Abstract
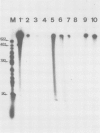
In analogy to the Escherichia coli replicative DNA polymerase III we define two forms of DNA polymerase alpha: the core enzyme and the holoenzyme. The core enzyme is not able to elongate efficiently primed single-stranded DNA templates, in contrast to the holoenzyme which functions well on in vivo-like template. Using these criteria, we have identified and partially purified DNA polymerase alpha holoenzyme from calf thymus and have compared it to the corresponding homogeneous DNA polymerase alpha (defined as the core enzyme) from the same tissue. The holoenzyme is able to use single-stranded parvoviral DNA and M13 DNA with a single RNA primer as template. The core enzyme, on the other hand, although active on DNAs treated with deoxyribonuclease to create random gaps, is unable to act on these two long, single-stranded DNAs. E. coli DNA polymerase III holoenzyme also copies the two in vivo-like templates, while the core enzyme is virtually inactive. The homologous single-stranded DNA-binding proteins from calf thymus and from E. coli stimulate the respective holoenzymes and inhibit the core enzymes. These results suggest a cooperation between a DNA polymerase holoenzyme and its homologous single-stranded DNA-binding protein. The prokaryotic and the mammalian holoenzyme behave similarly in several chromatographic systems.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert W., Grummt F., Hübscher U., Wilson S. H. Structural homology among calf thymus alpha-polymerase polypeptides. Nucleic Acids Res. 1982 Feb 11;10(3):935–946. doi: 10.1093/nar/10.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N., Kornberg A. Rep protein as a helicase in an active, isolatable replication fork of duplex phi X174 DNA. J Biol Chem. 1981 May 25;256(10):5294–5298. [PubMed] [Google Scholar]
- Banks G. R., Boezi J. A., Lehman I. R. A high molecular weight DNA polymerase from Drosophila melanogaster embryos. Purification, structure, and partial characterization. J Biol Chem. 1979 Oct 10;254(19):9886–9892. [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brakel C. L., Blumenthal A. B. Multiple forms of Drosophila embryo DNA polymerase: evidence for proteolytic conversion. Biochemistry. 1977 Jul 12;16(14):3137–3143. doi: 10.1021/bi00633a016. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Plummer J., Huberman A. J., Evans M. J. Restriction fragment primed phi X174 single-stranded DNA as template for DNA polymerase alpha and beta. Detection and partial purification of a polymerase alpha stimulating factor. Biochim Biophys Acta. 1980 Sep 19;609(2):205–223. doi: 10.1016/0005-2787(80)90232-4. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Cobianchi F., Riva S., Mastromei G., Spadari S., Pedrali-Noy G., Falaschi A. Enhancement of the rate of DNA polymerase-alpha activity on duplex DNA by a DNA-binding protein and a DNA-dependent ATPase of mammalian cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):639–647. doi: 10.1101/sqb.1979.043.01.071. [DOI] [PubMed] [Google Scholar]
- Coleman M. S., Hutton J. J., De Simone P., Bollum F. J. Terminal deoxyribonucleotidyl transferase in human leukemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4404–4408. doi: 10.1073/pnas.71.11.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danse J. M., Egly J. M., Kempf J. In vitro activation of DNA polymerase-alpha by a protein kinase in chick embryo. FEBS Lett. 1981 Feb 9;124(1):84–88. doi: 10.1016/0014-5793(81)80059-2. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Duguet M., Soussi T., Rossignol J. M., Méchali M., De Recondo A. M. Stimulation of rat liver alpha- and beta-type DNA polymerases by an homologous DNA-unwinding protein. FEBS Lett. 1977 Jul 1;79(1):160–164. doi: 10.1016/0014-5793(77)80374-8. [DOI] [PubMed] [Google Scholar]
- Duguet M., de Recondo A. M. A deoxyribonucleic acid unwinding protein isolated from regenerating rat liver. Physical and functional properties. J Biol Chem. 1978 Mar 10;253(5):1660–1666. [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. Enzymatic replication of viral and complementary strands of duplex DNA of phage phiX174 proceeds by seprate mechanisms. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3151–3155. doi: 10.1073/pnas.73.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fisher P. A., Korn D. DNA polymerase-alpha. Common polypeptide core structure of three enzyme forms from human KB cells. J Biol Chem. 1982 Feb 25;257(4):2029–2040. [PubMed] [Google Scholar]
- Fisher P. A., Korn D. DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells. J Biol Chem. 1977 Sep 25;252(18):6528–6535. [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification and partial characterization of a DNA polymerase alpha species from calf thymus. Nucleic Acids Res. 1980 Dec 11;8(23):5703–5714. doi: 10.1093/nar/8.23.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification of a 9S DNA polymerase alpha species from calf thymus. Biochemistry. 1981 Sep 15;20(19):5470–5475. doi: 10.1021/bi00522a019. [DOI] [PubMed] [Google Scholar]
- Grummt F. Diadenosine 5',5'''-P1,P4-tetraphosphate triggers initiation of in vitro DNA replication in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):371–375. doi: 10.1073/pnas.75.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Waltl G., Jantzen H. M., Hamprecht K., Huebscher U., Kuenzle C. C. Diadenosine 5',5'''-P1,P4-tetraphosphate, a ligand of the 57-kilodalton subunit of DNA polymerase alpha. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6081–6085. doi: 10.1073/pnas.76.12.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann H. J., Lezius A. G. High-molecular-weight DNA polymerases from mouse myeloma. Purification and properties of three enzymes. Eur J Biochem. 1975 Jan 2;50(2):357–366. doi: 10.1111/j.1432-1033.1975.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Herrick G., Delius H., Alberts B. Single-stranded DNA structure and DNA polymerase activity in the presence of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2142–2146. [PubMed] [Google Scholar]
- Hesslewood I. P., Holmes A. M., Wakeling W. F., Johnston I. R. Studies on the purification and properties of a 6.8-S DNA polymerase activity found in calf-thymus DNA polymerase-alpha fraction. Eur J Biochem. 1978 Mar;84(1):123–131. doi: 10.1111/j.1432-1033.1978.tb12148.x. [DOI] [PubMed] [Google Scholar]
- Holmer A. M., Hesslewood I. P., Johnston I. R. The occurrence of multiple activities in the high-molecular-weight DNA polymerase fraction of mammalian tissues. A preliminary study of some of their properties. Eur J Biochem. 1974 Apr 16;43(3):487–499. doi: 10.1111/j.1432-1033.1974.tb03436.x. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Hesslewood I. P., Johnston I. R. Evidence that DNA polymerase-alpha of calf thymus contains a subunit of molecular weight 155000. Eur J Biochem. 1976 Feb 16;62(2):229–235. doi: 10.1111/j.1432-1033.1976.tb10152.x. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kornberg A. The delta subunit of Escherichia coli DNA polymerase III holoenzyme is the dnaX gene product. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6284–6288. doi: 10.1073/pnas.76.12.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Kornberg A. The dnaZ protein, the gamma subunit of DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980 Dec 25;255(24):11698–11703. [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Variation of DNA polymerases-alpha, -beta. and -gamma during perinatal tissue growth and differentiation. Nucleic Acids Res. 1977 Aug;4(8):2917–2929. doi: 10.1093/nar/4.8.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Spanos A., Albert W., Grummt F., Banks G. R. Evidence that a high molecular weight replicative DNA polymerase is conserved during evolution. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6771–6775. doi: 10.1073/pnas.78.11.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson K. O., McHenry C. S. Purification and characterization of the beta subunit of the DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10984–10990. [PubMed] [Google Scholar]
- Kaguni L. S., Clayton D. A. Template-directed pausing in in vitro DNA synthesis by DNA polymerase a from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Feb;79(4):983–987. doi: 10.1073/pnas.79.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Falk H. H. An oligoribonucleotide polymerase from SV40-infected cells with properties of a primase. Nucleic Acids Res. 1982 Apr 10;10(7):2309–2321. doi: 10.1093/nar/10.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Changes in DNA polymerases alpha, beta, and gamma during the replicative life span of cultured human fibroblasts. Biochemistry. 1982 Mar 2;21(5):1002–1009. doi: 10.1021/bi00534a027. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukage A., Sivarajan M., Wilson S. H. Studies on DNA alpha-polymerase of mouse myeloma: partial purification and comparison of three molecular forms of the enzyme. Biochemistry. 1976 Nov 30;15(24):5305–5314. doi: 10.1021/bi00669a017. [DOI] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- McHenry C. S. Purification and characterization of DNA polymerase III'. Identification of tau as a subunit of the DNA polymerase III holoenzyme. J Biol Chem. 1982 Mar 10;257(5):2657–2663. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Scott J. V., Kornberg A. A temperature-sensitive single-stranded DNA-binding protein from Escherichia coli. J Biol Chem. 1980 Apr 10;255(7):2897–2901. [PubMed] [Google Scholar]
- Morioka K., Terayama H. A conversion factor for cytoplasmic DNA polymerase of rat liver. Biochem Biophys Res Commun. 1974 Nov 27;61(2):568–575. doi: 10.1016/0006-291x(74)90995-4. [DOI] [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M. Detection of a DNA-binding factor associated with mammalian DNA polymerase-alpha. Biochem Biophys Res Commun. 1978 May 15;82(1):255–264. doi: 10.1016/0006-291x(78)90603-4. [DOI] [PubMed] [Google Scholar]
- Novak B., Baril E. F. HeLa DNA polymerase alpha activity in vitro: specific stimulation by a non-enzymic protein factor. Nucleic Acids Res. 1978 Jan;5(1):221–239. doi: 10.1093/nar/5.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B., Baynes M., Knippers R. A single-strand-specific DNA-binding protein from mouse cells that stimulates DNA polymerase. Eur J Biochem. 1977 Feb 15;73(1):17–24. doi: 10.1111/j.1432-1033.1977.tb11287.x. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C., Baril E. F. Association of diadenosine 5',5"'-P1,P4-tetraphosphate binding protein with HeLa cell DNA polymerase alpha. J Biol Chem. 1981 Dec 10;256(23):12148–12151. [PubMed] [Google Scholar]
- Richter A., Knippers R., Otto B. Interaction of a mammalian single strand specific DNA binding protein with DNA polymerase alpha. FEBS Lett. 1978 Jul 15;91(2):293–296. doi: 10.1016/0014-5793(78)81195-8. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Hurwitz J. Enzymatic breakage of deoxyribonucleic acid. II. Purification and properties of endonuclease IV from T4 phage-infected Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6192–6198. [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Spanos A., Sedgwick S. G., Yarranton G. T., Hübscher U., Banks G. R. Detection of the catalytic activities of DNA polymerases and their associated exonucleases following SDS-polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Apr 24;9(8):1825–1839. doi: 10.1093/nar/9.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani G., Fay P. J., Bambara R. A., Lehman I. R. Elongation of RNA-primed DNA templates by DNA polymerase alpha from Drosophila melanogaster embryos. J Biol Chem. 1981 Aug 10;256(15):8202–8207. [PubMed] [Google Scholar]
- Villani G., Sauer B., Lehman I. R. DNA polymerase alpha from Drosophila melanogaster embryos. Subunit structure. J Biol Chem. 1980 Oct 10;255(19):9479–9483. [PubMed] [Google Scholar]
- Weissbach A., Baltimore D., Bollum F., Gallo R., Korn D. Nomenclature of eukaryotic DNA polymerases. Science. 1975 Oct 24;190(4212):401–402. doi: 10.1126/science.1179222. [DOI] [PubMed] [Google Scholar]
- Weissbach A. The functional roles of mammalian DNA polymerase. Arch Biochem Biophys. 1979 Dec;198(2):386–396. doi: 10.1016/0003-9861(79)90511-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tanabe K., Takahashi T., Matsukage A. Chick embryo DNA polymerase alpha. Polypeptide components and their microheterogeneity. J Biol Chem. 1982 Apr 25;257(8):4484–4489. [PubMed] [Google Scholar]