Abstract
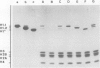
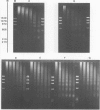
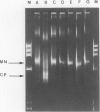
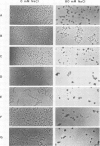
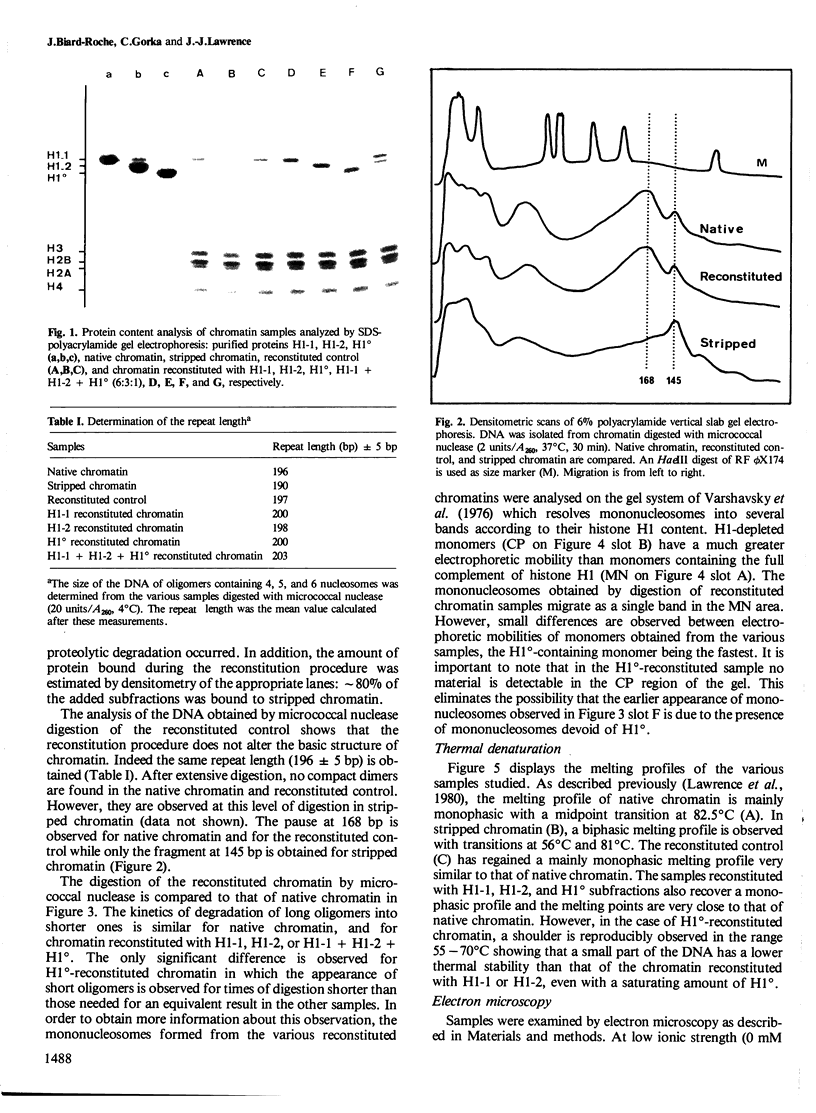
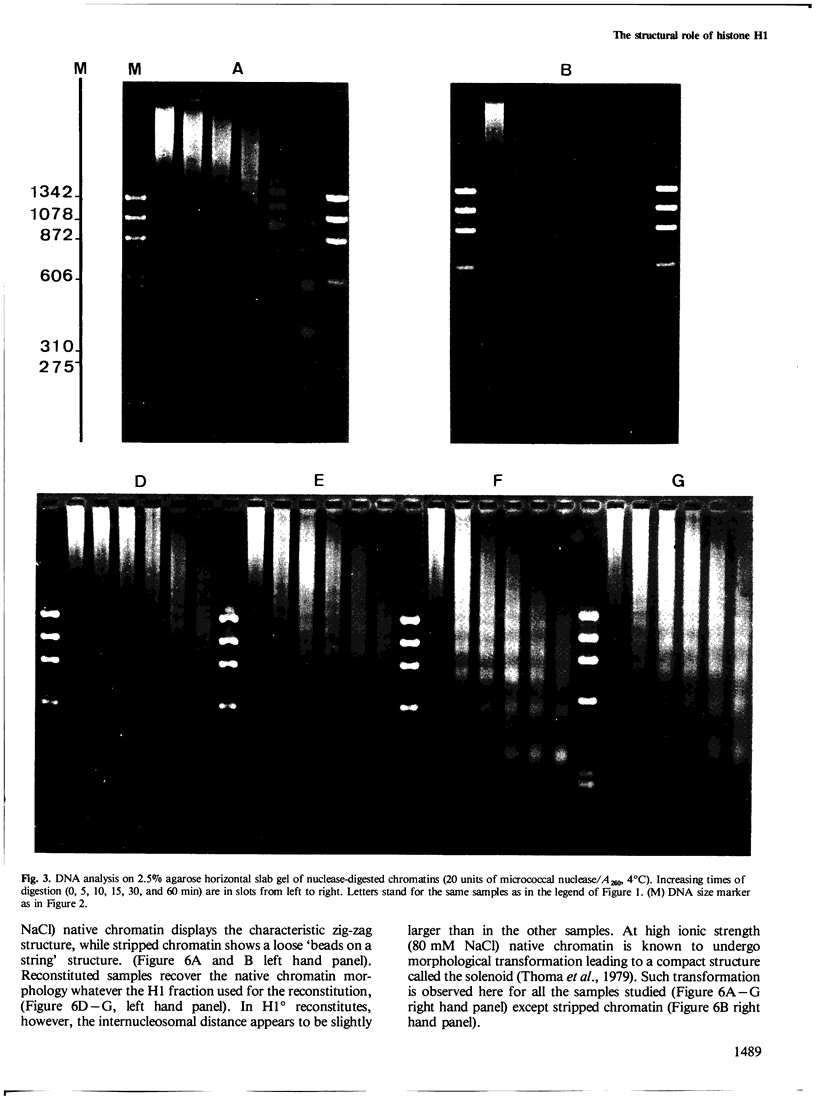
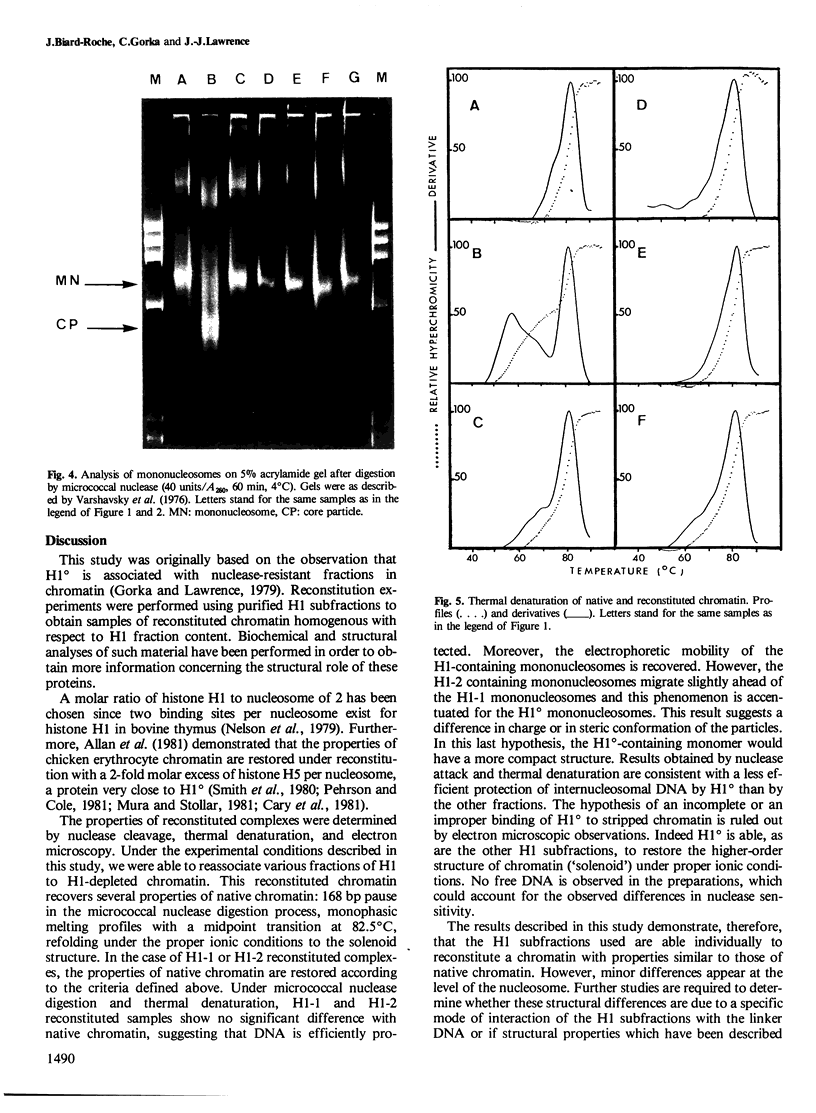
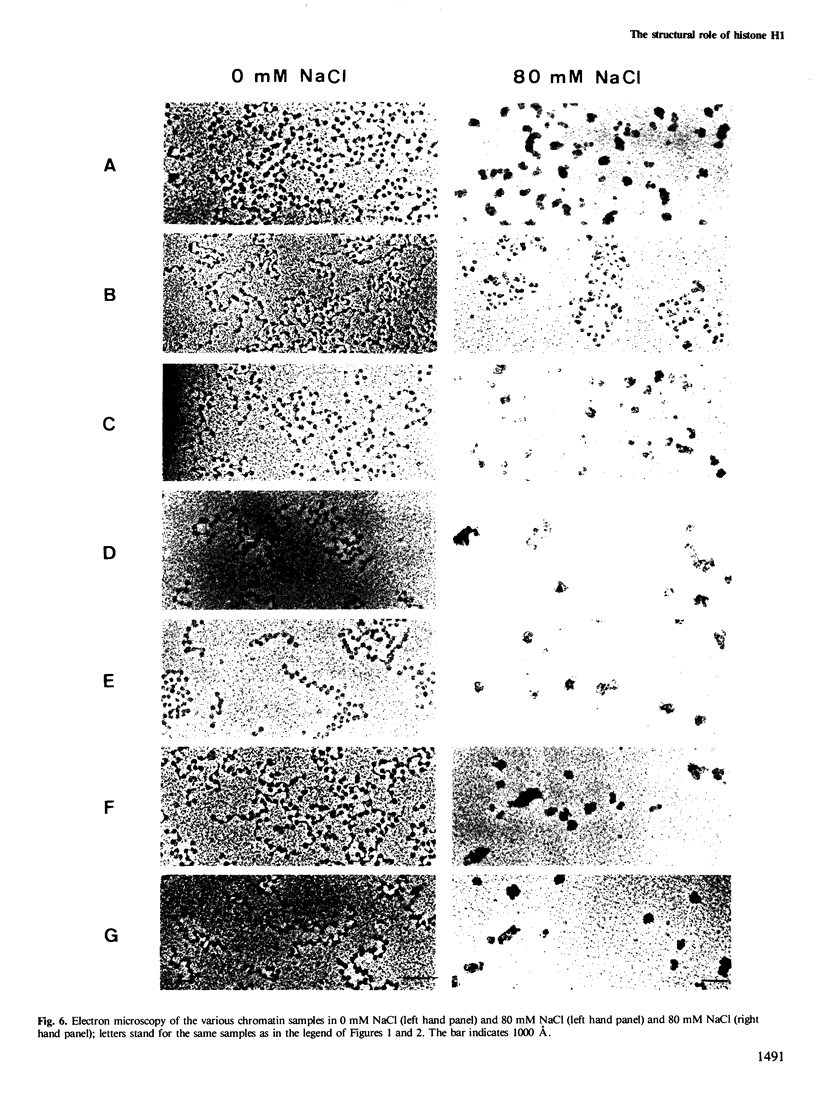
A previous study on the distribution of histone H1 subfractions in chromatin suggested that these proteins differ in the protection they confer to DNA. To elucidate further this suggestion, reconstitution experiments were carried out with purified H1 subfractions (H1-1, H1-2, H1o) and H1-depleted chromatin. We have studied the structural properties of H1o as compared to those of other H1 fractions by electrophoretic analysis of DNA and mononucleosomes obtained after micrococcal nuclease digestion, thermal denaturation, and electron microscopy. The three fractions studied reassociate to H1-depleted chromatin. However, differences in the extent of DNA protection are observed between H1o and the other fractions: H1o induces a more rapid degradation of long oligomers into mononucleosomes; these mononucleosomes bearing H1o only, have a greater electrophoretic mobility; furthermore, thermal denaturation shows that a small fraction of DNA is less efficiently protected by H1o than by the other fractions. Electron microscopy, on the other hand, shows that these differences are not due to areas of chromatin devoid of H1o in the reconstitute and that the reconstituted samples are able, under proper ionic conditions, to refold in a higher-order structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Cowling G. J., Harborne N., Cattini P., Craigie R., Gould H. Regulation of the higher-order structure of chromatin by histones H1 and H5. J Cell Biol. 1981 Aug;90(2):279–288. doi: 10.1083/jcb.90.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Hines M. L., Bradbury E. M., Smith B. J., Johns E. W. Conformation studies of histone H1(0) in comparison with histones H1 and H5. Eur J Biochem. 1981 Nov;120(2):371–377. doi: 10.1111/j.1432-1033.1981.tb05714.x. [DOI] [PubMed] [Google Scholar]
- Gjerset R., Gorka C., Hasthorpe S., Lawrence J. J., Eisen H. Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2333–2337. doi: 10.1073/pnas.79.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerset R., Ibarrando F., Saragosti S., Eisen H. Distribution of IP25 in chromatin and its possible involvement in chromatin condensation. Biochem Biophys Res Commun. 1981 Mar 31;99(2):349–357. doi: 10.1016/0006-291x(81)91752-6. [DOI] [PubMed] [Google Scholar]
- Gorka C., Lawrence J. J. The distribution of histone H1 subfractions in chromatin subunits. Nucleic Acids Res. 1979 Sep 25;7(2):347–359. doi: 10.1093/nar/7.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):653–656. doi: 10.1073/pnas.74.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Biochemical properties and localization of the chromosomal protein IP25. Eur J Biochem. 1979 Jun 1;96(3):477–482. doi: 10.1111/j.1432-1033.1979.tb13060.x. [DOI] [PubMed] [Google Scholar]
- Kinkade J. M., Jr, Cole R. D. The resolution of four lysine-rich histones derived from calf thymus. J Biol Chem. 1966 Dec 25;241(24):5790–5797. [PubMed] [Google Scholar]
- Kinkade J. M., Jr Qualitative species differences and quantitative tissue differences in the distribution of lysine-rich histones. J Biol Chem. 1969 Jun 25;244(12):3375–3386. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. J., Berne L., Ouvrier-Buffet J. L., Piette L. H. Spin-label study of histone H1-DNA interaction. Comparative properties of the central part of the molecule and the N and C-amino tails. Eur J Biochem. 1980;107(1):263–269. doi: 10.1111/j.1432-1033.1980.tb04646.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Marsh W. H., Fitzgerald P. J. Pancreas acinar cell regeneration. 13. Histone synthesis and modification. Fed Proc. 1973 Nov;32(11):2119–2125. [PubMed] [Google Scholar]
- Modak S. P., Lawrence J. J., Gorka C. Selective removal of histone H1 from nucleosomes at low ionic strength. Mol Biol Rep. 1980 Dec 31;6(4):235–243. doi: 10.1007/BF00777531. [DOI] [PubMed] [Google Scholar]
- Mura C. V., Stollar B. D. Serological detection of homologies of H1o with H5 and H1 histones. J Biol Chem. 1981 Oct 10;256(19):9767–9769. [PubMed] [Google Scholar]
- Nelson P. P., Albright S. C., Wiseman J. M., Garrard W. T. Reassociation of histone H1 with nucleosomes. J Biol Chem. 1979 Nov 25;254(22):11751–11760. [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. A new histone found only in mammalian tissues with little cell division. Biochem Biophys Res Commun. 1969 Dec 4;37(6):1042–1049. doi: 10.1016/0006-291x(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Pehrson J. R., Cole R. D. Bovine H10 histone subfractions contain an invariant sequence which matches histones H5 rather than H1. Biochemistry. 1981 Apr 14;20(8):2298–2301. doi: 10.1021/bi00511a035. [DOI] [PubMed] [Google Scholar]
- Pieler C., Adolf G. R., Swetly P. Accumulation of histone H1(0) during chemically induced differentiation of murine neuroblastoma cells. Eur J Biochem. 1981 Apr;115(2):329–333. doi: 10.1111/j.1432-1033.1981.tb05242.x. [DOI] [PubMed] [Google Scholar]
- Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Walker J. M., Johns E. W. Structural homology between a mammalian H1(0) subfraction and avian erythrocyte-specific histone H5. FEBS Lett. 1980 Mar 24;112(1):42–44. doi: 10.1016/0014-5793(80)80122-0. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova J., Swetly P. Uncoupled synthesis of histones and DNA during Friend cell differentiation. Nature. 1978 Nov 16;276(5685):276–277. doi: 10.1038/276276a0. [DOI] [PubMed] [Google Scholar]