Abstract
Whether inhibition is a unitary or multifaceted construct is still an open question. To clarify the electrophysiological distinction among the different types of inhibition, we used a modified flanker paradigm, in which interference inhibition, rule inhibition, and response inhibition were compared to non-inhibition condition. The results indicated that, compared to the non-inhibition condition (1) the interference inhibition condition induced larger negativities during N2 epoch at the frontal region, (2) the rule inhibition condition elicited a larger N1 at the posterior region, followed by a larger P3a at the frontal region, reflecting the function of proactive cognitive control in the new stimulus-reaction (S-R) association, and (3) the response inhibition condition evoked a larger P3b at the posterior region, reflecting the process of suppressing the old response and reprogramming the new action. These findings provide new evidence that distinct neural mechanisms underlie different types of inhibition.
Introduction
Inhibition is a core component of executive function1–6. Inhibition refers to the processes of suppressing information that is not (or no longer) relevant for the current processing in working memory7, 8. Inhibition also refers to suppressing a stimulus that pulls for a competing response so as to carry out a primary response or an appropriate action, suppressing distractors that might slow the primary response, or suppressing internal stimuli that may interfere with the current operations of working memory4–6, 8–10.
Whether inhibition is a unitary construct or a multidimensional construct has long been controversial. Recently, several theorists have proposed that inhibition-related processes are a family of functions rather than a single unitary construct11, 12. For example, Shiffrin and Schneider13 initially proposed controlled inhibition (the conscious and deliberate suppression of irrelevant stimuli or responses) and automatic inhibition (occurs without awareness and appears to be involuntary). Harnishfeger14 proposed that inhibition processes could be classified according to the following three dimensions: 1) whether they are intentional or unintentional, 2) whether inhibition takes place at a behavioral or cognitive level, and 3) the distinction between inhibition and resistance to interference. Nigg11 listed four types of inhibitory control functions, including interference control, cognitive inhibition, behavioral inhibition, and oculomotor inhibition. Recently, other types of inhibition including response or motor inhibition, lateral inhibition, prepulse inhibition, inhibition of return, and semantic inhibition have also been proposed6, 15–23. Moreover, Duncan’s Multiple Demand system theory24 and Niendam’s meta-analysis of fMRI data25 suggest a common network that underlies diverse inhibitory processes; however, a number of imaging studies found that the activation within the network differs between different types of inhibitory processes26–29.
Although inhibition-related functions may be conceptually distinguishable, the neural distinctions between different types of inhibition are not well understood. Recently, a few studies compared the neural mechanism of interference control with response inhibition. For example, Brydges et al.30 combined a flanker task and go/no-go task and revealed that slightly different brain areas are activated during interference suppression and response inhibition, supporting a neuroanatomical division12, 31–33. Groom and Cragg34 compared the response inhibition and conflict/flanker suppression in a hybrid go/no-go flanker task. They found that N2 amplitude was larger in incongruent than congruent trials; however, it was not enhanced by response inhibition when the stimulus array was congruent. Instead, P3 amplitude was greater in trials requiring response inhibition34.
In the current study, we attempted to test the distinctions among three types of inhibitions using a modified version of flanker task. The three inhibitions were flanker inhibition (i.e., interference control), rule (cognitive) inhibition, and response inhibition. It is necessary to note that, the response inhibition in the current study is different from that in the go/no-go or stop signal task. Response inhibition here refers to the process of implementing one response while inhibiting other alternative responses when there were two or more competitive responses35.
The flanker task is a typical task demanding interference control36–38. In a letter flanker task, participants have to focus on a central letter and ignore surrounding letters (i.e., the flankers) that could be identical (i.e., congruent) or different (i.e., incongruent) to the central letter. Increased reaction times (RTs) and decreased accuracies are observed for incongruent as compared to congruent stimuli37. It has been found that the amplitude of the N2 component, peaking 200–400 ms post-stimulus and typically maximum at the frontocentral scalp locations, is enhanced following incongruent relative to congruent arrays34, 39–42. The enhanced N2 have been interpreted as the suppression of irrelevant stimuli42–45.
Cognitive/rule inhibition has been widely studied in directed forgetting or set-shifting task11, 46, in which the suppression of irrelevant information or invalid rule from working memory is required. For example, in the set-shifting task, the task-set reconfiguration requires the inhibition of the prior task-set46, 47. Although psychometric studies suggest that set-shifting may be conceived of as separate executive functions2, 48, it is agreed that shifting performance necessarily involves inhibitory control25, 49–53. Previous event related potential (ERP) studies on set-shifting have shown that a number of ERP components, including the N1, P2, N2, and P3, are evoked by shifting trials. N1 at parietal sites is associated with attentional selection processes such as focusing on task-relevant stimuli54–56, and variation in N1 amplitude reflects a change in demand of transient inhibition by the proactive attention control57. The P2 may be sensitive to an early task-set updating process that would rapidly “detect” a relevant change in the task when a shift is involved58, 59. The shift-sensitive increase in N2 amplitude has been considered as an index of a process of inhibition of the currently irrelevant task-set60 and intentional control of motor responses, which is applied to enable the appropriate response61. Moreover, previous studies also revealed P3 attenuation on shifting trials, which is possibly related to higher task complexity compared to repeat trials59, 61, 62. P3 amplitude is larger in switch trials than non-switch trials when participants are required to keep more information active in working memory63–65.
If the current trial in a flanker task had the identical stimulus and the same response as the preceding trial, there would be a response repetition benefit66–70. In the classical choice RT tasks, identical stimulus-reaction (S-R) trials are usually associated with particularly fast responses, which is suggested to reflect remission of the same and the most recent response on detection of an identical stimulus reoccurrence71–73. In contrast, when the stimulus is different from the preceding trial, the preceding response might be partially primed before preparing the current response73–76. Thus, an inhibition of the preceding response and the subsequent reprogramming of new response would be triggered35, 77. It has been shown that P300 amplitudes under the response-changed condition were enhanced as compared to the response-unchanged condition35.
The goal of the current study was to elucidate the neural distinction between the above-mentioned three inhibition-related functions using a modified flanker task, in which the rules regarding the S-R association were switched unpredictably. Previous studies had adopted the flanker task in task-switching paradigm to address the performance monitoring, behavior adaptation, conflict adaptation, attentional switching, and task-set inhibition41, 61, 78–82. To our knowledge, no studies have explicitly compared these two inhibition functions (rule inhibition and flanker inhibition) and response inhibition in the same task. Particularly, we defined a baseline condition, under which the rule, stimulus, and response in the current trial were identical to the directly preceding trial. Besides, the flankers did not appear in the baseline condition.
Previous studies on flanker control usually considered whether the current trial is a congruent trial or not and did not exclude the influence of the preceding incongruent trial on the current trial. In our opinion, if the preceding trial was an incongruent trial, there would be a conflict-adaptation effect on the current trial, which was demonstrated by numerous studies83–86. Because of this consideration and the comparability between experimental conditions, we selected the incongruent trials that preceded with a congruent trial as the flanker trial in the current study. Thus, the flanker condition in present study can be seen as a pure flanker. Previous studies have found that the frontal N2 is more negative in the flanker condition relative to non-flanker34, 39–42. Accordingly, the flanker inhibition condition in the present study was also expected to induce a more negative N2 than the baseline.
Compared to the interference (flanker) inhibition, the rule inhibition is one type of executive control accompanied by working memory manipulation because subjects need to suppress the old rule in working memory while turning to and maintaining the new rule, so as to implement the new rule to the new stimuli quickly and accurately. Previous studies found that rule inhibition was mainly reflected during the N2-P3 epoch60–65, and particularly the frontal P3a evoked by attention-switching for incoming stimuli87–91. Hence, we expected that the rule inhibition trials might evoke a larger P3a than the baseline, reflecting the process of constructing and keeping a new S-R association in the working memory92. Moreover, in our study, the rule inhibition processes started earlier from the negative feedback of the preceding trial till the offset of the current trial. The proactive control is presumably involved in this somewhat long period of inhibition, and the central execution system might allocate more attention resources to the new task-set and stimuli. Correspondingly, we expected that, compared with the baseline, the rule inhibition condition might also elicit greater amplitudes in the early ERP components, such as N1 that was associated with attention allocation54–56 and P2 that was sensitive to the early rule change58, 59.
Previous relevant studies did not dissociate the response inhibition from flanker inhibition, so it is not easy to provide a specific anticipation about the ERPs characteristic of response inhibition in flanker task. Fleming, Mars, Gladwin, and Haggard (2009)35 found that response-change and response-unchanged conditions had distinct electrophysiological responses during the P3 component35. Accordingly, we expected that, in comparison to the baseline condition, the response inhibition condition would elicit a large P3b at the posterior brain, indexing the process of response inhibition and the reprogramming of a new action.
Results
Behavioral Data
Accuracy was calculated for each condition as the mean percentage of the correct responses of all trials. RTs were calculated as the mean RT for correct responses in each condition. RTs and accuracies for each condition (flanker inhibition, rule inhibition, response inhibition, and non-inhibition) are shown in Table 2 . The repeated measures analysis of variance (ANOVA) analysis on RTs and accuracies both showed a significant main effect of condition [F RT(3,57) = 22.78, p < 0.001, ƞ 2 = 0.55; F accuracy(3,57) = 21.42, p < 0.001, ƞ 2 = 0.53]. Post-hoc tests revealed that the RTs for flanker inhibition and response inhibition conditions were significantly longer than the RTs in non-inhibition condition respectively (p response = 0.022, p flanker < 0.001). The accuracies for flanker inhibition and rule inhibition conditions were significantly lower than that of non-inhibition condition respectively (both p < 0.001).
Table 2.
Mean (standard deviation) reaction time and accuracy for different conditions.
| Condition | Flanker Inhibition | Rule Inhibition | Response Inhibition | Non-inhibition |
|---|---|---|---|---|
| RT (ms) | 559 (11) | 485 (18) | 490 (10) | 470 (9) |
| Accuracy (%) | 87.0 (0.02) | 87.8 (0.02) | 95.3 (0.01) | 96.8 (0.01) |
ERP Data
The grand-average ERP waveforms and the different waveforms and topographic maps are shown in Figs 1 and 2. The ANOVA on the posterior N1 amplitude revealed a main effect of condition, F(3,57) = 5.12, p = 0.010, η 2 = 0.21. Post-hoc tests revealed that the rule inhibition condition elicited a larger N1 than non-inhibition condition (p = 0.038), whereas both the flanker inhibition and response inhibition conditions had no significant differences from the non-inhibition condition respectively (both p > 0.05); there was no significant difference between other pairs of conditions (all p > 0.05). The electrode also had a main effect, F(15,285) = 14.88, p < 0.001, η 2 = 0.44. There was an interaction between condition and electrode, F(45,855) = 6.14, p < 0.001, η 2 = 0.24 (Table 3). The simple effect analysis found that, larger N1 amplitudes were evoked by rule inhibition condition as compared with non-inhibition condition at the parietal electrodes including P3, P5, PO3, P4, P6, and PO4 (all p < 0.05); and increased N1 was evoked by rule inhibition condition as compared with the response inhibition at the most of right parietal-occipital electrodes including P2, P4, P6, PO4, and PO3 (all p < 0.05). Please see the supplementary Table S1 for the detailed results.
Figure 1.
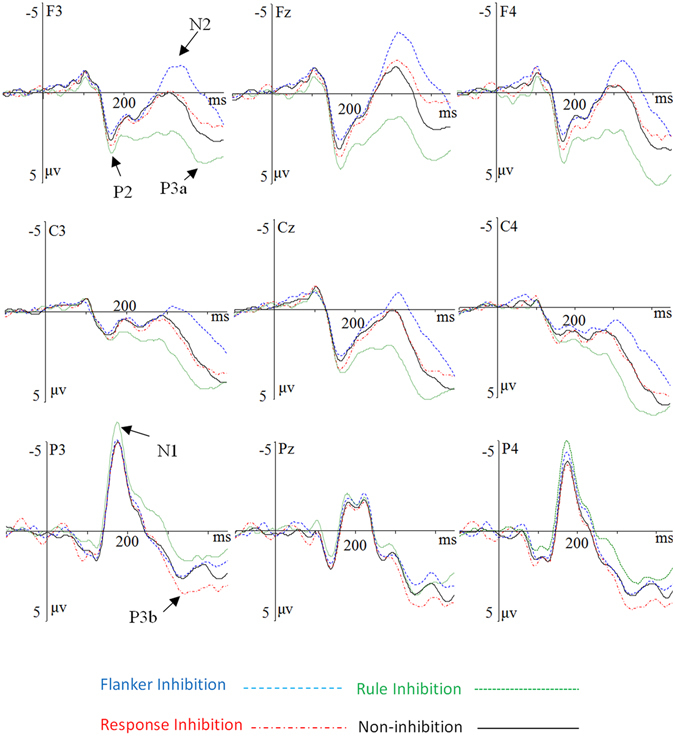
Grand-averaged ERP waveforms. The grand-averaged ERP waveforms are shown for each condition according to nine sites. The zero point on the time axis indicates the stimulus onset.
Figure 2.
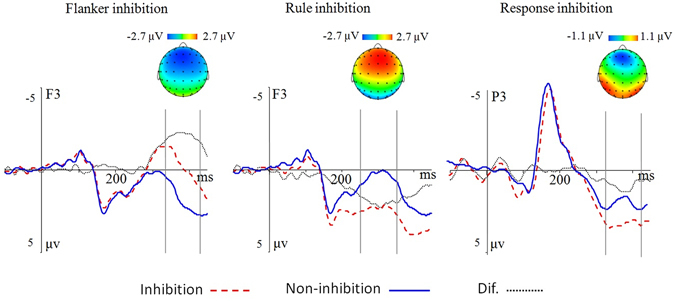
The difference waveforms and topographic map. The topographic map of difference waveforms under the three conditions. The zero point on the time axis indicates the onset of stimuli.
Table 3.
Statistical (F and P) values of ANOVA on each components.
| Time Window (ms) | Component | Main effect | Interaction effect | ||||
|---|---|---|---|---|---|---|---|
| Condition | Electrode | Condition × electrode | |||||
| F | P | F | P | F | P | ||
| 150–200 | Posterior N1 | 5.12 | 0.010 | 14.88 | 0.000 | 6.14 | 0.000 |
| 270–350 | Frontal P2 | 7.59 | 0.002 | 7.09 | 0.000 | 1.83 | 0.074 |
| Frontal N2 | 33.99 | 0.000 | 8.47 | 0.000 | 6.38 | 0.000 | |
| 350–450 | Frontal P3a | 38.24 | 0.000 | 6.94 | 0.000 | 3.57 | 0.001 |
| Posterior P3b | 4.89 | 0.017 | 6.52 | 0.001 | 9.33 | 0.000 | |
The ANOVA on the frontal P2 component showed a main effect of condition, F(3,57) = 7.59, p = 0.002, η 2 = 0.29. Post-hoc tests revealed that the rule inhibition condition elicited larger P2 amplitudes than the non-inhibition condition (p = 0.010) and flanker inhibition condition (p = 0.024), whereas the flanker inhibition and response inhibition conditions had no significant difference from the non-inhibition condition respectively (both p > 0.05); there was no significant difference between other pairs of conditions (all p > 0.05). The electrode also had a main effect, F(15,285) = 7.09, p < 0.001, η 2 = 0.27. There was no interaction between condition and electrode.
For the frontal N2, the results of ANOVA revealed a main effect of condition, F(3,57) = 33.99, p < 0.001, η 2 = 0.64. Post-hoc tests revealed that the flanker inhibition condition elicited the largest N2 amplitudes than other conditions (all p < 0.001); while the rule inhibition condition elicited the smallest N2 than other conditions (all p < 0.001). The response inhibition condition had no significant difference from the non-inhibition condition (p > 0.05). The electrode also had a main effect, F(15,285) = 8.47, p < 0.001, η 2 = 0.31. There was an interaction between condition and electrode, F(45,855) = 6.38, p < 0.001, η 2 = 0.25. The simple effect analysis found that flanker inhibition condition evoked larger N2 amplitudes than the response inhibition and rule inhibition conditions at all of the selected electrodes (all p < 0.01), and evoked an increased N2 than the non-inhibition condition at the most of electrodes excluding FC5; the rule inhibition condition had significantly attenuated N2 amplitude than the non-inhibition and the response inhibition condition at most of electrodes excluding FC6 (all p < 0.05).
The results of ANOVA revealed a main effect of condition during the P3a component, F(3,57) = 38.24, p < 0.001, η 2 = 0.67. Post-hoc tests showed that, the rule inhibition condition elicited a larger P3a than the non-inhibition condition and other two types of inhibitions (all p < 0.001), whereas the frontal P3a in the response inhibition condition did not differ significantly from that of the non-inhibition condition (p > 0.05). The electrode also had a main effect, F(15,285) = 6.94, p < 0.001, η 2 = 0.27. There was an interaction between condition and electrode, F(45,855) = 3.57, p < 0.001, η 2 = 0.16. The simple effect analysis did not revealed any suggestive results.
The ANOVA on the parietal P3b component showed a main effect of condition, F(3,57) = 4.89, p = 0.017, η 2 = 0.21. Post-hoc tests showed that the response inhibition condition elicited a larger P3b than the non-inhibition (p = 0.030) and the flanker inhibition condition (p = 0.002), whereas both the rule inhibition and flanker inhibition conditions overlapped with the non-inhibition condition (both p > 0.05). The electrode also had a main effect, F(15,285) = 6.52, p < 0.001, η 2 = 0.26. There was an interaction between condition and electrode, F(45,855) = 9.33, p < 0.001, η 2 = 0.33. The simple effect analysis revealed the marked difference between the response inhibition and non-inhibition conditions at the following sites, P3, P5, P6, and PO4 (all p < 0.05); the marked difference between the response inhibition and the flanker inhibition condition at the following sites, P1, P2, P3, Pz (all p < 0.05); and the marked difference between the response inhibition and rule inhibition at the P3, P5, PO3, P4, P6, and PO4 (all p < 0.05).
Discussion
In the present study, we recorded the ERPs evoked by different types of inhibitions in a modified Eriksen Flanker task. The RTs for the response inhibition and flanker inhibition trials were significantly longer than that of the non-inhibition trials. The accuracies for the flanker inhibition and rule inhibition conditions were significantly lower than that of the non-inhibition condition. The results of the behavior data indicates a profound increase in RT and a decrease in accuracy under the flanker inhibition condition, which is consistent with the typical finding on the flanker congruency effect36–38, 93. Compared to the non-inhibition condition, the rule inhibition trials were less correctly responded to, which was in line with the results of previous studies46, 94–96. The RTs of rule inhibition trials had no significant difference from the non-inhibition condition, which is different from the results of the previous studies46, 78. One possibility is that there is enough preparation time (at least 1800 ms between switching cue and target stimulus) to reduce the switch cost39, 62, 96, 97. Another possibility is that participants received the rule-switch signal via the negative feedback (e.g., red square) to the preceding trial; hence, a proactive control was triggered before the presentation of the current trials98. It is likely that these two possible factors cause the non-significant difference in RT between the rule inhibition and non-inhibition conditions. The RTs for the response inhibition condition was significantly longer than the non-inhibition condition, reflecting response inhibition and response change66, 67, 77.
We also found different brain responses under different inhibition conditions. The flanker inhibition condition elicited a larger N2 component than the non-inhibition condition at the anterior brain scalp. The rule inhibition elicited a positive-going component during the N2-P3 time window, at the anterior region. Particularly, an increased N1 was observed at the posterior region in the rule inhibition condition. The response inhibition condition evoked a larger posterior P3b than the non-inhibition condition and other two types of inhibitions.
The flanker task in the present study was performed in a rule switch paradigm. Consistent with our expectation, the flanker inhibition condition elicited a more negative N2 than the non-inhibition condition and other two types of inhibitions at the frontal sites. The N2, with a frontocentral topography, is associated with cognitive control35, 42, 98, 99, particularly related to the suppression of irrelevant stimuli40. Kopp et al.42 found that the N2 amplitude was larger in incongruent trials than congruent trials39. The incongruence effect correlation with N2 component has been replicated by a number of studies30, 34, 37, 38, 42, 100. The larger N2 evoked by flanker inhibition in the present study is consistent with the explanation in terms of interference control17, 28 and possibly reflects the function of reactive control101–103.
Proactive control might contribute to the significant difference between the rule inhibition and non-inhibition conditions at the whole epoch. First, the electrophysiological differences initially emerged at the posterior N1 (150 ms) component. At this time window, the rule inhibition condition elicited a larger N1 than the non-inhibition condition. N1 component found in the anterior and posterior sites differed in latencies and the underlying cognitive functions55. The frontal N1, which peaked around 100 ms, is often correlated with response-related processes, and the parietal-occipital N1 peaked around 160 ms, which might reflect the discriminative process and perceptual load104–106. N1 also reflects the demands of the recruitment of cognitive control after stimulus presentation107. We speculated that proactive control triggered in the rule inhibition condition might evoke increased cognitive control on the processing of the target that followed the rule-switching signal; subsequently, increased N1 amplitudes were evoked in the rule inhibition condition as compared to the non-inhibition condition56, 57, 107–109.
Under the rule inhibition condition, there was no flanker in the current and preceding trials. However, the direction of all arrows in the current trial was opposite to that of preceding trial (e.g., from ↑↑↑↑↑ to ↓↓↓↓↓). We found that larger anterior P2 amplitudes were elicited in the rule inhibition condition as compared to the non-inhibition and the flanker inhibition conditions, which might reflect the detection and processing of the global change, such as arrow direction of the stimuli array54, 110. Compared to the non-inhibition condition and the other two types of inhibitory processes, the rule inhibition condition induced a larger frontal P3a. Squires, Squires, and Hillyard87 first distinguished the P300 into the frontal P3a (300 to 400 ms) and posterior P3b (350–600 ms). The P3a has a central peak and seems to reflect shifts or allocation of perceptual attention, and this process reflects the top-down attention switching for incoming stimuli over response processes to distracters88–90. This indicates that the P3a may reflect the activation of a more general brain switching mechanism responsible for processing both stimuli and task novelty, and that an enhanced P3a is elicited by switching of a rule and hence might be an index for the detection of changes in task sets, which may require changes in responses91. In the present study, increased P3a observed in the rule inhibition condition might reflect the detection of changes in task requirement and the preparation for action reorganization of the new task-set.
At the posterior site, the response inhibition condition evoked a more positive P3b than the non-inhibition condition and the flanker inhibition condition. P3b has been suggested to be associated with the execution of the task or the process of responding. Donchin111 suggested that the posterior P3b reflects working memory context updating, the comparison of the attributes of incoming stimuli with an internal model, and the subsequent revision of the model63, 64, 88, 90, 91, 112, 113. P3b amplitude is proportional to the total working memory required during task performance. In comparison to the non-inhibition condition, in which stimulus and response in the current trial were both identical to the preceding trial, the stimulus and response changed from the preceding trial to the current trial in the response inhibition condition. Consequently, participants should inhibit the repetition of the foregoing response, while reprogramming a new action in working memory. Thus, the increased P3b in the response inhibition condition might reflect the inhibition of the old S-R association as well as the reprogramming of the action during response shifting35.
In summary, we found the different behavioral and brain responses across different brain regions in different time windows. Rule inhibition begins early in the posterior N1 components, reflecting the function of proactive control. During the N2 time window, the flanker inhibition condition evoked a larger negativity than other conditions at the frontal sites, reflecting a reactive control process. In comparison with non-inhibition condition, the rule inhibition condition induced a larger P3a, while the response inhibition condition evoked a larger P3b during the P3 time window. These finding implies that different types of inhibitions might be controlled by different brain regions, at least with different temporal courses. However, the following limitations should be addressed in future studies. First, the present study compared each type of inhibition with the baseline respectively, and only analyzed the somewhat pure flanker trials. In fact, flanker inhibition stimuli also incorporate response inhibition. Future study might calculate a difference wave between flanker inhibition and response inhibition to remove any aspects of response inhibition that may be included in the flanker inhibition trials. Second, as compared to other two types of inhibitions, rule inhibition condition not only involved the process of inhibiting the old rule but also accompanied with the process of shifting to the new rule; hence, future studies are needed to distinguish the shifting component from the rule inhibition condition. Third, the rule inhibition condition was necessarily triggered by a negative feedback; hence, the method of elucidating the effect of feedback also remains unaddressed.
Methods
Participants
After obtaining informed written consent, 20 undergraduate volunteers (8 male subjects, aged from 18 to 21 years, mean (M) = 19.35 years, standard deviation (SD) = 1.17) participated in this study. All subjects reported to be right-handed, and all had normal or corrected-to-normal eyesight and reported no neurological disorders. All participants were paid for their participation. The study was approved by the Ethics Committee of Jiangxi Normal University (China), and the investigation was carried out in accordance with the latest version of the Declaration of Helsinki.
Materials and procedures
In a modified Eriksen-Flanker task76, five vertical direction arrow-strings consisting of “↑” and “↓” were used as congruent (↑↑↑↑↑, ↓↓↓↓↓) and incongruent stimuli (↓↓↑↓↓, ↑↑↓↑↑). These stimuli were presented in a pseudorandom order with 55% incongruent stimuli of all trials. In terms of the relationship between the current trial and the directly preceding trial, the following four types of trials were defined. In the flanker inhibition trials, the preceding stimulus was a congruent one, whereas the current stimulus was an incongruent trial with the same S-R rule and the same button response to the preceding trial (Table 1). When an incongruent stimulus appeared in the current trial, if the S-R rule or the correct response button was not the same as that in the preceding trial, or if the preceding trial was not a congruent trial, these trials were not defined as flanker inhibition trials. In the rule inhibition trials, the first trial followed the switch signal and was a congruent stimulus with the same button response as the preceding trial. In the response inhibition trials, both the preceding and the current stimuli were congruent trials; however, the corresponding buttons were different. In non-inhibition (baseline) trials, the current stimulus was a congruent trial (non-flanker) and was identical to the preceding trial.
Table 1.
Sample stimuli used in different conditions.
| Condition | Preceding stimulus | Feedback | Current stimulus |
|---|---|---|---|
| Flanker Inhibition | ↓↓↓↓↓↓ |

|
↑↑↓↑↑ |
| Rule Inhibition | ↑↑↑↑↑ |

|
↓↓↓↓↓ |
| Response Inhibition | ↑↑↑↑↑ |

|
↓↓↓↓↓ |
| Non-inhibition | ↑↑↑↑↑ |

|
↑↑↑↑↑ |
With each trial, participants were instructed to respond to the central arrow by a button press with either the “F” or the “J” on the keyboard. Participants were not informed about a fixed S-R association. Instead, they had to figure out the currently valid S-R association via feedback, which was presented after each response. The feedback stimulus consisted of a colored square, which was green in case of the correct response and red for an incorrect response. After 6–10 trials, the rule regarding the stimulus–response association was switched. The subjects were not explicitly informed about this switch but had to derive the new rule from the feedback. The first negative feedback (e.g., a red square) functioned as a rule-switch was defined as “switch signal” informing participants to use the opposite S-R rule in the following trials (Fig. 3). Participants were instructed to respond as quickly and accurately as possible and to focus only on the centrally presented arrow (i.e., to ignore the flanker). If the response time exceeded the deadline of 1 s, a feedback comprising a gray square was provided (Fig. 3).
Figure 3.
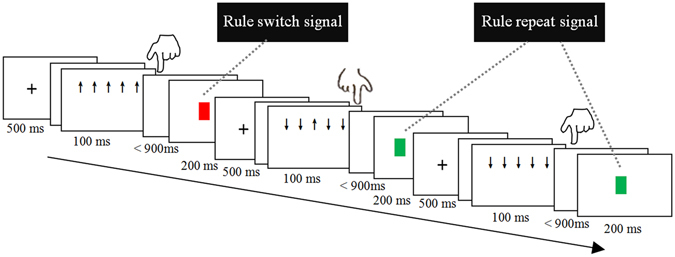
Experiment paradigm and procedure. A participant might respond to the central “↑” by pressing the key “F” with the left hand and received a red square (functioned as a negative feedback, signaling a switch of the task rule).
Tasks were presented using E-Prime presentation software (E-Prime 2.0 Professional, Psychology Software Tools, Inc.) with predefined stimuli lists. Stimuli were presented in white against a black background on a screen, viewed at a distance of approximately 60 cm. At the beginning of the task, participants performed training trials. Training was repeated until participants reached an accuracy of at least 60% correct responses. The formal experiment consisted of 1014 trials in total, which were organized into three blocks. Each block lasted about 15 min. The four types of trials (flanker inhibition, rule inhibition, response inhibition, and baseline) were assigned pseudorandomly into the same block, with 80 trials for each type. The same stimuli lists and the experimental procedure were presented to all participants.
A trial started with a cross fixation for 500 ms, followed by a blank screen for a random duration (800–1200 ms). Then, a stimulus was presented for 100 ms and followed by a response screen41, 61, 78. After the button press (or exceed 900 ms), a feedback square was displayed for 200 ms, finally, a blank screen (500–800 ms) appeared. The total experiment, including electroencephalogram (EEG) preparation, task training, task run, and breaks, took about 2.5 h.
Electrophysiological Recording and Analysis
The EEG was recorded using Brain Amp equipment (Brain Products, Germany) with 64 Ag/AgCl ring electrodes, which were mounted on a fabric cap according to the extended 10–20 system. The online reference electrode was placed over FCz, and the ground electrode was placed over AFz. An electrode placed under the right eye (EOG) allowed the monitoring of blinks and vertical eye movements. The impedance at each electrode was kept below 10 kΩ. Raw data were band-pass filtered between 0.01–250 Hz and digitized at a sampling rate of 500 Hz. The offline preprocessing of the EEG signal was performed in Brain Vision Analyzer 2.1 (Brain Products Gmb H). Blinks, ocular movements, and muscle artifacts were detected and removed using an independent component analysis (ICA), implemented in the Brain Vision Analyzer software. Next, extracted epochs (from −100 to 450 ms) of the correct trials were time-locked with the stimuli. The resulting data were baseline-corrected using windows from −100 to 0 ms. In addition, trials containing further artifacts were removed using an automatic procedure. The automatic detection criteria included an absolute difference between two sampling points exceeding 30 μV/ms, peak-to-peak deflections in a segment exceeding ±80 μV within intervals of 200 ms, amplitudes exceeding a value of ±80 μV, and activity lower than 0.1 μV within intervals of 200 ms. Then, data were re-referenced to the average of the two mastoid channels (TP9 and TP10), which are typically used as references in the ERP literature on task-switching. The averaging procedure was performed on the extracted epoch. The single-subject ERP averages for each of the four conditions were used for further analysis. The two mastoid channels were excluded from the analysis. In the final data set, at least 67 artifact-free trials per task condition were contained.
The stimulus-locked ERPs were measured at the following 32 electrodes: AF3, F1, F3, F5, FC1, FC3, FC5, AF4, F2, F4, F6, FC2, FC4, FC6, Fz, FCz, CP1, CP3, CP5, P1, P3, P5, PO3, CP2, CP4, CP6, P2, P4, P6, PO4, CPz, and Pz. Based on the relevant literature34, 36, 63, 64, 104 and a visual inspection of the grand-averages, the following ERP components were selected. The frontal P2 and posterior N1 were measured over the time window ranging from 150–200 ms after stimuli onset. The frontal N2 was measured during the 270–350-ms time window. The frontal P3a and posterior P3b both measured at 300–450 ms time window. No obvious peaks were found for some conditions in some time windows (e.g., in the P3a time window, only rule inhibition had a distinct peak), and the visual inspection showed that the latency difference was not obvious; hence, we did not analyze the latency of ERPs. The mean amplitudes of each component were all analyzed using two-factor ANOVA. The mean amplitudes of the frontal P2, N2, and P3a were subjected to a 4 (condition) × 16 (electrode: AF3, F1, F3, F5, FC1, FC3, FC5, AF4, F2, F4, F6, FC2, FC4, FC6, Fz, and FCz) repeated measures ANOVA, respectively. The mean amplitudes of the posterior N1, P3b were submitted to a 4 (condition) × 16 (electrode: CP1, CP3, CP5, P1, P3, P5, PO3, CP2, CP4, CP6, P2, P4, P6, PO4, CPz, and Pz) repeated measures ANOVA, respectively. Greenhouse-Geisser corrections were performed on the p values where necessary. Bonferroni correction was used for the multiple comparison.
Electronic supplementary material
Acknowledgements
This study was supported by grant of National Natural Science Foundation of China (NSFC, 31571118), and the graduate innovation fund project of Jiangxi provincial education office.
Author Contributions
X.L.F. and L.F.H. designed the experiment. R.M.F. and C.B.H. conducted the experiment and analyzed the data by supervision of L.F.H.; X.L.F. and L.F.H. wrote the manuscript; L.F.H. edited and revised manuscript; L.F.H. approved final version of manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04907-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Purpura DJ, Schmitt SA, Ganley CM. Foundations of mathematics and literacy: The role of executive functioning components. J Exp Child Psychol. 2017;153:15–34. doi: 10.1016/j.jecp.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 3.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Curr Dir Psychol Sci. 2012;21(1):8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn. Sci. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Jewsbury PA, Bowden SC, Strauss ME. Integrating the switching, inhibition, and updating model of executive function with the Cattell-Horn-Carroll model. J Exp Psychol Gen. 2016;145(2):220. doi: 10.1037/xge0000119. [DOI] [PubMed] [Google Scholar]
- 6.Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SP, Kipp K. The development of efficient inhibition: Evidence from directed-forgetting tasks. Dev Revl. 1998;18(1):86–123. doi: 10.1006/drev.1997.0445. [DOI] [Google Scholar]
- 8.Diamond JR, et al. Initial clinical sensitivity and acquired resistance to MET inhibition in MET-mutated papillary renal cell carcinoma. J Clin Oncol. 2013;31(16):e254–e258. doi: 10.1200/JCO.2012.46.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizio, A. A. & Dennis, N. A. Recollection after inhibition: The effects of intentional forgetting on the neural correlates of retrieval. Cogn Neurosci. 1–8 (2016). [DOI] [PubMed]
- 10.Brown WS, Perreault TS. Relation between temporal perception and inhibitory control in the Go/NO-Go task. Acta Psychol. 2017;173:87–93. doi: 10.1016/j.actpsy.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126(2):220. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- 12.Vuillier L, Bryce D, Szücs D, Whitebread D. The Maturation of Interference Suppression and Response Inhibition: ERP Analysis of a Cued Go/Nogo Task. PLoS One. 2016;11(11):e0165697. doi: 10.1371/journal.pone.0165697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychol Rev. 1977;84(2):127. doi: 10.1037/0033-295X.84.2.127. [DOI] [Google Scholar]
- 14.Harnishfeger, K. K. The development of cognitive inhibition. Interference and inhibition in cogn. 175–204 (1995).
- 15.Robinson OJ, Krimsky M, Grillon C. The impact of induced anxiety on response inhibition. Front. Hum. Neurosci. 2013;7:69. doi: 10.3389/fnhum.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridgeman B. Contributions of lateral inhibition to object substitution masking and attention. Vision Res. 2006;46(24):4075–4082. doi: 10.1016/j.visres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 18.Possin KL, Filoteo JV, Song DD, Salmon DP. Space-based but not object-based inhibition of return is impaired in Parkinson’s disease. Neuropsychologia. 2009;47(7):1694–1700. doi: 10.1016/j.neuropsychologia.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debruille JB. The N400 potential could index a semantic inhibition. Brain Res Rev. 2007;56(2):472–477. doi: 10.1016/j.brainresrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Yi Y, Friedman D. Event-related potential (ERP) measures reveal the timing of memory selection processes and proactive interference resolution in working memory. Brain Res. 2011;1411:41–56. doi: 10.1016/j.brainres.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Brown SW, Johnson TM, Sohl ME, Dumas MK. Executive attentional resources in timing: Effects of inhibitory control and cognitive aging. J Exp Psychol Human. 2015;41(4):1063. doi: 10.1037/xhp0000078. [DOI] [PubMed] [Google Scholar]
- 22.Rebetez MML, Rochat L, Billieux J, Gay P, Van der Linden M. Do emotional stimuli interfere with two distinct components of inhibition? Cognition Emotion. 2015;29(3):559–567. doi: 10.1080/02699931.2014.922054. [DOI] [PubMed] [Google Scholar]
- 23.Howard SJ, Johnson J, Pascual-Leone J. Clarifying inhibitory control: Diversity and development of attentional inhibition. Cognitive Dev. 2014;31:1–21. doi: 10.1016/j.cogdev.2014.03.001. [DOI] [Google Scholar]
- 24.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Ne. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampshire A, Sharp DJ. Contrasting network and modular perspectives on inhibitory control. Trends Cogn. Sci. 2015;19(8):445–452. doi: 10.1016/j.tics.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swick D, Chatham CH. Ten years of inhibition revisited. Front Hum Neurosci. 2014;8:329. doi: 10.3389/fnhum.2014.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erika-Florence M, Leech R, Hampshire A. A functional network perspective on response inhibition and attentional control. Nat Commun. 2014;5:4073. doi: 10.1038/ncomms5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brydges CR, et al. Dissociable components of cognitive control: an event-related potential (ERP) study of response inhibition and interference suppression. PloS one. 2012;7(3):e34482. doi: 10.1371/journal.pone.0034482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: A visual ERP investigation. Int J Psychophysiol. 2009;72(2):145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 32.van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive–compulsive spectrum disorders. Front Hum Neurosci. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brydges CR, Anderson M, Reid CL, Fox AM. Maturation of cognitive control: delineating response inhibition and interference suppression. PloS one. 2013;8(7):e69826. doi: 10.1371/journal.pone.0069826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groom MJ, Cragg L. Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain Cognition. 2015;97:1–9. doi: 10.1016/j.bandc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Fleming SM, Mars RB, Gladwin TE, Haggard P. When the brain changes its mind: flexibility of action selection in instructed and free choices. Cereb Cortex. 2009;19(10):2352–2360. doi: 10.1093/cercor/bhn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders AF, Lamers JM. The Eriksen flanker effect revisited. Acta Psychol. 2002;109(1):41–56. doi: 10.1016/S0001-6918(01)00048-8. [DOI] [PubMed] [Google Scholar]
- 37.Eriksen CW. The flankers task and response competition: A useful tool for investigating a variety of cognitive problems. Vis Cogn. 1995;2(2–3):101–118. doi: 10.1080/13506289508401726. [DOI] [Google Scholar]
- 38.Scharinger C, Soutschek A, Schubert T, Gerjets P. When flanker meets the n‐back: What EEG and pupil dilation data reveal about the interplay between the two central‐executive working memory functions inhibition and updating. Psychophysiology. 2015;52(10):1293–1304. doi: 10.1111/psyp.12500. [DOI] [PubMed] [Google Scholar]
- 39.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931. doi: 10.1037/0033-295X.111.4.931. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh S, Fang W. Elderly adults through compensatory responses can be just as capable as young adults in inhibiting the flanker influence. Biol Psychol. 2012;90(2):113–126. doi: 10.1016/j.biopsycho.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Purmann S, Badde S, Luna-Rodriguez A, Wendt M. Adaptation to frequent conflict in the Eriksen Flanker Task: An ERP study. J Psychophysiol. 2011;25:50–59. doi: 10.1027/0269-8803/a000041. [DOI] [Google Scholar]
- 42.Alguacil S, Tudela P, Ruz M. Cognitive and affective control in a flanker word task: Common and dissociable brain mechanisms. Neuropsychologia. 2013;51(9):1663–1672. doi: 10.1016/j.neuropsychologia.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Heil M, Osman A, Wiegelmann J, Rolke B, Hennighausen E. N200 in the Eriksen-task: Inhibitory executive process? J Psychophysiol. 2000;14(4):218. doi: 10.1027//0269-8803.14.4.218. [DOI] [Google Scholar]
- 44.Kopp B, Rist F, Mattler UWE. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33(3):282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 45.Luck, S. J. An introduction to the event-related potential technique. The MIT Press, Cambridge, Ma, pp. 84–85 (2005).
- 46.Monsell S. Task switching. Trends Cogn. Sci. 2003;7(3):134–140. doi: 10.1016/S1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 47.Allport, D. A., Styles, E. A. & Hsieh, S. Shifting intentional set: Exploring the dynamic control of tasks. In Umilta, C. & Moscovich, M. (Eds) Attention and performance XV (pp. 421–452) Cambridge, MA: MIT Press (1994).
- 48.Fournier-Vicente S, Larigauderie P, Gaonac’h D. More dissociations and interactions within central executive functioning: A comprehensive latent-variable analysis. Acta Psychol. 2008;129(1):32–48. doi: 10.1016/j.actpsy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Costa RE, Friedrich FJ. Inhibition, interference, and conflict in task switching. Psychon B Rev. 2012;19(6):1193–1201. doi: 10.3758/s13423-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 50.Kiesel A, et al. Control and interference in task switching—A review. Psychol Bull. 2010;136(5):849. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- 51.Koch I, Gade M, Schuch S, Philipp AM. The role of inhibition in task switching: A review. Psychon B Rev. 2010;17(1):1–14. doi: 10.3758/PBR.17.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Vandierendonck A, Liefooghe B, Verbruggen F. Task switching: interplay of reconfiguration and interference control. Psychol Bull. 2010;136(4):601. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- 53.Lien MC, Ruthruff E. Inhibition of task set: Converging evidence from task choice in the voluntary task-switching paradigm. Psychon B Rev. 2008;15(6):1111–1116. doi: 10.3758/PBR.15.6.1111. [DOI] [PubMed] [Google Scholar]
- 54.Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos T Roy Soc B. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luck SJ. Multiple mechanisms of visual-spatial attention: recent evidence from human electrophysiology. Behav Brain Res. 1995;71(1):113–123. doi: 10.1016/0166-4328(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav R. 2001;25(6):465–476. doi: 10.1016/S0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki K, Shinoda H. Transition from reactive control to proactive control across conflict adaptation: An sLORETA study. Brain Cognition. 2015;100:7–14. doi: 10.1016/j.bandc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Capizzi M, Fehér K, Penolazzi B, Vallesi A. Task-switching preparation across semantic and spatial domains: an event-related potential study. Biol Psychol. 2015;110:148–158. doi: 10.1016/j.biopsycho.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Tsai CL, Wang WL. Exercise-mode-related changes in task-switching performance in the elderly. Front behav Neurosci. 2015;9:56. doi: 10.3389/fnbeh.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. J Cognitive Neurosci. 2002;14(8):1139–1150. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- 61.Umebayashi K, Okita T. An ERP investigation of task switching using a flanker paradigm. Brain Res. 2010;1346:165–173. doi: 10.1016/j.brainres.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 62.Wang M, et al. Differential preparation intervals modulate repetition processes in task switching: an ERP study. Front Hum Neurosci. 2016;10:57. doi: 10.3389/fnhum.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barceló F, Periáñez JA, Knight RT. Think differently: A brain orienting response to task novelty. NeuroReport. 2002;13(15):1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- 64.Donchin E, Coles MG. Is the P300 component a manifestation of context updating. Behav Brain Sci. 1988;11(3):357–427. doi: 10.1017/S0140525X00058027. [DOI] [Google Scholar]
- 65.Schapkin SA, Gajewski PD, Freude G. Age Differences in Memory-Based Task Switching With and Without Cues. J Psychophysiol. 2014;28:187–201. doi: 10.1027/0269-8803/a000125. [DOI] [Google Scholar]
- 66.Hübner R, Druey MD. Multiple response codes play specific roles in response selection and inhibition under task switching. Psychol Res. 2008;72(4):415–424. doi: 10.1007/s00426-007-0118-2. [DOI] [PubMed] [Google Scholar]
- 67.Koch I, Schuch S, Vu KPL, Proctor RW. Response-repetition effects in task switching—Dissociating effects of anatomical and spatial response discriminability. Acta Psychol. 2011;136(3):399–404. doi: 10.1016/j.actpsy.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Wendt M, Heldmann M, Münte TF, Kluwe RH. Disentangling sequential effects of stimulus-and response-related conflict and stimulus-response repetition using brain potentials. J Cognitive Neurosci. 2007;19(7):1104–1112. doi: 10.1162/jocn.2007.19.7.1104. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt JR, Liefooghe B. Feature Integration and Task Switching: Diminished Switch Costs after Controlling for Stimulus, Response, and Cue Repetitions. PloS one. 2016;11(3):e0151188. doi: 10.1371/journal.pone.0151188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Druey MD. Stimulus-category and response-repetition effects in task switching: An evaluation of four explanations. J Exp Psychol Learn. 2014;40(1):125. doi: 10.1037/a0033868. [DOI] [PubMed] [Google Scholar]
- 71.Hommel B, Colzato L. Visual attention and the temporal dynamics of feature integration. Vis Cogn. 2004;11(4):483–521. doi: 10.1080/13506280344000400. [DOI] [Google Scholar]
- 72.Pashler H, Baylis GC. Procedural learning: II. Intertrial repetition effects in speeded-choice tasks. J Exp Psychol Learn. 1991;17(1):33. doi: 10.1037/0278-7393.17.1.33. [DOI] [Google Scholar]
- 73.Bertelson P. SR relationships and reaction times to new versus repeated signals in a serial task. J Exp Psychol. 1963;65(5):478. doi: 10.1037/h0047742. [DOI] [Google Scholar]
- 74.Meiran N. Modeling cognitive control in task-switching. Psychol Res. 2000;63(3–4):234–249. doi: 10.1007/s004269900004. [DOI] [PubMed] [Google Scholar]
- 75.Meiran N, Kessler Y, Adi-Japha E. Control by action representation and input selection (CARIS): A theoretical framework for task switching. Psychol Res. 2008;72(5):473–500. doi: 10.1007/s00426-008-0136-8. [DOI] [PubMed] [Google Scholar]
- 76.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- 77.Grzyb KR, Hübner R. Excessive response-repetition costs under task switching: How response inhibition amplifies response conflict. J Exp Psychol Learn. 2013;39(1):126. doi: 10.1037/a0028477. [DOI] [PubMed] [Google Scholar]
- 78.von der Gablentz J, Tempelmann C, Münte TF, Heldmann M. Performance monitoring and behavioral adaptation during task switching: An fMRI study. Neuroscience. 2015;285:227–235. doi: 10.1016/j.neuroscience.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 79.Morimoto HM, et al. On verbal/nonverbal modality dependence of left and right inferior prefrontal activation during performance of flanker interference task. J Cognitive Neurosci. 2008;20(11):2006–2014. doi: 10.1162/jocn.2008.20138. [DOI] [PubMed] [Google Scholar]
- 80.Dignath D, Kiesel A, Eder AB. Flexible conflict management: Conflict avoidance and conflict adjustment in reactive cognitive control. J Exp Psychol Learn. 2015;41(4):975. doi: 10.1037/xlm0000089. [DOI] [PubMed] [Google Scholar]
- 81.Li KZ, Dupuis K. Attentional switching in the sequential flanker task: Age, location, and time course effects. Acta Psychol. 2008;127(2):416–427. doi: 10.1016/j.actpsy.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 82.Kuhns D, Lien MC, Ruthruff E. Proactive versus reactive task-set inhibition: Evidence from flanker compatibility effects. Psychon B Rev. 2007;14(5):977–983. doi: 10.3758/BF03194131. [DOI] [PubMed] [Google Scholar]
- 83.Nieuwenhuis S, Stins JF, Posthuma D, Polderman TJ, Boomsma DI, de Geus EJ. Accounting for sequential trial effects in the flanker task: Conflict adaptation or associative priming? Mem Cognition. 2006;34(6):1260–1272. doi: 10.3758/BF03193270. [DOI] [PubMed] [Google Scholar]
- 84.Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6(5):450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- 85.Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: support for the conflict monitoring theory. Neuropsychologia. 2011;49(7):1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 86.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- 87.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroen Clin Neuro. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 88.Polich, J. Theoretical overview of P3a and P3b. In Detection of Change (pp. 83–98) Springer US (2003).
- 89.Hagen GF, Gatherwright JR, Lopez BA, Polich J. P3a from visual stimuli: task difficulty effects. Int J Psychophysiol. 2006;59(1):8–14. doi: 10.1016/j.ijpsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Barceló, F., Periáñez, J. A. & Knight, R. T. A new ERP paradigm for studying individual differences in the executive control of attention. The Cognitive Neuroscience of Individual Differences–New Perspectives, 47 (2003).
- 91.Barcelo F, Escera C, Corral MJ, Periáñez JA. Task switching and novelty processing activate a common neural network for cognitive control. J Cognitive Neurosci. 2006;18(10):1734–1748. doi: 10.1162/jocn.2006.18.10.1734. [DOI] [PubMed] [Google Scholar]
- 92.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luk G, Anderson JA, Craik FI, Grady C, Bialystok E. Distinct neural correlates for two types of inhibition in bilinguals: Response inhibition versus interference suppression. Brain Cognition. 2010;74(3):347–357. doi: 10.1016/j.bandc.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Jersild, A. T. Mental set and shift. Archives of psychology (1927).
- 95.Spector, A. & Biederman, I. Mental set and mental shift revisited. The American Journal of Psychology, 669–679 (1976).
- 96.Rogers RD, Monsell S. Costs of a predictible switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124(2):207. doi: 10.1037/0096-3445.124.2.207. [DOI] [Google Scholar]
- 97.Kieffaber PD, Hetrick WP. Event-related potential correlates of task switching and switch costs. Psychophysiology. 2005;42(1):56–71. doi: 10.1111/j.1469-8986.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 98.Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77(4):477–482. doi: 10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 99.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartholow BD, et al. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42(1):33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 101.West R, Choi P, Travers S. The influence of negative affect on the neural correlates of cognitive control. Int J Psychophysiol. 2010;76(2):107–117. doi: 10.1016/j.ijpsycho.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: Support for the conflict monitoring theory. Neuropsychologia. 2011;49(7):1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 103.Larson MJ, Clayson PE, Baldwin SA. Performance monitoring following conflict: Internal adjustments in cognitive control? Neuropsychologia. 2012;50(3):426–433. doi: 10.1016/j.neuropsychologia.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 104.Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37(02):190–203. doi: 10.1111/1469-8986.3720190. [DOI] [PubMed] [Google Scholar]
- 105.Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. P Natl Acad Sci. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32(1):4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- 107.Di Russo F, Martínez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex. 2003;13(5):486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- 108.Boehler CN, et al. Sensory MEG responses predict successful and failed inhibition in a stop-signal task. Cereb Cortex. 2009;19(1):134–145. doi: 10.1093/cercor/bhn063. [DOI] [PubMed] [Google Scholar]
- 109.Greenhouse I, Wessel JR. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology. 2013;50(9):900–908. doi: 10.1111/psyp.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bigman Z, Pratt H. Time course and nature of stimulus evaluation in category induction as revealed by visual event-related potentials. Biol Psychol. 2004;66(2):99–128. doi: 10.1016/j.biopsycho.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 111.Donchin E. Surprise!… surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 112.Scisco JL, Leynes PA, Kang J. Cardiovascular fitness and executive control during task-switching: An ERP study. Int J Psychophysiol. 2008;69(1):52–60. doi: 10.1016/j.ijpsycho.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 113.Periáñez, J. A. & Barceló, F. Updating sensory versus task representations during task-switching: Insights from cognitive brain potentials in humans. Neuropsychologia47(4), 1160–1172 (2009). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


