Abstract
Encountering another’s suffering can elicit both empathic distress and empathic care—the warm desire to affiliate. It remains unclear whether these two feelings can be accurately and differentially predicted from neural activity and to what extent their neural substrates can be distinguished. We developed fMRI markers predicting moment-by-moment intensity levels of care and distress intensity while participants (N=66) listened to true biographies describing human suffering. Both markers’ predictions correlated strongly with self-report in out-of-sample participants (r = .59 and r = .63, ps<.00001), and both markers predicted later trial-by-trial charitable donation amounts (ps<.05). Empathic care was preferentially associated with nucleus accumbens and medial orbitofrontal cortex activity, while distress was preferentially associated with premotor and somatosensory cortical activity. In tests of marker specificity with an independent behavioral sample (N=200), the empathic care marker was associated with a mixed-valence feeling state while the empathic distress marker was specific to negative emotion.
Keywords: empathy, compassion, altruism, biomarker, prosocial
eTOC blurb
Ashar et al. present fMRI markers predicting the intensity of two different empathic emotions in response to naturalistic, dynamic stimuli. The markers predicted charitable donations, were supported by distinct large-scale brain systems, and were differentially related to eight other feelings.
Introduction
Distinctions among multiple systems are central to recent conceptualizations of empathy and compassion (Ashar, Andrews-Hanna, Dimidjian, & Wager, 2016; de Waal, 2008; Kanske, Böckler, Trautwein, Lesemann, & Singer, 2016; Shamay-Tsoory, Aharon-Peretz, & Perry, 2009; Singer & Klimecki, 2014; Zaki & Ochsner, 2012). An ‘affective empathy’ system is believed to support the sharing or simulation of others’ affective experiences, often leading to personal distress for the empathizer. A ‘cognitive empathy’ system, related to mentalizing and perspective taking, supports the conceptual understanding of others’ internal states. And an ‘empathic care’ system supports responding to others’ distress with warmth and care, and is closely associated with sympathy and compassion. These distinctions—especially between empathic care and empathic distress—are at the heart of several recent debates. Scientists have argued that shared experiences of distress are a poor guide for moral behavior and public policy, which ought to instead rely on empathic care and perspective-taking (Bloom, 2016; Lamm & Majdandžić, 2015); that empathic distress causes burnout among professional caregivers while empathic care leads to sustained functioning and fulfillment (Klimecki & Singer, 2011; Zenasni, Boujut, Woerner, & Sultan, 2012); and that empathic distress leads to avoidance, while empathic care leads to helping behavior (Batson, 2011; Batson, Fultz, & Schoenrade, 1987; Eisenberg et al., 1989).
Neuroscientific evidence suggests that empathic care and distress are supported by distinct brain systems. Neuroimaging investigations of altruistic and prosocial behavior, which may be preferentially linked to care rather than to distress, have linked these behaviors to the mesolimbic dopamine system, including the ventral tegmental area (VTA), ventral striatum (VS), and ventromedial prefrontal cortex (vmPFC) (Harbaugh, Mayr, & Burghart, 2007; Hare, Camerer, Knoepfle, & Rangel, 2010; Moll et al., 2006; Morelli, Sacchet, & Zaki, 2015; Zaki & Mitchell, 2011), especially in the context of positive affect (Genevsky & Knutson, 2015; Genevsky, Västfjäll, Slovic, & Knutson, 2013). In addition, a septal/anterior hypothalamic circuit has been linked to a range of affiliative emotions and behaviors across species, including maternal and juvenile play behavior in rats (Bredewold, Schiavo, van der Hart, Verreij, & Veenema, 2015; Numan, 1988), and in humans, trust (Krueger et al., 2007), charitable donation (Moll et al., 2006), prosocial behavior (Morelli, Rameson, & Lieberman, 2014), support giving (Inagaki & Eisenberger, 2012), and affiliative emotion (Moll et al., 2012, 2014).
Empathic distress—the negative affect arising in response to others’ suffering—has been investigated most often by presenting participants with cues of others’ physical or emotional pain, such as a photograph of an injured hand. Meta-analyses of these studies, which are thought to elicit more distress than care, report robust activation of the anterior midcingulate (aMCC) and anterior insula (aIns) (Fan, Duncan, de Greck, & Northoff, 2011; Lamm, Decety, & Singer, 2011). Observing emotional pain also has been linked to activity in regions associated with mentalizing, including the dorsomedial prefrontal cortex (dmPFC), posterior cingulate (PCC), precuneus, and temporo-parietal junction (TPJ) (Bruneau, Dufour, & Saxe, 2012; Bruneau, Pluta, & Saxe, 2012; Immordino-Yang, McColl, Damasio, & Damasio, 2009; Krishnan et al., 2016; Lutz, Brefczynski-Lewis, Johnstone, & Davidson, 2008; Masten, Morelli, & Eisenberger, 2011; Meyer, Masten, & Ma, 2013; Morelli et al., 2014). Empathic distress may stem partly from the sharing or simulation of the other’s suffering (Batson, 2011; Decety & Lamm, 2011; Raz et al., 2013), and thus could be supported by putative “mirror” regions, including premotor cortex, inferior parietal lobe, and the inferior frontal gyrus (Gallese, Keysers, & Rizzolatti, 2004; Iacoboni & Dapretto, 2006; Molenberghs, Cunnington, & Mattingley, 2012; Mukamel, Ekstrom, Kaplan, Iacoboni, & Fried, 2010), as well as somatosensory cortex when somatosensation is involved (Keysers, Kaas, & Gazzola, 2010).
We addressed three open neuroscientific questions regarding empathic care and distress. First, can activity in different brain systems accurately and differentially predict the reported intensity of care and distress, and are the brain systems involved consistent enough across individuals to provide accurate predictions in new individuals? Previous investigations have typically studied these emotions in isolation. And while brain patterns have been shown to reliably track basic affective states in novel individuals (Chang, Gianaros, Manuck, Krishnan, & Wager, 2015; Kassam, Markey, Cherkassky, Loewenstein, & Just, 2013; Knutson, Rick, Wimmer, Prelec, & Loewenstein, 2007; Kragel & LaBar, 2015; Saarimaki et al., 2015; Wager et al., 2015), it remains unknown whether the same is possible for conceptually elaborated and empathy-related feelings (but see Kassam et al., 2013). Patterns that are predictive in novel individuals have several translational applications. They can be tested for generalizability and validity across diverse task paradigms, be used for prognosis and subtyping of individuals, and can serve as targets for interventions (Woo, Chang, Lindquist, & Wager, 2017).
Second, it remains unclear how brain systems support moment-by-moment changes in empathic responses to ecological stimuli. Prior research has largely ignored the temporal dynamics of empathic processes, instead focusing on the overall affective response to brief stimuli. The temporal dimension of affect is critical for healthy functioning and decision-making (Houben, Van Den Noortgate, & Kuppens, 2015; Kahneman, Fredrickson, Schreiber, & Redelmeier, 1993) though it has been understudied in neuroscientific investigations (Admon & Pizzagalli, 2015; Heller et al., 2013).
Third, it remains unclear how empathic care and distress differentially motivate helping. Conflicting evidence suggests that distress predicts both escape behavior (Batson, 2011; Batson et al., 1987) and helping behavior (Ashar, Andrews-Hanna, Yarkoni, et al., 2016) and that charitable donation is motivated by brain activity related to both positive affect (Genevsky & Knutson, 2015; Genevsky et al., 2013) and negative affect (Sawe & Knutson, 2015).
To address these three questions, we developed a novel study paradigm in which participants (N = 66) listened to true biographies describing a range of human suffering during functional Magnetic Resonance Imaging (fMRI). Biography content emphasized ecological validity and stimulus diversity, describing children born with congenital disease, adults struggling with cancer, experiences of homelessness, and other hardships. Participants provided moment-by-moment ratings of empathic care and distress while listening to these biographies a second time, after the scanning session. We then developed two whole-brain neural markers tracking these moment-by-moment ratings of empathic care and distress, and their ability to predict reported emotional experience from the brain activity of out-of-sample test subjects.
To better understand how empathic care and distress are related to a range of other emotions (Condon & Barrett, 2013; Shaver, Schwartz, Kirson, & O’Connor, 1987), we collected an independent behavioral dataset (N = 200) for testing marker specificity relative to sadness, fear, disgust, anger, happiness, surprise, positive valence, and negative valence. An advantage of our analytic approach, which used normative (group-average) time courses to develop the care and distress brain markers, is that it allowed us to map normative time courses for the eight other feelings collected in the behavioral sample on to the neuroimaging data. We then tested to what extent the empathic care and distress brain markers predicted these other feelings.
Finally, we gave participants the opportunity to make charitable donations from their participation earnings ($0 – $100) and tested the extent to which the care and distress brain marker responses predicted trial-by-trial variation in charitable donation amounts. To assess incremental validity, we compared the markers’ predictions of donation with predictions derived from self-reported care and distress and from two regions of interest (the vmPFC and NAc) previously linked to charitable donation.
Results
Reported care and distress and charitable donation behavior
Empathic care and distress ratings were made moment-by-moment using a visual analogue scale while participants listened to the biographies for a second time outside of the scanner, to avoid rating-related confounds during imaging. Each participant was assigned to rate care for half the biographies and distress for the other half, with a randomized counter-balanced assignment of biographies to care or distress across participants. Ratings of care and distress increased strongly during biography presentation, with an average peak care rating of M = 74.90, SD = 8.17 and distress rating of M = 68.86, SD = 6.50, on a 1 – 100 scale anchored from “not at all” to “extremely”. Distress typically peaked at the midpoint of each biography, Mtime of peak = 17.33 s, SD = 5.62 s, while empathic care typically peaked towards the end of each story, Mtime of peak = 30.71 s, SD = 5.66 s, although intensities and temporal profiles differed across biographies (Figure 1a, Figure S1, biography texts listed in Table S1). Ratings of care and distress were positively correlated at an average of r(22) = .25, SD = .49 across biographies, indicating partial dissociability of these two emotions.
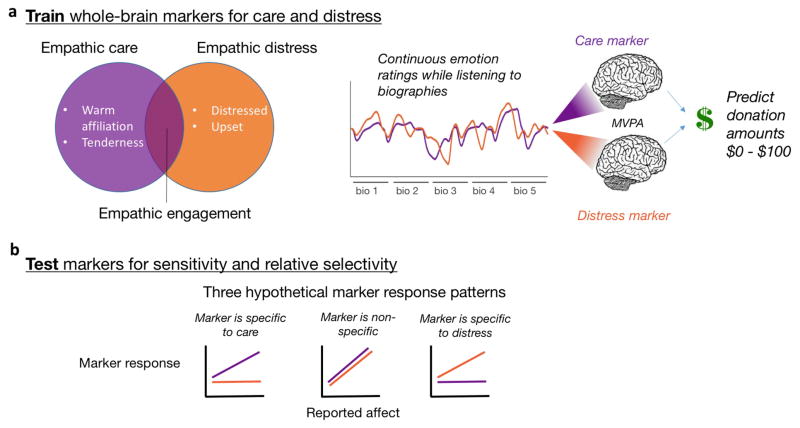
Figure 1.
Theoretical and analytic framework. (a) The encounter with a suffering person can elicit both empathic care and empathic distress. Participants provided continuous ratings of these emotions while listening to biographies describing true stories of human suffering. We developed whole-brain patterns predicting ratings of empathic care and distress from fMRI activity, and used marker responses to predict real-money trial by trial charitable donation amounts. (b) In leave-one-out cross-validation, we tested the sensitivity and relative selectivity of both markers, applying them to held-out test data for empathic care and distress.
Participants donated an average of $21 per trial, with variability across participants (SD = $17.36) and biographies (SD = $4.00). Participants knew that one donation trial would be randomly chosen and implemented at the end of the experimental session (Hare et al., 2010), resulting in $1,480 total donated to charitable organizations.
Neural markers of empathic care and distress
We used machine-learning-based regression techniques to identify two whole-brain patterns (“ brain markers”) that quantitatively predicted the intensity of moment-by-moment empathic care and empathic distress ratings (Figure 1a). We first computed the group-average time course of care and distress for each biography, to increase the reliability of the continuous ratings and because each subject had only rated either care or distress for a given biography. We then binned the care and distress group-average time courses into five quintiles representing five intensity levels across all biographies, to reduce the influence of extreme values and to make the data more computationally manageable. We estimated contrast images representing these five intensity levels of care and distress for each subject, and submitted these to a Support Vector Regression (SVR) predicting emotion intensity level (1 – 5) in out-of-sample participants (leave-one-subject-out cross-validation) (Chang et al., 2015; Kassam et al., 2013; Kragel & LaBar, 2015; Saarimaki et al., 2015; Wager et al., 2013). In the held-out participant, the contrast images representing five emotion intensity levels were estimated using that participant’s continuous ratings of care and distress, and not the group-average ratings that were used to create the training data. Finally, to localize the brain regions most reliably involved in prediction (Figure 2a), we bootstrap-resampled the SVR analyses 5,000 times, generating p-values for each voxel (Wager et al., 2013), and applied a voxel-wise threshold of false discovery rate (FDR) q < .05 (Genovese, Lazar, & Nichols, 2002).
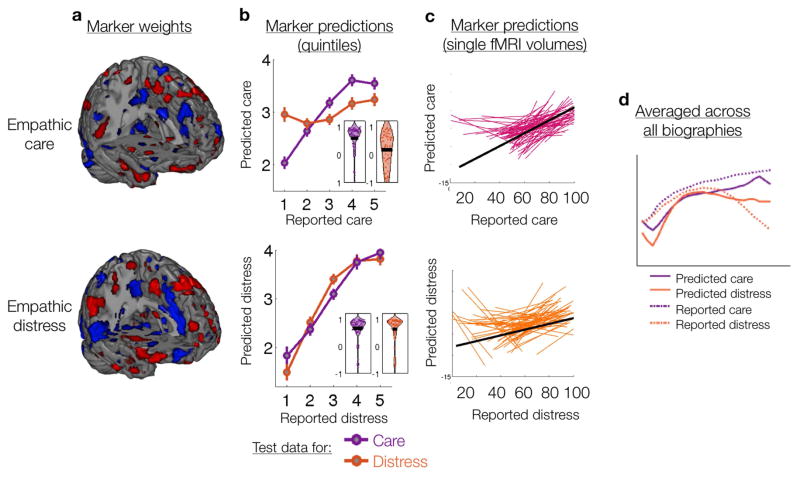
Figure 2.
Marker weights and predictions. (a) Marker voxel weights reliably contributing to prediction across 5,000 bootstrap samples thresholded at FDR q < .05. Positive (red) and negative (blue) weights indicate that more intense emotion was predicted by increased and reduced activity, respectively. (b) Using leave-one-subject-out cross-validation, markers predictions were computed for both empathic care and distress test data across five emotion intensity levels (mean ± SEM). Both markers were strongly predictive of the emotion they were trained to track, and the empathic care marker was relatively selective in predicting empathic care. The inset violin plots show the distribution of within-subject prediction-outcome correlations. (c) Markers predicted fluctuations in emotional intensity at the resolution of single fMRI volumes. With leave-one-subject-out cross-validation, we compared marker responses to single fMRI volumes with the emotional intensity reported at that moment. Each subject is represented by a colored line, and the black line shows the group average relationship. (d) Predicted empathic care and distress tracked the temporal unfolding of emotional experience during biography presentation. Shown here is the average across all biographies; results for each biography are presented in Figure S1. (e) Marker responses during biography presentation predicted later charitable donation to that biography at the trial level.
Significantly predictive brain regions were distributed across multiple brain systems for both the empathic care and distress markers (Figure 2a, Tables S4 and S5). Significant positive and negative weights indicated that more intense emotion was predicted by increased and reduced activity, respectively. The two markers had overlapping positive weights in the anterior mPFC, OFC, VS, supplementary motor area, precuneus, and bilateral mid- and posterior insula (Figure S2a). These regions have been implicated in mentalizing, empathy, and affect more broadly (Ashar, Andrews-Hanna, Dimidjian, et al., 2016; Zaki & Ochsner, 2012). Brain regions preferentially related to either care or distress were probed in further analyses described below.
We assessed both the sensitivity and relative specificity of each marker—the extent to which it tracked the emotion it was trained to track, and did not track the other emotion (Chang et al., 2015; Kassam et al., 2013; Koutsouleris et al., 2015; Kragel & LaBar, 2015; Pantazatos, Talati, Schneier, & Hirsch, 2014; Peelen, Atkinson, & Vuilleumier, 2010; Saarimaki et al., 2015; Wager et al., 2013; Woo et al., 2014). Because predictions were unbiased (tested in independent participants), two likely outcomes were that a marker would (a) track only the emotion it was trained on, demonstrating emotion-specificity; or (b) track both emotions, thus capturing aspects of emotional engagement common to both care and distress (Figure 1b). The marker trained on care predicted care in out-of-sample subjects with high accuracy, with an average within-subject correlation of r(3) = .59, p <.001, while its predictions of distress were significantly worse, average r(3) = .18, p < .001; care vs. distress: T(65) = 4.79, p < .001 (Figure 2b). The marker was thus sensitive and specific to care relative to distress. In contrast, the marker trained on distress predicted both distress and care with similarly high accuracy in novel subjects, with average within-subject correlations of r(3) = .63 and r(3) = .66 respectively, ps <.001; care vs. distress: T(65) = −0.55, p > .1 (Figure 2b). Thus, the distress marker did not exhibit specificity to distress relative to care in this analysis.
We further tested the extent to which these markers, which were trained on binned data (quintiles), would track changes in empathic emotion at the resolution of single fMRI volumes. For held-out subjects, we compared marker predictions for each fMRI volume to self-reported emotion intensity at that moment (Figure 2c). Prediction-outcome correlations were significantly greater than zero at single-volume resolution, with an average within-subject correlation of r(310) = .24, p < .000001 for care and r(310) = .10, p < .0001 for distress. Additionally, the more group-average reported care and distress diverged from each other during listening to a particular biography, the more the group-average fMRI marker activity over successive volumes diverged for that biography as well, r(22) = .38, p = .04 (Figure S1). These results show that the fMRI markers could reliably track the moment-by-moment temporal dynamics of empathic care and distress.
Brain regions selective for empathic care and distress
We searched for brain regions selectively related to empathic care and to empathic distress to better understand their distinct brain substrates. To identify regions selective for empathic care, we identified those that (a) had significantly more positive weights for the empathic care marker than for the distress marker in the multivariate patterns, and (b) were significantly positively correlated with empathic care in univariate voxel-by-voxel analyses, both when controlling and not controlling for distress (see Experimental Procedures). This conjunction revealed that empathic care was preferentially related to activity in mOFC, vmPFC, VS, and septal area (Figure 3a, Figure 4, Table S2), consistent with prior associations of these regions with positive empathic affect, prosocial behavior, and affiliative emotion and behavior (Bredewold et al., 2015; Genevsky & Knutson, 2015; Genevsky et al., 2013; Harbaugh et al., 2007; Hare et al., 2010; Inagaki & Eisenberger, 2012; Klimecki, Leiberg, Lamm, & Singer, 2012; Klimecki, Leiberg, Ricard, & Singer, 2014; Krueger et al., 2007; Moll et al., 2012, 2014, 2006; Morelli et al., 2015; Numan, 1988; Zaki & Mitchell, 2011). Empathic care also was associated with precuneus/posterior cingulate activity, a key node of the mentalizing system often active in response to observing emotional suffering (Bruneau, Dufour, & Saxe, 2013; Bruneau, Dufour, et al., 2012; Bruneau, Pluta, et al., 2012; Immordino-Yang et al., 2009; Masten et al., 2011; Meyer et al., 2013; Morelli et al., 2014).
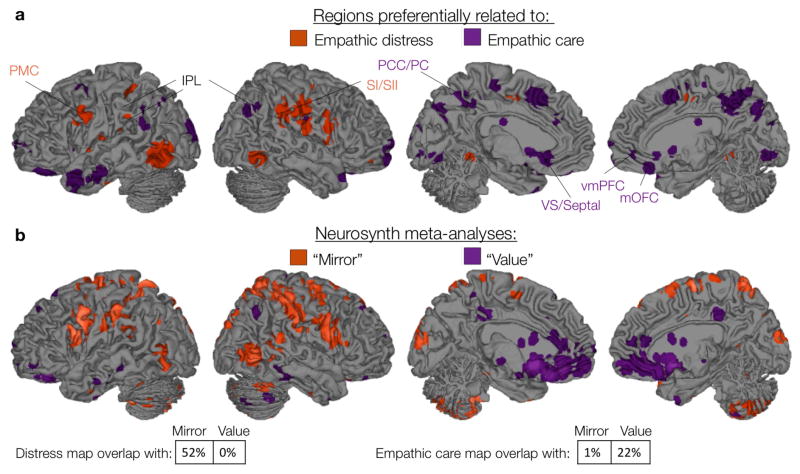
Figure 3.
Brains systems preferentially related to empathic care or distress overlap with brain systems related to value and mirroring, respectively. (a) Regions sensitive and relatively selective for empathic care and distress, in both multivariate and univariate analyses (see text for details). (b) Meta-analytic maps for the terms “mirror” and “value” (reverse inference), thresholded at FDR q = .01 (Yarkoni et al., 2011). PMC = premotor cortex, IPL = inferior parietal lobe, SI/SII = primary/secondary somatosensory cortex, PCC = posterior cingulate, PC = precuneus, VS = ventral striatum, vmPFC = ventromedial prefrontal cortex, mOFC = medial orbitofrontal cortex.
Figure 4.
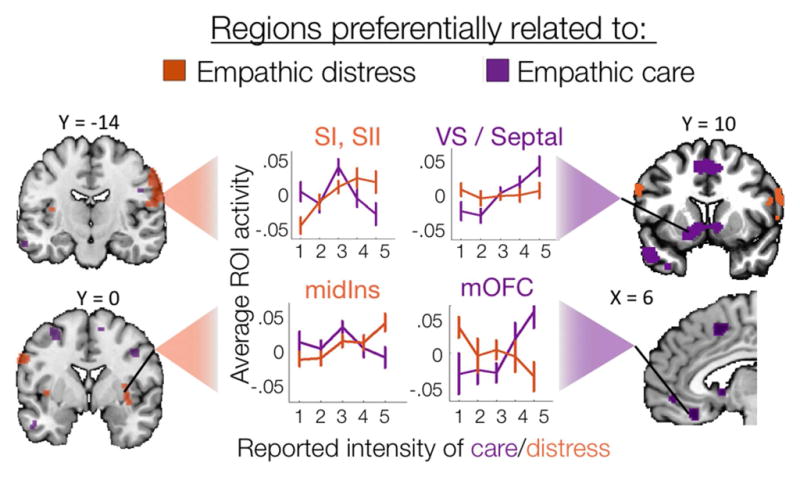
Regions identified in previous analyses (see Figure 3) as sensitive and specific to empathic care or distress show the expected pattern of response across emotion intensity levels. Plots depict ROI-average activity across increasing intensity levels of care (purple) and distress (orange) (1 = lowest intensity, 5 = highest intensity, mean ± 95% CIs).
We conducted corresponding analyses to identify brain regions sensitive and relatively specific to empathic distress. We found that distress was preferentially associated with activity in the left ventral premotor cortex, bilateral inferior parietal lobe (IPL), and bilateral somatosensory cortex (SI and SII) (Figure 3a, Figure 4, Table S3). Meta-analyses have linked these somatosensory and premotor regions to both the experience and the observation of actions, sensations, and emotional facial expression (Keysers et al., 2010; Molenberghs et al., 2012), leading them to be considered as part of a “mirror” system (Gallese et al., 2004). Empathic distress was also preferentially related to left mid-insula and a right hemisphere cluster spanning mid-insula, claustrum, and putamen. This is consistent with previous work in which participants asked to imagine themselves in painful situations (vs. imagining someone else in those painful situations) reported increased distress and recruited a bilateral mid-insula region overlapping with the activation reported here (Jackson, Brunet, Meltzoff, & Decety, 2006; Lamm, Batson, & Decety, 2007).
We confirmed that these regions were in fact sensitive and relatively selective to empathic care or distress by plotting their average activity across binned intensity levels (quintiles) of both care and distress (Figure 4). We also confirmed our interpretation of the care-specific regions as related to valuation processes and the distress-specific regions as related to mirroring processes by comparing our results to reverse inference meta-analytic maps from Neurosynth for the terms “value” and “mirror” (Yarkoni, Poldrack, Nichols, & Wager, 2011) (Figure 3b). Neurosynth reverse-inference maps show brain regions that are selectively related to a given term (i.e., “value” or “mirror”) relative to the hundreds of other terms in the database collected from more than 10,000 fMRI studies.
Relation of empathic care and distress to other feelings
Encountering others’ suffering can elicit a complex array of feelings. To understand how the empathic care and distress markers related to other feelings, we asked an independent nation-wide sample of participants (N = 200) to listen to the same biographies and provide moment-by-moment ratings of: sadness, fear, disgust, anger, happiness, surprise, positive valence, negative valence, as well as replication ratings of care and distress for testing reliability across the two samples. In an online experiment (with no neuroimaging) participants rated one feeling for each biography, with a different random assignment of biographies to the feeling rated for each participant. The time courses of group-average care and distress ratings were highly correlated across the fMRI and behavioral samples, average r = .81, T(23) = 10.67, p < .00001 for care, and average r = .82, T(23) = 15.15, p < .00001 for distress (averaged and tested across biographies). This indicates that the emotional responses elicited by the biographies were similar for both samples.
To investigate the interrelationship of the ten rated feelings, we computed the normative (group-average) time course of each feeling for each biography, with n = 20 participants contributing data for each biography-feeling pair (time courses for sample stimuli shown in Figure 5a, all stimuli show in Figure S3). We then calculated the pairwise inter-correlations for each of the ten feelings for each biography, and averaged them across biographies into a 10 × 10 matrix. We submitted this matrix to a hierarchical clustering analysis. This revealed three feeling clusters: 1) a negative feeling cluster, including sadness, empathic distress, anger, fear, disgust, and negativity, which were correlated on average r = .80 +/− .02 (SEM); 2) a positive feeling cluster, including happiness and positivity, which were highly correlated at r = .95; and 3) a bivalent cluster including empathic care and surprise, which were correlated at r = .56 (Figure S4). The biographies thus elicited a blend of emotions. In this blend, empathic care emerged as a distinct measure, differentiable from both distress (average r = .29 +/− .11 SEM across biographies) and positive emotion (average r = .63 +/− .04 SEM across biographies). During presentation of the biographies, empathic care tended to steadily increase, negative emotions tended to rise and fall, and positive emotions tended to rise towards the end of biographies (Figure 5a and Figure S3). Overall, our findings agrees with previous work showing that compassion and affiliative emotion do not have a clear, consistent valence (Condon & Barrett, 2013; Moll et al., 2012, 2014).
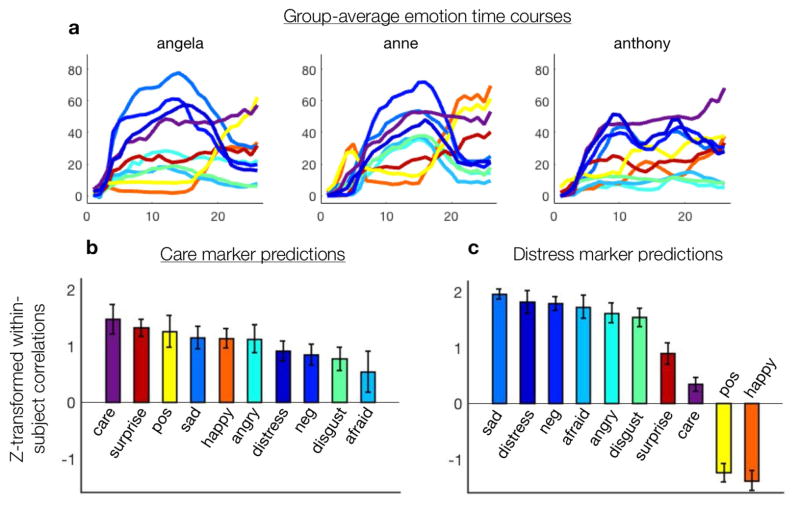
Figure 5.
Relation of empathic care and distress to eight other feelings. a) Group-average continuous time courses (in seconds) of feelings provided by an independent behavioral sample (N = 200), shown for three sample biographies. These time courses were mapped onto neuroimaging data from the fMRI sample (N = 66). b) To assess the specificity of the empathic care and distress brain markers, we tested to what extent each marker correlated with the group-average time course of each feeling. This approach tracks the time course of feelings but not the absolute intensity of feelings. The care marker tracked empathic care significantly more strongly than any other emotion, and it was positively correlated with a range of positively and negatively valenced emotions. c) The distress marker was positively correlated with a cluster of negative emotions, negatively correlated with the two positive emotions, and had a relatively weak relationship with care and surprise, the two emotions with ambiguous valence.
We then tested to what extent the care and distress markers tracked the time courses of these ten different feelings, to better characterize their specificity. An advantage of our approach, which used normative time courses to develop brain markers that can be applied to out-of-sample individuals, is that it allowed us to map the data collected in the online sample with the neuroimaging data. Paralleling the methods described above, we binned the group-average time courses for each feeling into five quintiles representing increasing intensity levels of that feeling. This approach was insensitive to absolute intensity differences between feelings, and was only sensitive to relative changes in intensity within each emotion. We then computed contrast images for each subject for each intensity level for each feeling, creating ten different sets of images representing normative intensity for the ten different feelings. Finally, we applied the care and distress markers to these sets of images, assessing how closely the care and distress markers tracked each of the ten normative feeling time-courses.
The empathic care marker tracked ratings of empathic care from the online sample significantly more strongly than it tracked any of the other 9 emotions collected in that sample (Figure 5b) (care vs. next closest emotion, surprise, T(65) = 1.77, p = .04 one-tailed). The empathic care marker also correlated to a varying degree with a range of other feelings, including those with positive and with negative valence. This suggests that empathic care marker is most closely related to empathic care per se, but also reflects to some extent aspects of other emotions. This may be because these emotions partially share underlying neural systems (Figure 1a) and/or because these emotions were only partially dissociated in our ecological stimulus set.
The empathic distress marker was positively correlated with a cluster of negatively valenced emotions (sadness, anger, disgust, fear, and general negativity) and was negatively correlated with happiness and general positivity (Figure 5c). It was also positively correlated with empathic care, though more weakly than in the original neuroimaging sample. These results suggest that the empathic distress marker is most closely related to a non-specific negative arousal in response to others’ suffering, in line with how we and others have conceptualized empathic distress (Ashar, Andrews-Hanna, Yarkoni, et al., 2016; Ashar, Andrews-Hanna, Dimidjian, et al., 2016; Batson, 2011; Singer & Klimecki, 2014).
Predicting charitable donation amounts from the neural markers
Using leave-one-subject-out cross-validation, we tested whether brain marker response over the course of a biography could predict later charitable donation amounts to the charity associated with that biography. We computed for each subject the average empathic care and distress marker responses during each biography. In the training data, we estimated a model predicting donation from the brain markers using a multi-level regression. We then applied this model to the test subject and correlated predicted and observed donations to generate cross-validated R2 values for each subject. When considered separately (i.e., including only one marker as a predictor in the regression), the response of both brain markers positively predicted later charitable donation amounts at a trial-by-trial level in out-of-sample-subjects, βcare = 0.03, p = .04, average within-subject R2care = .05, 95% CI [0.03 0.06]; βdistress = 0.02, p = .02, average within-subject R2distress = .03, 95% CI [0.02 0.05]. When the two markers were included in the same model, both positively predicted helping behavior, though each fell short of independent statistical significance, βcare = 0.02, p = .14, βdistress = 0.01, p = .15, likely due to collinearity between the marker responses, which had an average within-subject correlation of r(22) = .34, p < .0001. The model including both brain markers yielded an average cross-validated within-subject R2 = .04, 95% CI = [0.03 0.05], T(60) = 6.54, p < .00001.
To test whether the brain markers improved upon donation predictions derived from self-report alone, we conducted a multi-level regression predicting trial-by-trial donation amounts from brain marker activity and continuous care and distress ratings, using the means of those measures across time for each biography. Since participants only provided either care or distress ratings on each trial, we split each participant’s data according to which self-report rating was available for a given trial and conducted a regression on each half of the data (i.e., 12 trials per subject). Self-reported care was a very strong predictor of donation, T(60) = 7.75, p < .00001, controlling for both brain markers. Controlling for self-reported care, the distress marker also positively predicted donation, T(60) = 2.22, p = .03, while the care marker did not, p > .4. In the other half of the data, average self-reported distress predicted increased donation, T(60) = 1.81, p = .075, though with only marginal significance, and neither the care nor the distress marker significantly predicted donation when controlling for self-reported distress, p’s > .1. Overall, comparisons to self-report revealed that self-reported care was the strongest predictor of donation, and that the distress marker contributed to the prediction of donation above and beyond self-report, although the regressions with only half the data had low statistical power.
We also tested the ability of the brain markers to predict donation relative to two ROIs previously predictive of donation—the nucleus accumbens (NAc) (i.e., Harbaugh et al., 2007; Genevsky et al. 2013, 2015) and the vmPFC (i.e., Moll et al., 2006; Hare et al 2010; Cooper et al., 2010). For both the NAc and vmPFC, we submitted the average ROI activity during each trial to a multi-level regression predicting charitable donation amounts on a trial by trial level. We found that NAc activity significantly predicted charitable donation amounts, p = .045 one-tailed. The vmPFC ROI-average activity was not reliably related to charitable donation amounts, p > .6. When including the care marker, distress marker, and NAc all in the same model, the distress marker was marginally significant, p = .08, while the care marker and the NAC were not significant controlling for the other predictors. This was likely influenced by collinearity between the predictors—NAc activity was positively correlated with the care marker, average r(22) = .13, p < .001 across subjects, and negatively correlated with the distress marker, r(22) = −.06, p < .05. Overall, these results confirm the link between the NAc and charitable donation (i.e., Harbaugh et al., 2007; Genevsky et al. 2013, 2015), show that the NAc activity and the care marker share variance, and suggest that the empathic distress marker captures additional variance in donation not explained by NAc activity or by empathic care.
Discussion
Recent debates about empathy and compassion have hinged on distinctions between multiple systems. A critical distinction is between empathic care and distress—‘feeling for’ vs. ‘feeling with’—which some have labeled as compassion vs. empathy (Ashar, Andrews-Hanna, Dimidjian, et al., 2016; Batson, 2011; Bloom, 2016; Lamm & Majdandžić, 2015; Shamay-Tsoory et al., 2009; Singer & Klimecki, 2014; Zaki & Ochsner, 2012). Here, we described for the first time brain markers accurately predicting the intensity of empathic care and distress in novel, out-of-sample participants. These markers tracked fluctuations in different, simultaneously unfolding empathic responses to ecological stimuli, predicted later charitable donation decisions, and were differently associated with eight other feelings. We also identified distinct large-scale brain systems preferentially related to empathic care and to empathic distress.
Both the empathic care and distress brain markers were strongly predictive of care and distress, respectively, in novel subjects. In tests of marker specificity, the empathic care marker was most strongly correlated with empathic care ratings. It also correlated positively with other positive and negative feelings to varying degrees, suggesting that the empathic care marker reflected a mixed-valence feeling state. The empathic distress marker was strongly positively correlated with a cluster of negatively valenced emotions, indicating that is relates preferentially to negative emotion. It was also positively correlated with empathic care, more strongly in the neuroimaging sample and relatively weakly in the online behavioral sample. Overall, the empathic care and distress markers demonstrated partial—but not full—specificity to the targeted emotion, possibly due to common underlying brain systems across emotions (Figure S2)(K. A. Lindquist, Satpute, Wager, Weber, & Barrett, 2015; K. A. Lindquist, Wager, Kober, Bliss-moreau, & Barrett, 2012) and/or to the blended nature of emotional responses to naturalistic stimuli. These findings extend previous work on brain-based prediction of emotion (Chang et al., 2015; Kassam et al., 2013; Kragel & Labar, 2016; Kragel & LaBar, 2015; Saarimaki et al., 2015; Wager et al., 2013) by demonstrating the accurate prediction of empathic emotions in response to complex social stimuli.
Predictive markers of empathic processes have several advantages that may position them to contribute to translational neuroscience (Woo et al., 2017). By integrating information across the entire brain, multivariate approaches can improve our ability to infer mental states from brain activity: Information pertaining to mental states can be broadly distributed across the brain (Chang et al., 2015; Huth, Heer, Griffiths, Theunissen, & Jack, 2016; Kragel & Labar, 2016; K. A. Lindquist et al., 2012; Wager et al., 2015). Multivariate approaches also produce potential research products: markers or patterns that can be easily shared between labs, applied to new samples, and tested for sensitivity and specificity across a continually expanding range of conditions. This can enable a cumulative science that ultimately produces rigorously validated markers ready for translational use. In the domain of empathy, validated predictive markers may aid in the diagnosis or prognosis of empathy-related disorders (i.e., autism or psychopathy), serve as proximal biological targets for interventions seeking to enhance compassion (Ashar, Andrews-Hanna, Yarkoni, et al., 2016; Klimecki et al., 2012, 2014; Lutz et al., 2008; Weng et al., 2013), or as targets for biofeedback-based training programs aiming to enhance empathy (Moll et al., 2014; Yao et al., 2016). The markers presented here are a first step in a cumulative process of refinement and validation.
Univariate approaches complement the multivariate approach, and are valuable for explaining the function of particular brain regions. We found that empathic care rather than distress was preferentially related to activity in the vmPFC, mOFC, and VS. This is consistent with the previously reports linking the mesolimbic dopaminergic system to prosocial behavior (Genevsky & Knutson, 2015; Genevsky et al., 2013; Harbaugh et al., 2007; Moll et al., 2006; Morelli et al., 2015), positive feelings toward suffering others (Genevsky & Knutson, 2015; Genevsky et al., 2013; Moll et al., 2012), support giving (Inagaki & Eisenberger, 2012), and compassionate states induced by loving-kindness meditation in contemplative experts and in meditation trainees (Engen & Singer, 2015; Klimecki et al., 2012, 2014). The present findings extend previous work by linking these brain regions more specifically to empathic care rather than the closely related empathic distress. Medial prefrontal and VS activity has been linked to computations of value (Levy & Glimcher, 2012), the degree of closeness with other people (Harris & Fiske, 2007; Krienen, Tu, & Buckner, 2010), thinking about the self (Denny, Kober, Wager, & Ochsner, 2012; Murray, Schaer, & Debbané, 2012), and vicarious reward (Mobbs et al., 2009; Morelli et al., 2015). Taken together, these findings suggest that empathic care, rather than empathic distress, has a preferential relationship with ventromedial prefrontal-striatal systems supporting valuation, affiliation, and self-relevance (Ashar, Chang, & Wager, 2017).
Empathic care was also preferentially related to brain activity in the septal area of the basal forebrain. Converging evidence suggests that this region plays a unique role in affiliative behavior across species. In humans, multiple fMRI investigations have linked this area with trust (Krueger et al., 2007), charitable donation (Moll et al., 2006), prosocial behavior (Morelli et al., 2014), giving support to a romantic partner (Inagaki & Eisenberger, 2012), and affiliative emotion (Moll et al., 2012, 2014). In rats, lesioning and pharmacological manipulations of the septal area suggest a causal relationship with the expression of various affiliative and social behaviors, especially maternal behaviors (Sheehan & Numan, 2000). The septal area also adjoins and is densely interconnected with the anterior hypothalamus (Zaborszky et al., 2008), a region rich in oxytocin and vasopressin magnocellular neurons implicated in maternal and affiliative behavior (Insel, 2010).
Empathic distress, on the other hand, was preferentially related to premotor and somatosensory regions involved in representing both one’s own and others’ bodily states (Iacoboni & Dapretto, 2006). In a meta-analysis of over 100 fMRI studies, these regions were found to reliably activate both during the observation and the execution of actions, sensations, and emotional facial expressions (Molenberghs et al., 2012). Electrophysiological recordings in humans have further confirmed the presence of single neurons within these regions with these same mirroring properties (Mukamel et al., 2010). This brain system may critically support our understanding of others by allowing us to share in or simulate their experiences (Gallese et al., 2004; Keysers et al., 2010; Zaki & Ochsner, 2012). Sharing in or simulating the experience of a suffering other would naturally lead to distress, and the present results suggest that empathic distress rather than empathic care is tied to premotor and somatosensory activity potentially reflecting mirroring-like processes. Since participants did not provide ratings while listening to the biographies in the scanner, these results are unlikely to be due to task-related motor demands.
Both markers also prospectively predicted later charitable donation amounts at a trial-by-trial level, validating the emotion markers against an independent behavioral task. The distress marker predicted donation controlling for self-reported care and for NAc activity. This supports the notion that the distress marker captured negative affect-related brain processes not reflected in NAc activity, as the NAc has been linked specifically to positive affect motivating charitable donation (Genevsky & Knutson, 2015; Genevsky et al., 2013). Separately, we found that self-reported care and distress both positively predicted increased donation, although care was a stronger predictor. This both confirms previous reports that increased distress predicts helping (i.e., Ashar, Andrews-Hanna, Yarkoni, et al., 2016) and lends credence to prior arguments that empathic care rather than distress is the primary motivator of altruism (Ashar, Andrews-Hanna, Dimidjian, et al., 2016; Batson, 2011; Singer & Klimecki, 2014).
We also demonstrated that predictive brain markers can track the temporal unfolding of relatively more complex emotional experiences. Our investigation of dynamic affect may offer a different perspective on affective brain function. For example, we did not find the aMCC or the aIns to be central to empathic care or distress, in distinction from prior work (Corradi-Dell’Acqua, Tusche, Vuilleumier, & Singer, 2016; Fan et al., 2011; Klimecki et al., 2014; Lamm et al., 2011; Singer & Klimecki, 2014). One reason for this may be that the cingulate and insula respond to salient events of multiple modalities, and play a domain general role in detecting and orienting which may not specific to empathy (Legrain, Iannetti, Plaghki, & Mouraux, 2011; Uddin, 2014; Yarkoni et al., 2011). The fluctuations in unfolding empathic emotion modeled here may not have required the same detecting/orienting as required by the brief, highly salient stimuli often used in previous studies. Additionally, the aMCC and aIns may have been more recruited by the task which served as a baseline comparison for fMRI contrasts (see Experimental Procedures).
In conclusion, the current findings demonstrate the feasibility of predicting temporally dynamic empathic emotion elicited by ecological stimuli from the brain activity in novel individuals, with partial specificity with respect to other emotions. Future studies are needed to test the generalizability of brain markers across different psychological contexts. The development of validated predictive markers may facilitate a translational neuroscience of empathy, and ultimately, may help create a kinder, more compassionate society.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Yoni Ashar (yoniashar@gmail.com).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
71 healthy right-handed adults participated between January and July of 2012. Along with the typical magnetic resonance exclusions (e.g., metal in the body), subjects were required to have no history of major psychiatric illness and no current psychiatric conditions. Participants who refused in advance to make charitable donations from their participation earnings were excluded, to increase the likelihood of collecting informative data at the expense of generalizability. Five participants were excluded from analyses due to technical issues, leading to 66 analyzed participants. Of these, n = 26 were men and n = 40 were women, with Mage = 30.1 and age range = 23 – 55. An additional four participants donated the same amount to every biography and thus could not be included in donation analyses—they had no variance in the outcome of interest—yielding 62 participants for the donation analyses. Since participants were later randomized to an intervention, some additional inclusion criteria relevant to the intervention were applied (see Ashar et al., 2016). The University of Colorado Institutional Review Board approved all procedures, including informed consent.
METHOD DETAILS
Biographies of suffering others
During fMRI imaging, participants listened to 24 randomly ordered biographies describing true stories of suffering individuals, such as orphaned children, adults with cancer, and homeless veterans (see Table S1 for examples). Biographies were composed from information posted on charity websites, and were audio recorded by a female member of the research team as clips 26 to 33s in duration. An authentic face photograph of each individual, also drawn from the charity website, was displayed while participants listened to that individual’s biography. The individuals described in the biographies were evenly balanced on age (child or adult), race (Black or White), and sex. Real stories and photographs were used to increase ecological validity. Biographies also concluded with a description of how an (unnamed) charitable organization provided some measure of relief from suffering, to motivate charitable donation. Following each biography, participants made either a rating of overall empathic care or distress using a trackball on a visual analog scale ranging from “not at all” to “extremely” (5 sec); these ratings were not used in the analyses presented here. The rating was followed by a jittered period of 3, 6, 9, or 12 seconds during which we presented arrows to participants and asked them to indicate whether the arrow was left- or right-facing. The arrows task served as a non-social non-affective baseline contrast for the biographies task, and was chosen to interfere with any continued reflection on the biographies between trials. The [biographies – arrows] contrast is depicted in Figure S5.
Charitable donation task
After listening to and rating all the biographies as described above, participants then listened to an abbreviated audio-recorded “reminder” of each biography (8 – 11 s), followed by a donation decision screen with a visual analog scale for selecting the desired donation amount (5 s), followed by the arrows task described above. To encourage participants to make independent choices on each trial, they were informed that at the end of the experimental session, exactly one of their donations would be randomly selected, subtracted from their endowment, and donated to the organization that had helped the individual described in the biography (Hare et al., 2010). $1,500 was distributed to charitable organizations at the conclusion of this study.
Continuous ratings of empathic care and distress
Participants were then removed from the fMRI scanner and listened to the full version of all 24 biographies a second time in a behavioral testing room. While listening, participants provided moment-by-moment ratings of either empathic care or empathic distress, balanced across subjects. Ratings were provided on a visual analog scale ranging from “not at all” to “extremely”, and the slider was initiated to the mid-point of the scale for every trial.
We operationalized care and distress by asking participants to rate “how tender/distressed do you feel right now?”. We used the word “tender” because of prior research strongly linking this word to empathic care (Batson et al., 1987), and because we thought this word would clearly communicate the construct of interest to participants. To ensure understanding, prior to the scan participants were given the following instructions: “Anytime we use the word “tender”, we mean a feeling of caring, openness, and warmth towards another person. When we use the word distress, we mean a feeling of discomfort or suffering for you, as someone listening to these stories.” Participants were then prompted to describe the difference between tender and distress in their own words, and the researcher reviewed the above definitions if the participant had misunderstood our use of these terms.
After each biography, participants also answered six questions selected to measure feelings, attributions, and similarities relevant to charitable donation, and completed surveys measuring traits of interest (results presented in Ashar et al., 2016).
fMRI data acquisition
Images were acquired with a 3.0 T Siemens Trio Tim magnetic resonance imaging scanner using a 16 channel head coil. Twenty-six 3.0-mm-thick slices (in-plane resolution 3.4 × 3.4 × 3.0, 1 mm gap, ascending sequential acquisition) extended axially from the mid-pons to the top of the brain, providing whole-brain coverage. Functional scans were acquired with a T2*-weighted gradient echo pulse sequence (TR = 1.3 s, TE = 25 ms, flip= 75°, field of view = 220 mm, matrix size = 64 × 64 × 26). The biographies task extended over three runs of 284 volumes each, and the charitable donation task extended over two runs of 231 volumes each. High-resolution structural scans were acquired prior to the functional runs with a T1-weighted MP RAGE pulse sequence while participants practiced using the in-scanner response devices (TR = 2530 ms, TE = 1.64 ms, flip = 7°, 192 slices, 1 × 1 × 1 mm). Parallel image reconstruction (GRAPPA) with an acceleration factor of 2 was used.
fMRI data preprocessing
We removed the five initial volumes of each run to allow for image intensity stabilization. To identify volumes with signal values that were outliers within the time series (“spikes”), we then computed both the mean and the standard deviation of intensity values across each slice, for each image. Mahalanobis distances for the matrix of (concatenated) slice-wise mean and standard deviation values by functional volumes (over time) were then computed. Any values with a significant χ2 value (corrected for multiple comparisons based on the more stringent of either false discovery rate (FDR) or Bonferroni methods) were considered outliers. In practice, approximately 1–2% of images were deemed outliers. The outputs of this procedure were later included as nuisance covariates in the first level models. Head motion was then estimated for each subject using SPM8, and 24 head motion covariates were entered into each first level model (displacement in six dimensions, displacement squared, derivatives of displacement, and derivatives squared). Next, functional images were corrected for differences in the acquisition timing of each slice and were motion-corrected (realigned) using SPM8. The functional images were then coregistered to the structural T1-weighted images using the iterative mutual information-based algorithm implemented in SPM8, then interpolated to 2×2×2 mm3 voxels and smoothed with an 8 mm FWHM Gaussian kernel. Lastly, the structural images were warped to SPM’s normative atlas using SPM8 (warping parameters estimated from coregistered, high-resolution structural images), and these warping parameters were applied to the functional data, normalizing it to MNI space.
Continuous ratings of other feelings collected online
A nation-wide sample of participants recruited from Mechanical Turk (N = 200) completed an online experiment in which they listened to the same biographies and provided continuous ratings of sadness, fear, disgust, anger, happiness, surprise, positive valence, negative valence, care, or distress. Quality control measures were used to screen out careless responders, including simple questions to check that participants were paying attention (Meade & Craig, 2012). Participants rated one feeling for each biography, with a different random assignment of biographies to feelings for each participant.
Ratings were provided on a visual analog scale ranging from “not at all” to “extremely”. The slider was initiated to “not at all” for every trial, as we imagined that participants would not be feeling some of these emotions at all at the trial onset. This stands in distinction to the neuroimaging sample, for whom the slider was initiated to the midpoint of the scale. This difference in the slider’s initial position may explain why the empathic distress marker was differently correlated with reported empathic care across the two samples.
The online experiment was programmed in Qualtrics. Participants were compensated $3 for participation and given a $1 endowment for donation to the biographies (donation data from this sample not analyzed here). Demographic data were not collected in this sample.
QUANTIFICATION AND STATISTICAL ANALYSIS
Brain markers of empathic care and empathic distress
We used a two-step framework to identify patterns (“markers”) of fMRI activity predicting empathic care and distress intensity levels in novel individuals, since computational limitations rendered direct MVPA analysis of the full subject-level data unfeasible. This two-step approach has previously been used to successfully predict affect in novel individuals (Chang et al., 2015; Wager et al., 2013; Woo et al., 2014).
We computed the group-average time course of care and distress for each biography and binned these time courses into five quintiles representing five intensity levels of reported care and distress. We then estimated for each subject five contrast images representing each emotion intensity level, using a General Linear Model (GLM) in SPM8. This procedure was designed to limit the influence of outlying time points and to reduce data to a computationally manageable size. Each subject’s GLM included five binary regressors for each emotion intensity level, as well several regressors of no interest: A Listen condition, a Rate condition, 24 head motion covariates, spike indicator covariates, run intercepts, and a linear trend for each run. The Listen condition modeled the presentation of biographies, and the Rate condition modeled the rating of overall empathic care or distress. The emotion time courses were delayed by four volumes (5.2s) prior to binning to account for hemodynamic lag (Wager et al., 2004).
We then submitted the five contrast images from each subject to a cross-subject Support Vector Regression (Spider package for MATLAB) predicting intensity level (1, 2, 3, 4, or 5). Contrast images were first mean-centered within-person within-voxel, as we sought to focus on the dynamics of within-person emotional experience, and because outcome data (i.e., 1 – 5) did not include between-subject variability. The MVPA was conducted in a template whole-brain gray matter mask, to exclude extraneous signal from the feature set. In leave-one-subject-out cross-validation, the marker developed on the training data was applied to the test data by computing a dot product. To help visualize and interpret neuroanatomical features of the marker, we boot-strapped this analysis 5,000 times. We then conducted a one-sample T-test at each voxel, testing the voxel weights across bootstrapped samples against zero. This yielded p-values at each voxel, which were thresholded at FDR q < .05 (Genovese et al., 2002).
The markers were trained on group average timecourse ratings (a form of Empirical Bayes regularization), because doing so improves accuracy to the extent affective responses are consistent across individuals (M. A. Lindquist et al., 2015), and because it was not feasible for participants to provide continuous ratings on both empathic care and distress simultaneously and we thus had idiographic care and distress ratings for only half the trials. The markers were tested, however, on the idiographic emotion timecourse ratings provided by each participant, though data was only available for half the trials. The test data set included contrast images representing quintiles of emotional intensity derived from the self-report of the held-out subject.
To generate marker predictions for individual fMRI volumes, we regressed out the Listen, Rate, and nuisance regressors as described above from the testing subject’s data, and then computed the dot product between each volume of the test data and the markers developed in the training sample (leave-one-subject-out cross-validation). To assess predictive accuracy at the single volume resolution, we 1) correlated each subject’s reported and predicted emotional intensity for each volume (Figure 2c), and 2) compared predicted and reported emotion, averaging across biographies (Figure 1d, Figure S1). For these biography-average predictions, we correlated the divergence between predicted care and distress with the divergence between reported care and distress. This test was conducted one-tailed, as our hypothesis was in one direction only. For ease of visual interpretation, the moment-by-moment predictions shown in Figure 2d and Figure S1 were smoothed (moving average, with a window of 5 volumes).
Analyses to identify regions preferentially supporting empathic care and distress
To identify the regions reliably and specific associated with care, we identified brain regions that were 1) significantly more positively related to care than to distress in multivariate analyses, comparing multivariate marker weights across 5,000 bootstrapped samples (care marker – distress marker), 2) predictive of care in univariate analyses (GLM), and 3) predictive of care controlling for distress in univariate analyses (GLM). Each of these three maps was thresholded at FDR q < .05 (Genovese et al., 2002). The conjunction of these maps represented regions that were positively related to care, and more related to care than to distress, both when considered in isolation and when controlling for activity in other brain regions, allowing for more robust interpretation. Corresponding analyses were also conducted for distress.
Predicting other feelings from marker activity
We used identical procedures as described above for the analyses of care and distress to estimate five contrast images for each subject corresponding to increasing intensity levels of each of the ten feelings. We used for this the group-average rating for each feeling for each biography provided by the online sample.
Predicting charitable donation
We estimated single-trial images for each biography, and computed the dot product between these images and the brain markers. This yielded an empathic care and empathic distress marker response for each biography for each subject. We then conducted a multi-level GLM using the marker responses to predict trial-by-trial variation in charitable donation amounts. Charitable donations were Z-scored within-subject prior to the GLM, to adjust for the large variability in donation patterns in different subjects. For generating unbiased estimates of variance explained in donation behavior, we conducted the multi-level regressions in the context of leave-one-subject-out cross-validation. In the training sample, we fit a model predicting donation from the brain markers. We fit this model to the test sample, generating predicted donations which we then correlated with observed donations. The brain markers trained on the entire sample were used for this analysis, as they were developed independently of the donation data.
Similar analyses were conducted for predicting donation from the NAc and vmPFC, with one difference: we extracted ROI activity from each single trial image (representing average activity over the course of the trial) instead of computing the marker dot product. The NAc was anatomically defined from the Harvard-Oxford atlas, and the vmPFC ROI was created from a 10mm sphere around the average cluster peaks reported by Hare et al 2010 and Cooper et al. 2010, which reported adjacent cluster peaks of [−3 39 −3] and [0 42 −8], MNI coordinates.
For predicting donation from self-report, we computed the average care and distress rating for each biography for each subject, and submitted these to a multi-level GLM. Since subjects provided only care or distress continuous ratings for a particular biography, we divided each subject’s data in half and conducted regressions on each half of the data.
Supplementary Material
Table S4. Clusters in the empathic care marker surviving the FDR q < .05 threshold, related to Figure 2a (top row). Z-values reflect single-sample T-test against zero of the voxel weights across 5,000 bootstrap samples.
Table S5. Clusters in the empathic distress marker surviving the FDR q < .05 threshold, related to Figure 2a (bottom row). Z-values reflect single-sample T-test against zero of the voxel weights across 5,000 bootstrap samples.
Highlights.
fMRI markers predicted the intensity of empathic emotion in novel subjects
The markers dissociated empathic care from distress and predicted charitable giving
Empathic care was preferentially associated with vmPFC, mOFC, and NAcc activity
Empathic distress is associated with premotor and somatosensory cortical activity
Acknowledgments
Gratitude to research assistants Jenifer Mutari, Robin Kay, Scott Meyers, Nicholas Peterson, and Brandin Williams for their help in data collection, to June Gruber for comments on an earlier version of the manuscript, and to Desmond Ong for technical help with Qualtrics. Funded by John Templeton Foundation. No conflicts of interest exist for any author regarding this manuscript.
Footnotes
Author contributions: T. D. W. and S. D. developed the study concept. T. D. W., S. D., Y. K. A., and J. R. A. contributed to the study design. Y. K. A. developed the experimental tasks and materials, under the supervision of T. D. W. and S. D. Data collection was performed by Y. K. A. and J. R. A. Analyses were conducted by Y. K. Ashar under the supervision of T. D. W. Y. K. A. drafted the manuscript with guidance from T. D. W, and all authors provided revisions. All authors approved the final version of the manuscript for submission.
DATA AND SOFTWARE AVAILABILITY
fMRI data were analyzed using freely available tools from the Wager lab, available at: https://github.com/canlab/CanlabCore.
Thequintile images representing five emotion intensity levels for every subject for empathic care are available at: http://neurovault.org/collections/2444/.
The quintile images representing five emotion intensity levels for every subject for empathic distress are available at: http://neurovault.org/collections/2448/.
The brain markers are available at: http://neurovault.org/collections/2450/.
Maps of brain regions preferentially related to empathic care and distress are available at: http://neurovault.org/collections/2450/.
ADDITIONAL RESOURCES
The biographies/charitable donation task is available at: https://github.com/canlab/Paradigms_Public/tree/master/Ashar_2016_CompassionMeditation/Compassion_current.
The online experiment measuring the intensity of ten different emotions in response to the biographies is available at: https://cuboulder.qualtrics.com/jfe/form/SV_cRX2bvedsWDgdlb.
Continuous sliders were implemented in Qualtrics using a freely available toolbox, available at: https://github.com/desmond-ong/psychWidgets/blob/master/continuous_time_slider/QualtricsReadme.md.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Admon R, Pizzagalli DA. Corticostriatal pathways contribute to the natural time course of positive mood. Nature Communications. 2015;6(May):10065. doi: 10.1038/ncomms10065. http://doi.org/10.1038/ncomms10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashar YK, Andrews-Hanna JR, Dimidjian S, Wager TD. Towards a neuroscience of compassion: A brain systems-based model and research agenda. In: Greene JD, editor. Positive Neuroscience. Oxford University Press; 2016. pp. 1–27. [Google Scholar]
- Ashar YK, Andrews-Hanna JR, Yarkoni T, Sills J, Halifax J, Dimidjian S, Wager TD. Effects of Compassion Meditation on a psychological model of charitable donation. Emotion. 2016 doi: 10.1037/emo0000119. [DOI] [PubMed] [Google Scholar]
- Ashar YK, Chang LJ, Wager TD. Mechanisms of the placebo: an affective appraisal account. Annual Reviews Clinical Psychology. 2017 doi: 10.1146/annurev-clinpsy-021815-093015. [DOI] [PubMed] [Google Scholar]
- Batson CD. Altruism in humans. Oxford University Press; 2011. [Google Scholar]
- Batson CD, Fultz J, Schoenrade Pa. Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. Journal of Personality. 1987;55(1):19–39. doi: 10.1111/j.1467-6494.1987.tb00426.x. http://doi.org/10.1111/j.1467-6494.1987.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Bloom P. Against Empathy: The Case for Rational Compassion. Ecco 2016 [Google Scholar]
- Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema A. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Journal of Neuroscience. 2015;8(5):583–592. doi: 10.1016/j.neuroscience.2015.08.052. http://doi.org/10.1002/aur.1474.Replication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Dufour N, Saxe R. Social cognition in members of conflict groups: behavioural and neural responses in Arabs, Israelis and South Americans to each other’s misfortunes. Philosophical Transactions of the Royal Society of London. 3; Series B, Biological Sciences. 2012;367(1589):717–30. doi: 10.1098/rstb.2011.0293. http://doi.org/10.1098/rstb.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Dufour N, Saxe R. How We Know It Hurts: Item Analysis of Written Narratives Reveals Distinct Neural Responses to Others’ Physical Pain and Emotional Suffering. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0063085. http://doi.org/10.1371/journal.pone.0063085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Pluta A, Saxe R. Distinct roles of the “Shared Pain” and “Theory of Mind” networks in processing others’ emotional suffering. Neuropsychologia. 2012;50(2):219–231. doi: 10.1016/j.neuropsychologia.2011.11.008. http://doi.org/10.1016/j.neuropsychologia.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Gianaros PJ, Manuck SB, Krishnan A, Wager TD. A Sensitive and Specific Neural Signature for Picture-Induced Negative Affect. PLOS Biology. 2015;13(6):e1002180. doi: 10.1371/journal.pbio.1002180. http://doi.org/10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon P, Barrett LF. Conceptualizing and experiencing compassion. Emotion. 2013 doi: 10.1037/a0033747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Tusche A, Vuilleumier P, Singer T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. 2016 doi: 10.1038/ncomms10904. http://doi.org/10.1038/ncomms10904. [DOI] [PMC free article] [PubMed]
- de Waal FBM. Putting the altruism back into altruism: the evolution of empathy. Annual Review of Psychology. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. http://doi.org/10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. http://doi.org/10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes Ra, Miller Pa, Fultz J, Shell R, Mathy RM, Reno RR. Relation of sympathy and personal distress to prosocial behavior: a multimethod study. Journal of Personality and Social Psychology. 1989;57(1):55–66. doi: 10.1037//0022-3514.57.1.55. http://doi.org/10.1037/0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- Engen HG, Singer T. Compassion-based emotion regulation up-regulates experienced positive affect and associated neural networks. Social Cognitive and Affective Neuroscience. 2015;10(9):1291–1301. doi: 10.1093/scan/nsv008. http://doi.org/10.1093/scan/nsv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. http://doi.org/10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. http://doi.org/10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Genevsky A, Knutson B. Neural Affective Mechanisms Predict Market-Level Microlending. Psychological Science. 2015 doi: 10.1177/0956797615588467. 0956797615588467-. http://doi.org/10.1177/0956797615588467. [DOI] [PMC free article] [PubMed]
- Genevsky A, Västfjäll D, Slovic P, Knutson B. Neural underpinnings of the identifiable victim effect: affect shifts preferences for giving. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(43):17188–96. doi: 10.1523/JNEUROSCI.2348-13.2013. http://doi.org/10.1523/JNEUROSCI.2348-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar Na, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. http://doi.org/10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science (New York, NY) 2007;316(5831):1622–5. doi: 10.1126/science.1140738. http://doi.org/10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(2):583–90. doi: 10.1523/JNEUROSCI.4089-09.2010. http://doi.org/10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social groups that elicit disgust are differentially processed in mPFC. Social Cognitive and Affective Neuroscience. 2007;2(1):45–51. doi: 10.1093/scan/nsl037. http://doi.org/10.1093/scan/nsl037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. The American Journal of Psychiatry. 2013;170(2):197–206. doi: 10.1176/appi.ajp.2012.12010014. http://doi.org/10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological Bulletin. 2015;141(4):901–930. doi: 10.1037/a0038822. http://doi.org/10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- Huth AG, De Heer WA, Griffiths TL, Theunissen FE, Jack L. Natural speech reveals the semantic maps that tile human cerebral cortex. Nature. 2016;532(7600):453–458. doi: 10.1038/nature17637. http://doi.org/10.1038/nature17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(December):942–951. doi: 10.1038/nrn2024. http://doi.org/10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8021–8026. doi: 10.1073/pnas.0810363106. http://doi.org/10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Eisenberger NI. Neural Correlates of Giving Support to a Loved One. Psychosomatic Medicine. 2012;74(1):3–7. doi: 10.1097/PSY.0b013e3182359335. http://doi.org/10.1097/PSY.0b013e3182359335. [DOI] [PubMed] [Google Scholar]
- Insel TR. The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. http://doi.org/10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. http://doi.org/10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Fredrickson BL, Schreiber CA, Redelmeier DA. When More Pain Is Preferred To Less: Adding a Better End. Psychological Science. 1993;4(6):401–405. http://doi.org/10.1111/j.1467-9280.1993.tb00589.x. [Google Scholar]
- Kanske P, Böckler A, Trautwein F-M, Lesemann FHP, Singer T. Are strong empathizers better mentalizers? Evidence for independence and interaction between the routes of social cognition. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw052. http://doi.org/10.1093/scan/nsw052. [DOI] [PMC free article] [PubMed]
- Kassam KS, Markey AR, Cherkassky VL, Loewenstein G, Just MA. Identifying Emotions on the Basis of Neural Activation. PLoS ONE. 2013;8(6):e66032. doi: 10.1371/journal.pone.0066032. http://doi.org/10.1371/journal.pone.0066032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews. Neuroscience. 2010;11(6):417–428. doi: 10.1038/nrn2833. http://doi.org/10.1038/nrn2919. [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. Functional Neural Plasticity and Associated Changes in Positive Affect After Compassion Training. Cerebral Cortex (New York, NY3: 1991) 2012;23:1–10. doi: 10.1093/cercor/bhs142. http://doi.org/10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Ricard M, Singer T. Differential pattern of functional brain plasticity after compassion and empathy training. Social Cognitive and Affective Neuroscience. 2014;9(6):873–9. doi: 10.1093/scan/nst060. http://doi.org/10.1093/scan/nst060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki OM, Singer T. Empathic Distress Fatigue Rather Than Compassion Fatigue? Integrating Findings from Empathy Research in Psychology and Social Neuroscience. In: Oakley B, Knafo A, Madhavan G, Wilson DS, editors. Pathological altruism. New York: Oxford University Press; 2011. pp. 368–383. [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53(1):147–56. doi: 10.1016/j.neuron.2006.11.010. http://doi.org/10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Meisenzahl EM, Borgwardt S, Riecher-Rössler A, Frodl T, Kambeitz J, … Davatzikos C. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain: A Journal of Neurology. 2015;138(Pt 7):2059–73. doi: 10.1093/brain/awv111. http://doi.org/10.1093/brain/awv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Labar KS. Decoding the Nature of Emotion in the Brain. Trends in Cognitive Sciences. 2016;xx:1–12. doi: 10.1016/j.tics.2016.03.011. http://doi.org/10.1016/j.tics.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, LaBar KS. Multivariate neural biomarkers of emotional states are categorically distinct. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsv032. nsv032-. http://doi.org/10.1093/scan/nsv032. [DOI] [PMC free article] [PubMed]
- Krienen FM, Tu PC, Buckner RL. Clan Mentality: Evidence That the Medial Prefrontal Cortex Responds to Close Others. Journal of Neuroscience. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. http://doi.org/10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo CW, Chang LJ, Ruzic L, Gu X, López-Solà M, … Wager TD. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife. 2016;5(JUN2016):1–42. doi: 10.7554/eLife.15166. http://doi.org/10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Mccabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, … Grafman J. Neural correlates of trust. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20084–20089. doi: 10.1073/pnas.0710103104. http://doi.org/10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. http://doi.org/10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. http://doi.org/10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality in empathy - A critical comment. Neuroscience Research. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. http://doi.org/10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Progress in Neurobiology. 2011;93(1):111–24. doi: 10.1016/j.pneurobio.2010.10.005. http://doi.org/10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Current Opinion in Neurobiology. 2012;22(6):1027–38. doi: 10.1016/j.conb.2012.06.001. http://doi.org/10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cerebral Cortex. 2015:1–13. doi: 10.1093/cercor/bhv001. http://doi.org/10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed]
- Lindquist KA, Wager TD, Kober H, Bliss-moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. Behavioral and brain sciences. 2012 doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Krishnan A, Lopez-Sola M, Jepma M, Woo C-W, Koban L, … Wager TD. Group-regularized individual prediction: theory and application to pain. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.10.074. http://doi.org/10.1016/j.neuroimage.2015.10.074. [DOI] [PMC free article] [PubMed]
- Lutz A, Brefczynski-Lewis J, Johnstone T, Davidson RJ. Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PloS ONE. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. http://doi.org/10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli Sa, Eisenberger NI. An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage. 2011;55(1):381–388. doi: 10.1016/j.neuroimage.2010.11.060. http://doi.org/10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Meade AW, Craig SB. Identifying careless responses in survey data. Psychological Methods. 2012;17(3):437–55. doi: 10.1037/a0028085. http://doi.org/10.1037/a0028085. [DOI] [PubMed] [Google Scholar]
- Meyer M, Masten C, Ma Y. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive …. 2013 doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, … Dalgleish T. A key role for similarity in vicarious reward. Science (New York, NY) 2009;324(5929):900. doi: 10.1126/science.1170539. http://doi.org/10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. http://doi.org/10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Moll J, Bado P, de Oliveira-Souza R, Bramati IE, Lima DO, Paiva FF, … Zahn R. A Neural Signature of Affiliative Emotion in the Human Septohypothalamic Area. Journal of Neuroscience. 2012;32(36):12499–12505. doi: 10.1523/JNEUROSCI.6508-11.2012. http://doi.org/10.1523/JNEUROSCI.6508-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(42):15623–8. doi: 10.1073/pnas.0604475103. http://doi.org/10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Weingartner JH, Bado P, Basilio R, Sato JR, Melo BR, … Zahn R. Voluntary Enhancement of Neural Signatures of Affiliative Emotion Using fMRI Neurofeedback. PLoS ONE. 2014;9(5):e97343. doi: 10.1371/journal.pone.0097343. http://doi.org/10.1371/journal.pone.0097343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli Sa, Rameson LT, Lieberman MD. The neural components of empathy: Predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience. 2014;9(1):39–47. doi: 10.1093/scan/nss088. http://doi.org/10.1093/scan/nss088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli Sa, Sacchet MD, Zaki J. Common and distinct neural correlates of personal and vicarious reward: A quantitative meta-analysis. NeuroImage. 2015;112:244–253. doi: 10.1016/j.neuroimage.2014.12.056. http://doi.org/10.1016/j.neuroimage.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-Neuron Responses in Humans during Execution and Observation of Actions. Current Biology. 2010;20(8):750–756. doi: 10.1016/j.cub.2010.02.045. http://doi.org/10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews. 2012;36(3):1043–59. doi: 10.1016/j.neubiorev.2011.12.013. http://doi.org/10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13(1–2):47–62. doi: 10.1016/0306-4530(88)90006-6. http://doi.org/10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Talati A, Schneier FR, Hirsch J. Reduced anterior temporal and hippocampal functional connectivity during face processing discriminates individuals with social anxiety disorder from healthy controls and panic disorder, and increases following treatment. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39(2):425–34. doi: 10.1038/npp.2013.211. http://doi.org/10.1038/npp.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(30):10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. http://doi.org/10.1523/jneurosci.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarimaki H, Gotsopoulos A, Jaaskelainen IP, Lampinen J, Vuilleumier P, Hari R, … Nummenmaa L. Discrete Neural Signatures of Basic Emotions. Cerebral Cortex. 2015:1–11. doi: 10.1093/cercor/bhv086. http://doi.org/10.1093/cercor/bhv086. [DOI] [PubMed]
- Sawe N, Knutson B. Neural valuation of environmental resources. NeuroImage. 2015;122:87–95. doi: 10.1016/j.neuroimage.2015.08.010. http://doi.org/10.1016/j.neuroimage.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain: A Journal of Neurology. 2009;132(Pt 3):617–27. doi: 10.1093/brain/awn279. http://doi.org/10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shaver P, Schwartz J, Kirson D, O’Connor C. Emotion knowledge: Further exploration of a prototype approach. Journal of Personality and Social Psychology. 1987;52(6):1061–1086. doi: 10.1037//0022-3514.52.6.1061. http://doi.org/10.1037/0022-3514.52.6.1061. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Numan M. The Behavioral Neuroscience of the Septal Region. New York, NY: Springer New York; 2000. The Septal Region and Social Behavior; pp. 175–209. http://doi.org/10.1007/978-1-4612-1302-4_8. [Google Scholar]
- Singer T, Klimecki OM. Empathy and compassion. Current Biology: CB. 2014;24(18):R875–8. doi: 10.1016/j.cub.2014.06.054. http://doi.org/10.1016/j.cub.2014.06.054. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience. 2014;16(1):55–61. doi: 10.1038/nrn3857. http://doi.org/10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine. 2013;368(15):1388–97. doi: 10.1056/NEJMoa1204471. http://doi.org/10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF. A Bayesian Model of Category-Specific Emotional Brain Responses. PLOS Computational Biology. 2015:1–27. doi: 10.1371/journal.pcbi.1004066. http://doi.org/10.1371/journal.pcbi.1004066. [DOI] [PMC free article] [PubMed]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, … Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science (New York, NY) 2004;303(2004):1162–1167. doi: 10.1126/science.1093065. http://doi.org/10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Weng HY, Fox AS, Shackman AJ, Stodola DE, Caldwell JZK, Olson MC, … Davidson RJ. Compassion Training Alters Altruism and Neural Responses to Suffering. Psychological Science. 2013 doi: 10.1177/0956797612469537. http://doi.org/10.1177/0956797612469537. [DOI] [PMC free article] [PubMed]
- Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: Brain models in translational neuroimaging. Nature Neuroscience Reviews. 2017 doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, … Wager TD. Separate neural representations for physical pain and social rejection. Nature Communications. 2014;5(May):5380. doi: 10.1038/ncomms6380. http://doi.org/10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Becker B, Geng Y, Zhao Z, Xu X, Zhao W, … Kendrick KM. Voluntary control of anterior insula and its functional connections is feedback-independent and increases pain empathy. NeuroImage. 2016;130:230–240. doi: 10.1016/j.neuroimage.2016.02.035. http://doi.org/10.1016/j.neuroimage.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack R, Nichols T, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8(8) doi: 10.1038/nmeth.1635. http://doi.org/10.1038/NMETH.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage. 2008;42(3):1127–1141. doi: 10.1016/j.neuroimage.2008.05.055. http://doi.org/10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Mitchell JP. Equitable decision making is associated with neural markers of intrinsic value. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(49):19761–6. doi: 10.1073/pnas.1112324108. http://doi.org/10.1073/pnas.1112324108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience. 2012;15(5):675–80. doi: 10.1038/nn.3085. http://doi.org/10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zenasni F, Boujut E, Woerner A, Sultan S. Burnout and empathy in primary care. British Journal of General Practice. 2012;62(602):462. doi: 10.3399/bjgp12X652193. http://doi.org/10.3399/bjgp12X654515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4. Clusters in the empathic care marker surviving the FDR q < .05 threshold, related to Figure 2a (top row). Z-values reflect single-sample T-test against zero of the voxel weights across 5,000 bootstrap samples.
Table S5. Clusters in the empathic distress marker surviving the FDR q < .05 threshold, related to Figure 2a (bottom row). Z-values reflect single-sample T-test against zero of the voxel weights across 5,000 bootstrap samples.