Abstract
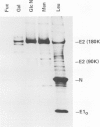
The intracellular sites of biosynthesis of the structural proteins of murine hepatitis virus A59 have been analyzed using cell fractionation techniques. The nucleocapsid protein N is synthesized on free polysomes, whereas the envelope glycoproteins E1 and E2 are translated on the rough endoplasmic reticulum (RER). Glycoprotein E2 present in the RER contains N-glycosidically linked oligosaccharides of the mannose-rich type, supporting the concept that glycosylation of this protein is initiated at the co-translational level. In contrast, O-glycosylation of E1 occurs after transfer of the protein to smooth intracellular membranes. Monensin does not interfere with virus budding from the membranes of the endoplasmic reticulum, but it inhibits virus release and fusion of infected cells. The oligosaccharide side chains of E2 obtained under these conditions are resistant to endoglycosidase H and lack fucose suggesting that transport of this glycoprotein is inhibited between the trans Golgi cisternae and the cell surface. Glycoprotein E1 synthesized in the presence of monensin is completely carbohydrate-free. This observation suggests that the intracellular transport of this glycoprotein is also blocked by monensin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODANSKY O., SCHWARTZ M. K. COMPARATIVE EFFECTS OF L-HISTIDINE ON THE ACTIVITIES OF 5'-NUCLEOTIDASE AND ALKALINE PHOSPHATASE. J Biol Chem. 1963 Oct;238:3420–3427. [PubMed] [Google Scholar]
- Becker W. B., McIntosh K., Dees J. H., Chanock R. M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E). J Virol. 1967 Oct;1(5):1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Shaper J. H., Olsen K. W., Trayer I. P., Hill R. L. Cross-linking of the components of lactose synthetase with dimethylpimelimidate. J Biol Chem. 1975 Feb 25;250(4):1434–1444. [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Carić-Lazar M., Schwarz R. T., Scholtissek C. Influence of the infection with lipid-containing viruses on the metabolism and pools of phospholipid precursors in animal cells. Eur J Biochem. 1978 Nov 15;91(2):351–361. doi: 10.1111/j.1432-1033.1978.tb12687.x. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Knobler R. L., Powell H., Buchmeier M. J. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell--cell fusion. Virology. 1982 Jun;119(2):358–371. doi: 10.1016/0042-6822(82)90095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levin J. G., Grimley P. M., Berezesky I. K. Membrane-associated replication complex in arbovirus infection. J Virol. 1972 Sep;10(3):504–515. doi: 10.1128/jvi.10.3.504-515.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. VI. Early and late glycopolypeptides. Virology. 1974 Apr;58(2):345–361. doi: 10.1016/0042-6822(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Koennecke I., Boschek C. B., Scholtissek C. Isolation and properties of a temperature-sensitive mutant (ts 412) of an influenza A virus recombinant with a ts lesion in the gene coding for the nonstructural protein. Virology. 1981 Apr 15;110(1):16–25. doi: 10.1016/0042-6822(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
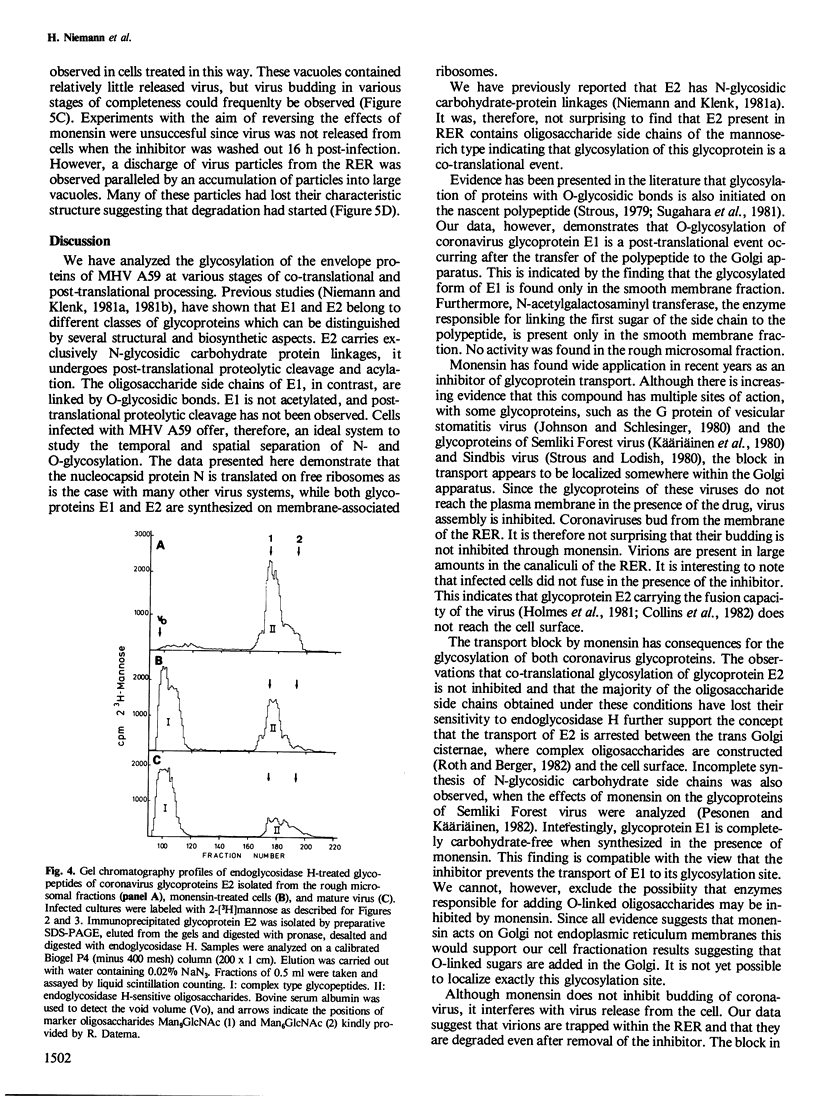
- Niemann H., Klenk H. D. Coronavirus glycoprotein E1, a new type of viral glycoprotein. J Mol Biol. 1981 Dec 25;153(4):993–1010. doi: 10.1016/0022-2836(81)90463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Jeansson S., Lycke E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J Virol. 1981 May;38(2):564–570. doi: 10.1128/jvi.38.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Käriäinen L. Incomplete complex oligosaccharides in semliki forest virus envelope proteins arrested within the cell in the presence of monensin. J Mol Biol. 1982 Jun 25;158(2):213–230. doi: 10.1016/0022-2836(82)90430-2. [DOI] [PubMed] [Google Scholar]
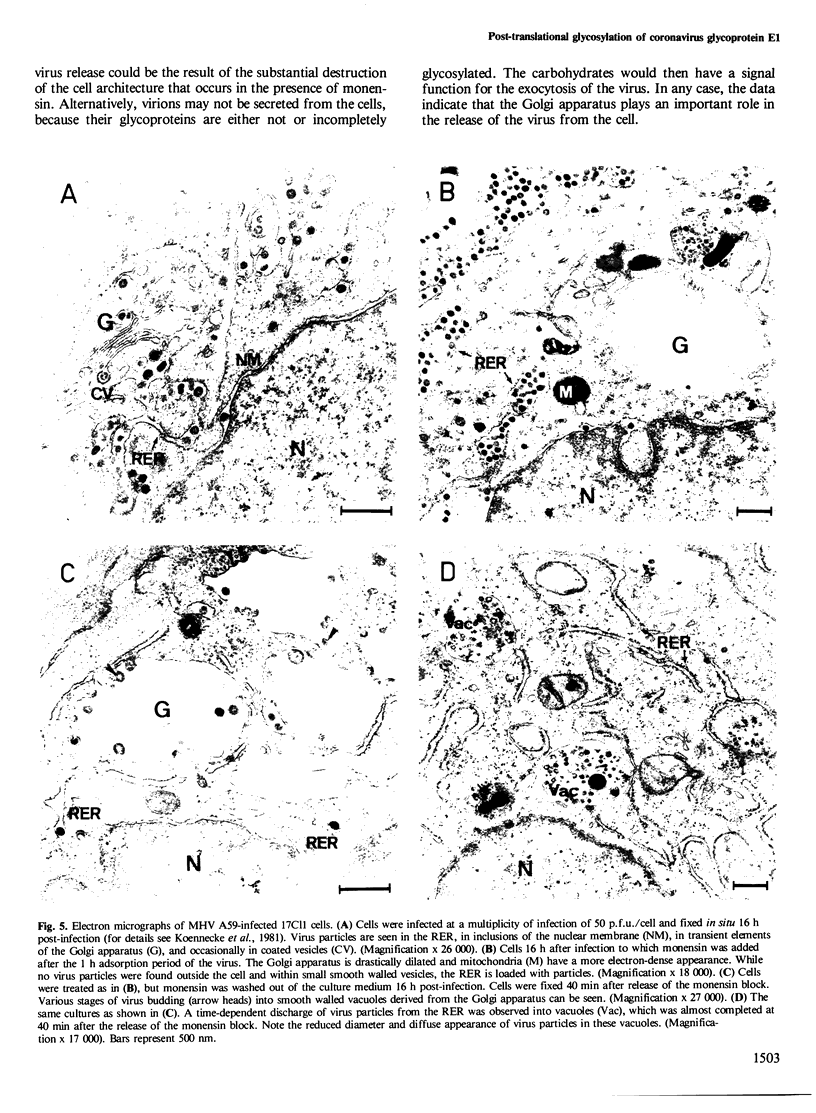
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Fries E. Transport of newly synthesized vesicular stomatitis viral glycoprotein to purified Golgi membranes. J Cell Biol. 1981 Apr;89(1):162–168. doi: 10.1083/jcb.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Lai M. M. Phosphoproteins of murine hepatitis viruses. J Virol. 1979 Nov;32(2):672–675. doi: 10.1128/jvi.32.2.672-675.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2694–2698. doi: 10.1073/pnas.76.6.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]