Abstract
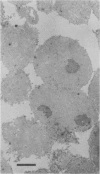
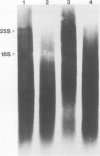
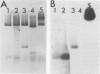
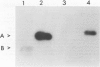
Nuclei isolated from both light-grown and dark-grown leaves of Pisum sativum by Percoll density gradient centrifugation incorporate labelled UTP into RNA when supplemented with the other three nucleoside triphosphates. The RNA is heterodisperse, with transcripts up to at least 25S in size. Among these transcripts are sequences hybridizing to cloned DNA probes for wheat rRNA and two abundant chloroplast polypeptides of Pisum, viz. the small subunit of ribulose bisphosphate carboxylase and a polypeptide of the light-harvesting chlorophyll a/b binding complex. Transcription of small subunit and light-harvesting complex sequences is greater (18-fold and 9-fold, respectively) in nuclei from light-grown leaves than in nuclei from dark-grown leaves. Transcription of ribosomal genes, by contrast, is only doubled by growth in the light. Small subunit and light-harvesting complex sequences transcribed in dark-grown nuclei are not degraded in a 120 min chase. These results suggest that the stimulation of accumulation of small subunit and light-harvesting complex mRNAs by exposure of Pisum seedlings to light is mediated by an increase in transcription rather than by a decrease in RNA degradation.
Keywords: chloroplast polypeptides, hybridization analysis, isolated nuclei, light-stimulation, transcription
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R. Reiteration frequency of the gene coding for the small subunit of ribulose--1,5--bisphosphate carboxylase. Cell. 1979 Jun;17(2):383–388. doi: 10.1016/0092-8674(79)90164-8. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert D. A., Knoll B. J., Woo S. L., Mace M. L., Tsai M. J., O'Malley B. W. Differential hormonal responsiveness of the ovalbumin gene and its pseudogenes in the chick oviduct. Biochemistry. 1980 Nov 25;19(24):5586–5592. doi: 10.1021/bi00565a020. [DOI] [PubMed] [Google Scholar]
- Cuming A. C., Bennett J. Biosynthesis of the light-harvesting chlorophyll a/b protein. Control of messenger RNA activity by light. Eur J Biochem. 1981 Aug;118(1):71–80. doi: 10.1111/j.1432-1033.1981.tb05487.x. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Bellard M., Chambon P. Clustering of RNA polymerase B molecules in the 5' moiety of the adult beta-globin gene of hen erythrocytes. Nucleic Acids Res. 1981 Jun 11;9(11):2589–2598. doi: 10.1093/nar/9.11.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Künsch U., Temperli A. Simple rapid procedures for isolation of tobacco leaf nuclei. Anal Biochem. 1972 Sep;49(1):48–57. doi: 10.1016/0003-2697(72)90241-2. [DOI] [PubMed] [Google Scholar]
- Ingle J., Sinclair J. Ribosomal RNA genes and plant development. Nature. 1972 Jan 7;235(5332):30–32. doi: 10.1038/235030a0. [DOI] [PubMed] [Google Scholar]
- Jendrisak J., Guilfoyle T. J. Eukaryotic RNA polymerase: comparative subunit structures, immunological properties, and alpha-amanitin sensitivities of the class II enzymes from higher plants. Biochemistry. 1978 Apr 4;17(7):1322–1327. doi: 10.1021/bi00600a029. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: II. CHARACTERIZATION OF RNA SYNTHESIZED IN VITRO. Plant Physiol. 1980 Feb;65(2):309–313. doi: 10.1104/pp.65.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Palmiter R. D., Lee D. C. Regulation of gene transcription by estrogen and progesterone. Lack of hormonal effects on transcription by Escherichia coli RNA polymerase. J Biol Chem. 1980 Oct 25;255(20):9693–9698. [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Sasaki Y., Ishiye M., Sakihama T., Kamikubo T. Light-induced increase of mRNA activity coding for the small subunit of ribulose-1,5-bisphosphate carboxylase. J Biol Chem. 1981 Mar 10;256(5):2315–2320. [PubMed] [Google Scholar]
- Smith S. M., Ellis R. J. Light-stimulated accumulation of transcripts of nuclear and chloroplast genes for ribulosebisphosphate carboxylase. J Mol Appl Genet. 1981;1(2):127–137. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M. Light regulation of specific mRNA species in Lemna gibba L. G-3. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4749–4753. doi: 10.1073/pnas.75.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M., Suttie J. L. Light Effects on the Synthesis of Ribulose-1,5-Bisphosphate Carboxylase in Lemna gibba L. G-3. Plant Physiol. 1980 Apr;65(4):641–647. doi: 10.1104/pp.65.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M. White Light Effects on the mRNA for the Light-Harvesting Chlorophyll a/b-Protein in Lemna gibba L. G-3. Plant Physiol. 1981 Jun;67(6):1078–1083. doi: 10.1104/pp.67.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Roop D. R., Tsai M. J., Stein J. P., Means A. R., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Regulation of the ovomucoid gene. Biochemistry. 1978 Dec 26;17(26):5773–5780. doi: 10.1021/bi00619a026. [DOI] [PubMed] [Google Scholar]
- Turner P. C., Woodland H. R. H3 and H4 histone cDNA sequences from Xenopus: a sequence comparison of H4 genes. Nucleic Acids Res. 1982 Jun 25;10(12):3769–3780. doi: 10.1093/nar/10.12.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wilson P. S., Bennett J. Transcription in nuclei isolated from a higher plant. Biochem Soc Trans. 1976;4(4):812–813. doi: 10.1042/bst0040812. [DOI] [PubMed] [Google Scholar]