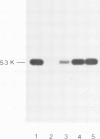
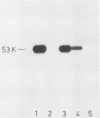
Abstract
A monoclonal mouse antibody has been prepared against a synthetic peptide corresponding to the six carboxy-terminal amino acids (C' peptide) of the src gene product pp60v -src of Rous sarcoma virus (RSV). The antibody was able to precipitate pp60v -src and to bind pp60v -src kinase activity in a competition test, indicating that this peptide can serve as an antibody-binding site (epitope). Furthermore, the finding that three out of 28 pp60src-specific tumor-bearing rabbit (TBR) sera contained antibody against the C' peptide argues for an in vivo role for the carboxy terminus of pp60src. C' peptide-specific IgG was purified from one TBR serum using affinity chromatography, and was shown to precipitate significant amounts of pp60src, and bind most of the pp60src kinase activity from SRA, PrA, and B77-C strains of avian sarcoma virus (ASV), but not endogenous pp60c -src, a cellular homologue to the viral pp60v -src. Similar results were obtained with IgG isolated from a C' peptide immune rabbit serum. None of the three C' peptide-specific IgGs could serve as a phosphate acceptor in an immune complex protein kinase reaction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschek C. B., Jockusch B. M., Friis R. R., Back R., Grundmann E., Bauer H. Early changes in the distribution and organization of microfilament proteins during cell transformation. Cell. 1981 Apr;24(1):175–184. doi: 10.1016/0092-8674(81)90513-4. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calothy G., Pessac B. Growth stimulation of chicl embryo neuroretinal cells infected with Rous sarcoma virus: relationship to viral replication and morphological transformation. Virology. 1976 May;71(1):336–345. doi: 10.1016/0042-6822(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Four different classes of retroviruses induce phosphorylation of tyrosines present in similar cellular proteins. Mol Cell Biol. 1981 May;1(5):394–407. doi: 10.1128/mcb.1.5.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. I., Collett M. S., Erikson E., Purchio A. F., Brugge J. S. Protein phosphorylation mediated by partially purified avian sarcoma virus transforming-gene product. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):907–917. doi: 10.1101/sqb.1980.044.01.098. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Mason W. S., Chen Y. C., Halpern M. S. A replication defective mutant of Rous sarcoma virus which fails to make a functional reverse transcriptase. Virology. 1975 Mar;64(1):49–62. doi: 10.1016/0042-6822(75)90078-1. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Schwarz R. T., Schmidt M. F. Phenotypes of Rous sarcoma virus-transformed fibroblasts: an argument for a multifunctional Src gene product. Med Microbiol Immunol. 1977;164(1-3):155–165. doi: 10.1007/BF02121311. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Presek P., Glossmann H., Eigenbrodt E., Schoner W., Rübsamen H., Friis R. R., Bauer H. Similarities between a phosphoprotein (pp60src)-associated protein kinase of Rous sarcoma virus and a cyclic adenosine 3':5'-monophosphate-independent protein kinase that phosphorylates pyruvate kinase type M2. Cancer Res. 1980 May;40(5):1733–1741. [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Saltenberger K., Friis R. R., Eigenbrodt E. Cytosolic malic dehydrogenase activity is associated with a putative substrate for the transforming gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):228–232. doi: 10.1073/pnas.79.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Liu F. T., Niman H. L., Lerner R. A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980 Oct 30;287(5785):801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- THURZO V., SMIDA J., SMIDOVA-KOVAROVA V., SIMKOVIC D. Some properties of the fowl virus tumour B77. Acta Unio Int Contra Cancrum. 1963;19:304–305. [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Antibodies specific for the polyoma virus middle-size tumor antigen. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4882–4886. doi: 10.1073/pnas.78.8.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Scheidtmann K. H., Carbone A., Laudano A. P., Doolittle R. F. Antibodies specific for the carboxy- and amino-terminal regions of simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5197–5200. doi: 10.1073/pnas.77.9.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Synthetic peptide fragment of src gene product inhibits the src protein kinase and crossreacts immunologically with avian onc kinases and cellular phosphoproteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7412–7416. doi: 10.1073/pnas.78.12.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Friis R. R. Simultaneous injection of newborn rabbits with the Schmidt-Ruppin and prague strains of Rous sarcoma virus induces antibodies which recognize the pp60src of both strains. J Gen Virol. 1980 Sep;50(1):211–216. doi: 10.1099/0022-1317-50-1-211. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]