Abstract
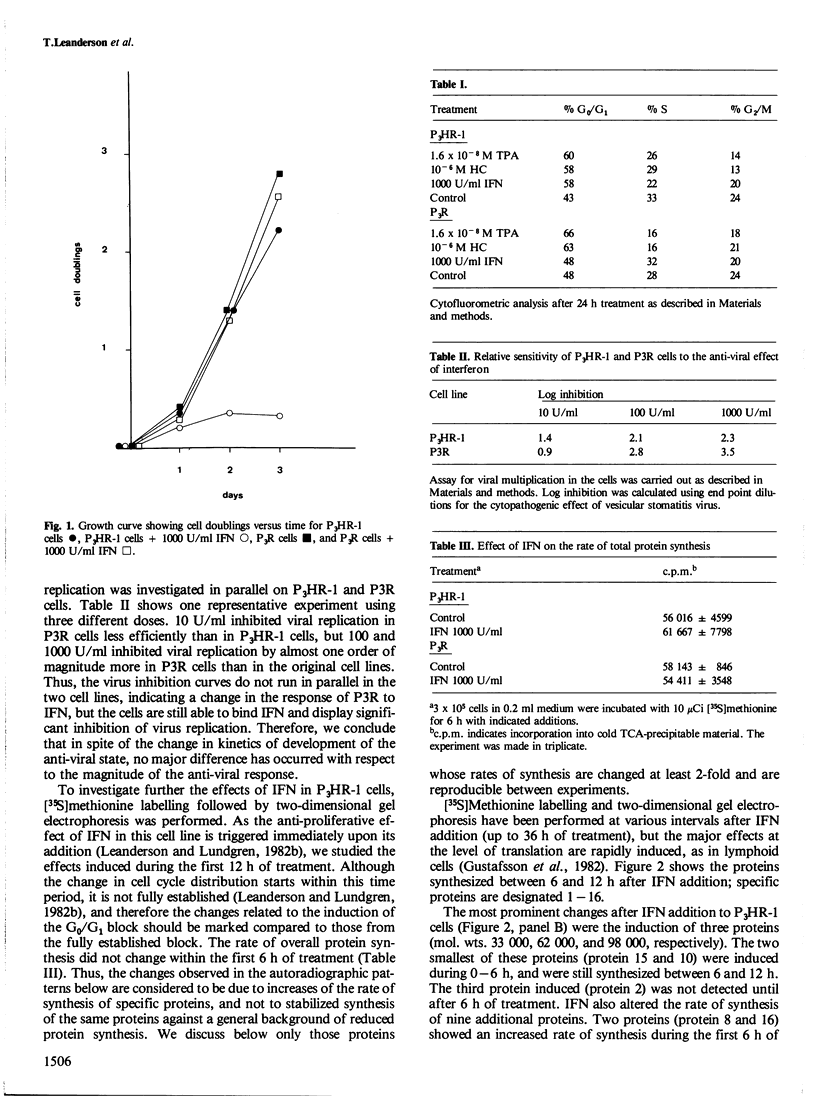
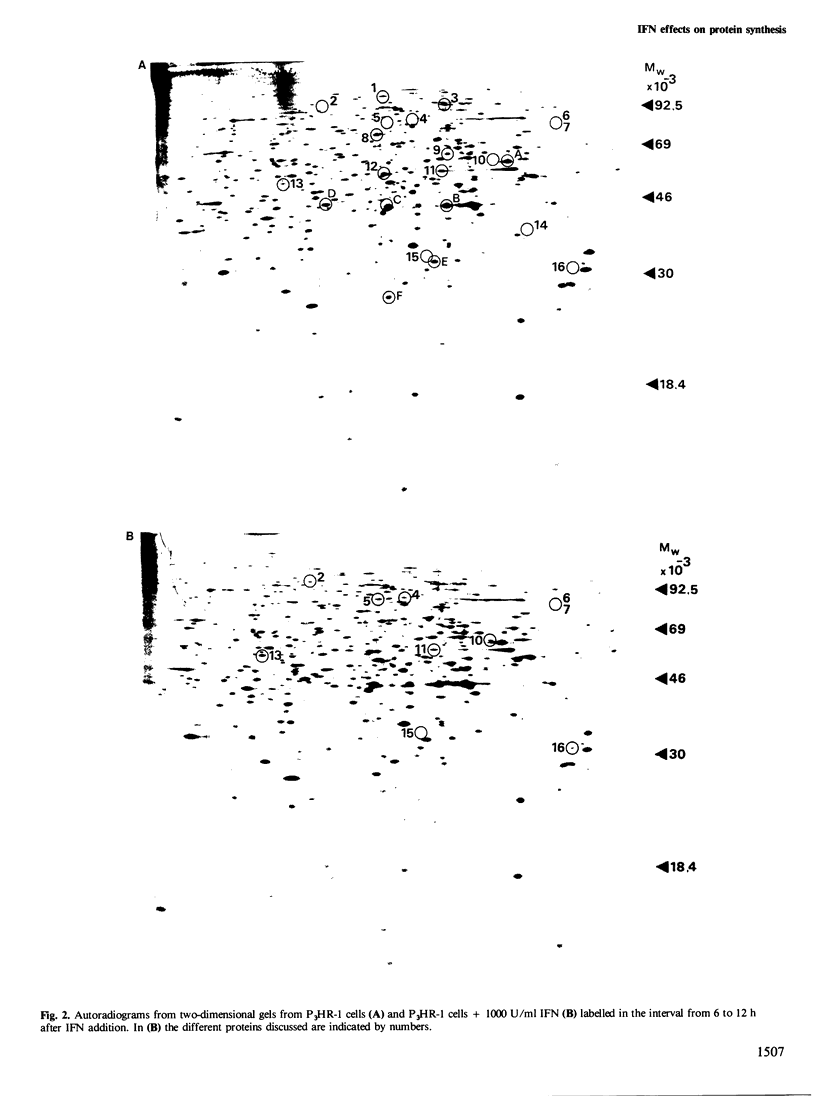
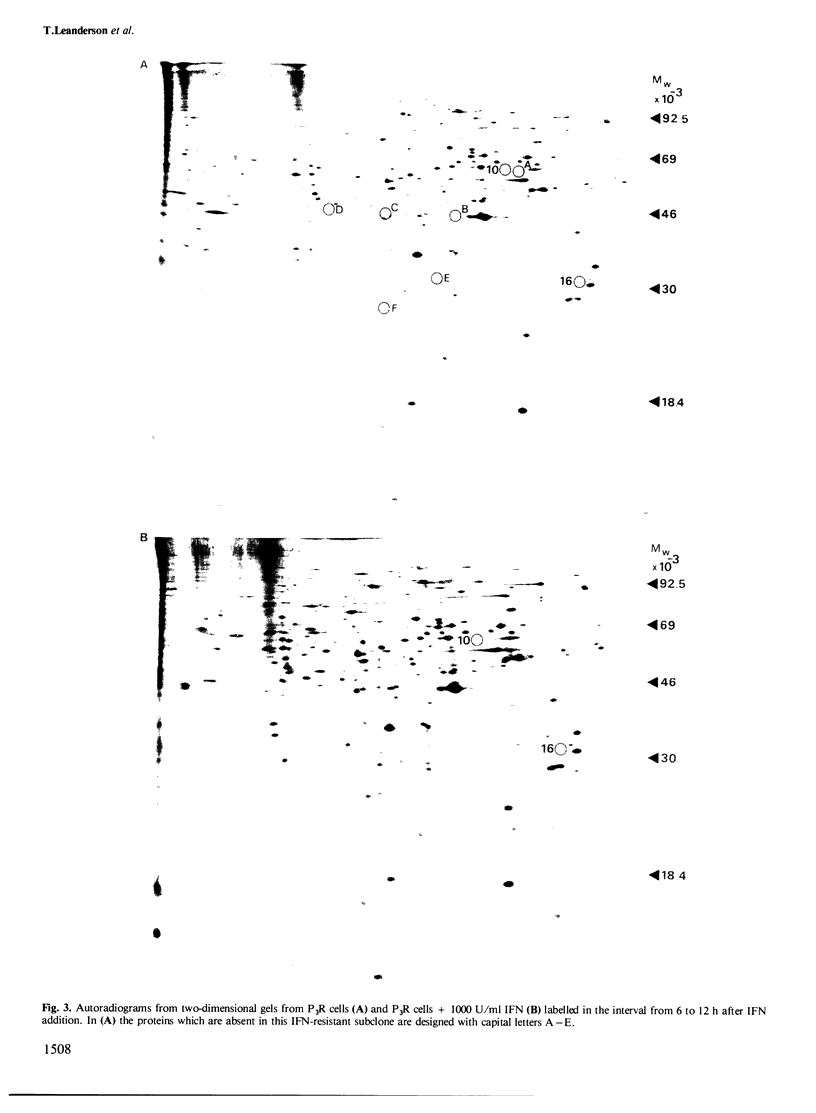
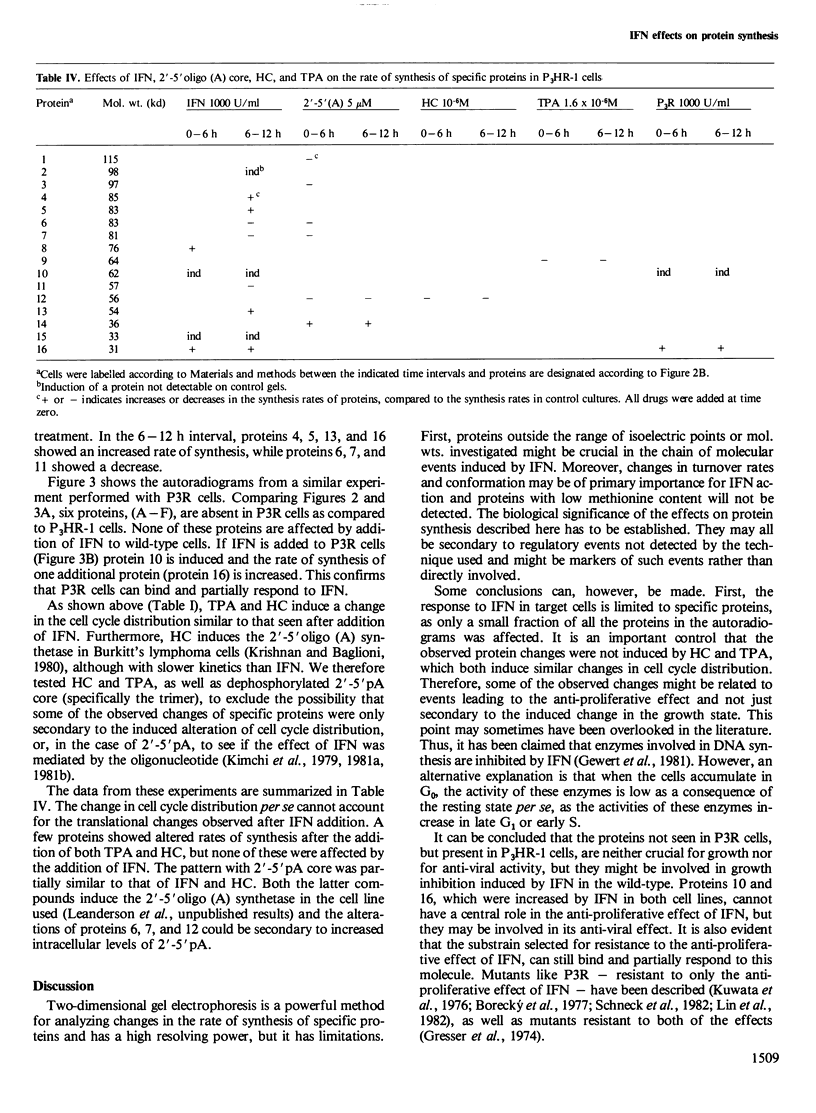
The effect of interferon (IFN) on protein synthesis was studied in the Burkitt's lymphoma cell line P3HR-1 by [35S]methionine labelling of the cells, followed by two-dimensional gel electrophoresis of cell extracts. De novo synthesis of three proteins (mol. wts. 33 000, 62 000, and 98 000, respectively) and alterations in the rate of synthesis for a small number of additional proteins were observed during the first 12 h of treatment, while the rate of overall protein synthesis was unaffected. Treatment of P3HR-1 cells with 12-O-tetradecanoyl-phorbol-13-acetate (TPA) or hydrocortisone (HC), which induce similar changes in cell cycle distribution as does IFN, did not induce comparable changes in the rates of protein synthesis. Thus, the effects were specific for IFN and not induced by the change in cell cycle distribution per se, i.e., accumulation in G0. Treatment of cells with 2'-5' pA core did not mimic the effect of IFN at the translational level. A substrain of P3HR-1 cells, selected for resistance to the anti-proliferative effect of IFN, lacked six proteins found in the wild-type. The 62 000 mol. wt. protein was induced in this substrain as well as in native P3HR-1 cells on addition of IFN. The resistant substrain still developed an anti-viral effect in response to IFN. Thus, it seems as if the anti-proliferative and anti-viral effects of IFN, at least in some cells are mediated by different intracellular molecular mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Borecký L., Fuchsberger N., Hajnická-Gondová V., Clampor F., Altaner C. The cell-growth inhibitory effect of interferon. Studies of a resistant cell-subline. Neoplasma. 1977;24:125–137. [PubMed] [Google Scholar]
- Colonno R. J. Accumulation of newly synthesized mRNAs in response to human fibroblast (beta) interferon. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4763–4766. doi: 10.1073/pnas.78.8.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Broeze R. J., Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979 Jun 7;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Fuse T. K., Morinaga N. Effects of interferon on cell and virus growth in transformed human cell lines. J Gen Virol. 1976 Oct;33(1):7–15. doi: 10.1099/0022-1317-33-1-7. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Shah S., Clemens M. J. Inhibition of cell division by interferons: Changes in the transport and intracellular metabolism of thymidine in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1981 Jun 1;116(3):487–492. doi: 10.1111/j.1432-1033.1981.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-Boyé D. Interferon and cell division. IX. Interferon-resistant L1210 cells: characteristics and origin. J Natl Cancer Inst. 1974 Feb;52(2):553–559. doi: 10.1093/jnci/52.2.553. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Johannsen R. Sensitivity of various human lymphoblastoid cells to the antiviral and anticellular activity of human leukocyte interferon. Arch Virol. 1977;54(3):271–277. doi: 10.1007/BF01314795. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Kimchi A. Increased levels of interferon-induced (2'--5') oligo-isoadenylate synthetase in mature T-lymphocytes and in differentiated Friend-erythroleukemic cells. J Interferon Res. 1981;1(4):559–569. doi: 10.1089/jir.1981.1.559. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Lapidot Y., Rapoport S., Panet A., Revel M. Antimitogenic effects of interferon and (2'-5')-oligoadenylate in synchronized 3T3 fibroblasts. FEBS Lett. 1981 Nov 16;134(2):212–216. doi: 10.1016/0014-5793(81)80604-7. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Regulation of lymphocyte mitogenesis by (2'--5') oligo-isoadenylate. Nature. 1979 Dec 20;282(5741):849–851. doi: 10.1038/282849a0. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. Fibroblast interferon induces synthesis of four proteins in human fibroblast cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1824–1827. doi: 10.1073/pnas.76.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. Increased levels of (2'-5')oligo(A) polymerase activity in human lymphoblastoid cells treated with glucocorticoids. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6506–6510. doi: 10.1073/pnas.77.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanderson T., Hillörn V., Holmberg D., Larsson E. L., Lundgren E. Selective effects of interferon on distinct sites of the T lymphocyte triggering process. J Immunol. 1982 Aug;129(2):490–494. [PubMed] [Google Scholar]
- Leanderson T., Lundgren E. Growth inhibition by IFN achieved by collecting cells in G0. J Interferon Res. 1982;2(1):21–29. doi: 10.1089/jir.1982.2.21. [DOI] [PubMed] [Google Scholar]
- Leanderson T., Nordfelth R., Lundgren E. Antiproliferative effect of (2'-5') oligoadenylate distinct from that of interferon in lymphoid cells. Biochem Biophys Res Commun. 1982 Jul 30;107(2):511–517. doi: 10.1016/0006-291x(82)91521-2. [DOI] [PubMed] [Google Scholar]
- Leandersson T., Lundgren E. Antiproliferative effect on interferon on a Burkitt's lymphoma cell line. Exp Cell Res. 1980 Dec;130(2):421–426. doi: 10.1016/0014-4827(80)90020-8. [DOI] [PubMed] [Google Scholar]
- Leandersson T., Lundgren E. Cell growth regulation during density inhibition and interferon treatment. Exp Cell Res. 1982 Mar;138(1):167–174. doi: 10.1016/0014-4827(82)90102-1. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on cell proliferation and Epstein-Barr virus DNA replication. Virology. 1982 Feb;117(1):186–194. doi: 10.1016/0042-6822(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Greene J. J., Ts'o P. O., Carter W. A. Sensitivity and resistance of human tumor cells to interferon and rIn . rCn. Nature. 1982 Jun 3;297(5865):417–419. doi: 10.1038/297417a0. [DOI] [PubMed] [Google Scholar]
- Lundgren E., Larsson I., Miörner H., Strannegård O. Effects of leukocyte and fibroblast interferon on events in the fibroblast cell cycle. J Gen Virol. 1979 Mar;42(3):589–595. doi: 10.1099/0022-1317-42-3-589. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck J., Rager-Zisman B., Rosen O. M., Bloom B. R. Genetic analysis of the role of cAMP in mediating effects of interferon. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1879–1883. doi: 10.1073/pnas.79.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Crabtree G. R., Kennedy S. J., Munck A. U. Glucocorticoid receptors and glucocorticoid sensitivity of mitogen stimulated and unstimulated human lymphocytes. Nature. 1977 Jun 9;267(5611):523–526. doi: 10.1038/267523a0. [DOI] [PubMed] [Google Scholar]
- Vandenbussche P., Content J., Lebleu B., Werenne J. Comparison of interferon action in interferon resistant and sensitive L1210 cells. J Gen Virol. 1978 Oct;41(1):161–166. doi: 10.1099/0022-1317-41-1-161. [DOI] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M. Inhibition of protein synthesis by 2'-5' linked adenine oligonucleotides in intact cells. Nature. 1978 Nov 2;276(5683):88–90. doi: 10.1038/276088a0. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Prives C., Hartman J. R., Winocour E., Revel M. Inhibition of viral protein synthesis in monkey cells treated with interferon late in simian virus 40 lytic cycle. Cell. 1977 Sep;12(1):73–81. doi: 10.1016/0092-8674(77)90186-6. [DOI] [PubMed] [Google Scholar]




