, and most NOACs had lower risks of both major bleeding and any bleeding.26 Although NOACs are more expensive than warfarin, cost-effective analyses showed that NOACs were more cost-effective than warfarin in various health-care settings.27 Because the risks of thromboembolism and major bleeding are higher in Asians than non-Asians, NOACs might be particularly cost-effective in Asians; for example, a Taiwan study found that dabigatran was highly cost-effective in a clinical practice setting.28
When we explored the predictors of poor anticoagulation control in several multivariable models, severe stroke indicated by an NIHSS score of >16 at admission was consistently associated with TTR <60%. Patients with severe stroke at presentation were likely to have more severe residual disability, which might contribute to poor INR control. In a large observation study involving 23,425 AF patients, TTR was significantly lower in patients with prior stroke than in those without prior stroke.29 A previous study that derived the SAMe-TT2R2 score showed that clinical factors of female sex, age <60 years, multiple comorbidities, treatment with interacting medications, tobacco use, and nonwhite race were associated with poor INR control. However, the SAMe-TT2R2 score was derived from and validated in AF populations in which more than 85% of patients had no history of stroke.30 In a Korean study that enrolled AF patients with ischemic stroke, the SAMe-TT2R2 score as well as other clinical factors were not associated with the quality of anticoagulation control as measured by TTR, whereas only VKORC1 genotype was an independent predictor. Because of the retrospective nature of the present study, we were not able to fully evaluate the genetic factors and clinical factors included in the SAMe-TT2R2 score. Furthermore, we could not adjust unmeasured confounders such as physician attitude, patient compliance, and diet change that potentially influence INR control. Therefore, our findings should be interpreted as exploratory.
This study has several limitations. The included patients were exclusively treated in academic or teaching hospitals, which limits the generalizability of our findings. The mean TTR of 49.1% was obtained from the long-term warfarin-treated cohort, and was lower in the warfarin-initiated cohort than in the warfarin-treated cohort alone. Of 2,431 patients who received warfarin at least once, 617 patients were not eligible for the TTR analysis, largely due to warfarin therapy being administered for less than 7 days or consecutive INR values being less than 3 after the adjustment period. Therefore, the overall quality of oral anticoagulation with warfarin would be worse for patients who are indicated for anticoagulation. This study had a retrospective design, and hence we were not able to analyze whether a low TTR was associated with clinical events. Finally, the findings from the exploration of independent factors for poor INR control should be interpreted with caution.
In conclusion, this study showed that, in Korean AF patients who had experienced stroke, the quality of oral anticoagulation with warfarin was low and might be inadequate to effectively prevent recurrent stroke or systemic embolism. Unless the quality of warfarin therapy substantially improves, a NOAC might be a preferred oral anticoagulant for secondary stroke prevention in Korean AF patients.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2017.13.3.273.
Demographics and baseline characteristics of the warfarin-initiated cohort
TTR and INR included in TTR analysis
TTR and INR 2.0–3.0 proportion included in TTR analysis of the warfarin-initiated cohort by each center
References
- 1.Jung KH, Lee SH, Kim BJ, Yu KH, Hong KS, Lee BC, et al. Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean Stroke Registry Study (secular trends in Korean stroke) Circ Cardiovasc Qual Outcomes. 2012;5:327–334. doi: 10.1161/CIRCOUTCOMES.111.963736. [DOI] [PubMed] [Google Scholar]
- 2.Kim BJ, Park JM, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. 2015;17:38–53. doi: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with nonvalvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–477. doi: 10.1136/hrt.2004.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost. 2011;106:968–977. doi: 10.1160/TH11-05-0353. [DOI] [PubMed] [Google Scholar]
- 5.Kim ES, Kim MS, Na WR, Sohn CM. Estimation of vitamin K intake in Koreans and determination of the primary vitamin K-containing food sources based on the fifth Korean National Health and Nutrition Examination Survey (2010-2011) Nutr Res Pract. 2013;7:503–509. doi: 10.4162/nrp.2013.7.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, et al. Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol. 2005;60:418–422. doi: 10.1111/j.1365-2125.2005.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaikwad T, Ghosh K, Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb Res. 2014;134:537–544. doi: 10.1016/j.thromres.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 9.Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2:e000067. doi: 10.1161/JAHA.112.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallentin L, Lopes RD, Hanna M, Thomas L, Hellkamp A, Nepal S, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation. 2013;127:2166–2176. doi: 10.1161/CIRCULATIONAHA.112.142158. [DOI] [PubMed] [Google Scholar]
- 11.Shimada YJ, Yamashita T, Koretsune Y, Kimura T, Abe K, Sasaki S, et al. Effects of regional differences in Asia on efficacy and safety of edoxaban compared with warfarin--insights from the ENGAGE AFTIMI 48 trial. Circ J. 2015;79:2560–2567. doi: 10.1253/circj.CJ-15-0574. [DOI] [PubMed] [Google Scholar]
- 12.Park YK, Lee MJ, Kim JH, Kim SJ, Kim JS, Lee SY, et al. Lack of association of clinical factors (SAMe-TT2R2) with CYP2C9/VKORC1 genotype and anticoagulation control quality. J Stroke. 2015;17:192–198. doi: 10.5853/jos.2015.17.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosendaal FR, Cannegieter SC, van der Meer MJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 14.Dixon WJ, Massey FJ. Introduction to Statistical Analysis. 4th ed. New York: McGraw-Hill; 1983. pp. 105–107. [Google Scholar]
- 15.Thane CW, Bolton-Smith C, Coward WA. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986-7 and 2000-1. Br J Nutr. 2006;96:1105–1115. doi: 10.1017/bjn20061971. [DOI] [PubMed] [Google Scholar]
- 16.Fulgoni VL, 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr. 2011;141:1847–1854. doi: 10.3945/jn.111.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboyama-Kasaoka N, Takizawa A, Tsubota-Utsugi M, Nakade M, Imai E, Kondo A, et al. Dietary intake of nutrients with adequate intake values in the dietary reference intakes for Japanese. J Nutr Sci Vitaminol (Tokyo) 2013;59:584–595. doi: 10.3177/jnsv.59.584. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111:789–797. doi: 10.1160/TH13-11-0948. [DOI] [PubMed] [Google Scholar]
- 19.JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2008): digest version. Circ J. 2010;74:2479–2500. doi: 10.1253/circj.cj-88-0001. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T Japanese Nonvalvular Atrial Fibrillation-Embolism Secondary Prevention Cooperative Study Group. Optimal intensity of warfarin therapy for secondary prevention of stroke in patients with nonvalvular atrial fibrillation: a multicenter, prospective, randomized trial. Stroke. 2000;31:817–821. doi: 10.1161/01.str.31.4.817. [DOI] [PubMed] [Google Scholar]
- 21.Yasaka M, Minematsu K, Yamaguchi T. Optimal intensity of international normalized ratio in warfarin therapy for secondary prevention of stroke in patients with non-valvular atrial fibrillation. Intern Med. 2001;40:1183–1188. doi: 10.2169/internalmedicine.40.1183. [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Okumura K, Atarashi H, Yamashita T, Origasa H, Kumagai N, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM registry. Circ J. 2013;77:2264–2270. doi: 10.1253/circj.cj-13-0290. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 24.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 25.Shin HW, Kim YN, Bae HJ, Lee HM, Cho HO, Cho YK, et al. Trends in oral anticoagulation therapy among Korean patients with atrial fibrillation: the KORean atrial fibrillation investigation. Korean Circ J. 2012;42:113–117. doi: 10.4070/kcj.2012.42.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 27.Kasmeridis C, Apostolakis S, Ehlers L, Rasmussen LH, Boriani G, Lip GY. Cost effectiveness of treatments for stroke prevention in atrial fibrillation: focus on the novel oral anticoagulants. Pharmacoeconomics. 2013;31:971–980. doi: 10.1007/s40273-013-0090-1. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Yang YH, Chen JH, Lin LJ. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation in Taiwan. Thromb Res. 2014;133:782–789. doi: 10.1016/j.thromres.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WW, Choi JC, Vanderpoel J, Damaraju CV, Wildgoose P, Fields LE, et al. Impact of co-morbidities and patient characteristics on international normalized ratio control over time in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2013;112:509–512. doi: 10.1016/j.amjcard.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest. 2013;144:1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographics and baseline characteristics of the warfarin-initiated cohort
TTR and INR included in TTR analysis
TTR and INR 2.0–3.0 proportion included in TTR analysis of the warfarin-initiated cohort by each center