Abstract
Cold environmental temperatures increase sympathetic nerve activity and blood pressure, and increase the risk of acute cardiovascular events in aged individuals. The acute risk of cardiovascular events increases with aortic pulse wave velocity as well as elevated central and peripheral pulse pressures. The aim of this study was to examine the independent influence of aortic pulse wave velocity upon central and peripheral pressor responses to sympathetic activation via the cold pressor test (CPT). Twenty‐two healthy subjects (age: 18–73 years) completed a CPT with the left hand immersed in 2–4°C water for 3 min. During the CPT, central (from: 36 ± 7 to: 51 ± 12 mmHg) and peripheral pulse pressure increased (from: 54 ± 7 to: 66 ± 11; both P < 0.05). In all subjects the increase in central pulse pressure during the CPT was independently associated with baseline aortic pulse wave velocity (r 2 = 0.221, P = 0.027) but not age (P > 0.05). In a subset of subjects with higher arterial stiffness, the increase in peripheral pulse pressure during the CPT was independently associated with baseline aortic pulse wave velocity (r 2 = 0.415, P = 0.032) but not age (P > 0.05). These data indicate that central and peripheral pulse pressure responses during sympathetic activation are positively and independently associated with aortic pulse wave velocity through a wide age range. Decreasing aortic pulse wave velocity in aged individuals with elevated arterial stiffness may help reduce the incidence of acute cardiovascular events upon exposure to cold environmental temperatures.
Keywords: Aortic pulse wave velocity, central pulse pressure, cold pressor test
Introduction
The risk of experiencing an acute cardiovascular event is elevated in aged individuals exposed to cold environmental temperatures (Danet et al. 1999; Abrignani et al. 2009; Wolf et al. 2009; Bhaskaran et al. 2010). Aged individuals experience greater increases in blood pressure, arterial stiffness (Hess et al. 2009; Wilson et al. 2010; Gao et al. 2012; Monahan et al. 2013) and sympathetic nerve activity (Greaney et al. 2014) relative to young individuals during whole body cold stress. These cold related pressor responses may further aggravate health risks in persons with cardiovascular diseases, such as hypertension (Keatinge et al. 1984; Mercer et al. 1999). Furthermore, the risk of incident cardiovascular events in aged individuals exposed to cold environments has been associated with increases in sympathetic nerve activity and consequent increases in blood pressure (Marchant et al. 1993; Culic 2007). Without simulating exposure to cold environmental temperatures, the cold pressor test increases sympathetic activity and blood pressure (Hines and Brown 1936; Victor et al. 1987; Seals 1990) thereby providing an opportunity to examine.
Aortic pulse wave velocity is a surrogate measure of arterial stiffness and is an independent predictor of acute cardiovascular event risk in a community population (Sutton‐Tyrrell et al. 2005; Mattace‐Raso et al. 2006; Willum‐Hansen et al. 2006; Mitchell et al. 2010). Increases in sympathetic nerve activity can acutely increase arterial stiffness (Boutouyrie et al. 1994; Swierblewska et al. 2010) and blood pressure during many different types of stressors (Lim et al. 2015; Maki‐Petaja et al. 2016). Baseline aortic pulse wave velocity increases through age (Mitchell et al. 2004; McEniery et al. 2005) and therefore, given its importance in predicting acute cardiovascular event risk, the consequence of an increased sympathetic nerve activation may be more significant for individuals with elevated arterial stiffness. Furthermore, central and peripheral pulse pressures are associated with incident cardiovascular events (Roman et al. 2007; Glasser et al. 2014). Central pulse pressure, for example, is a strong predictor of acute cardiovascular events including myocardial infarction over a 5 year period in individuals free of cardiovascular disease (Roman et al. 2007). Given this association with acute cardiovascular events, examining central and peripheral pressor responses to acute sympathetic stimulation via the cold pressor test throughout a wide range of age and aortic pulse wave velocities enables an insight into the influence of arterial stiffness upon the elevated cardiovascular event risk during cold exposure in aged individuals (Marchant et al. 1993; Culic 2007).
Arterial stiffness and central blood pressure increase in response to a cold induced stressor perturbation (Geleris et al. 2004; Edwards et al. 2006, 2008; Hess et al. 2009; Moriyama and Ifuku 2010; King et al. 2013; Hintsala et al. 2014; Lim et al. 2015) and this increase is related to baseline arterial stiffness in a young and an aged group (Hess et al. 2009). However, the association between baseline aortic pulse wave velocity and central blood pressure during a cold stressor in individuals representing a wide range of ages and arterial stiffness is unknown. Therefore, the aim of this study was to examine the influence of arterial stiffness, as indexed by aortic pulse wave velocity, upon the central and peripheral pressor responses to cold pressor test (Hines and Brown 1936) induced sympathetic activation (Victor et al. 1987; Seals 1990), in individuals spanning a wide range of chronological age (18–75 years) and arterial stiffness. We hypothesized that baseline pulse wave velocity would be an independent predictor of the pressor responses to the cold pressor test such that individuals with a higher pulse wave velocity would also demonstrate a higher central and peripheral pressor response.
Materials and Methods
Ethical approval
All subjects were informed of the purpose, procedures and risks of the study before providing their informed written consent. The Institutional Review Board at the University of Colorado Colorado Springs approved the protocol and consent. All procedures conformed to the standards set by the Declaration of Helsinki.
Subjects
Twenty‐two subjects (eight females) participated in this study. Subject characteristics were; age 41 ± 19 years; height 175 ± 9 cm; weight 76.7 ± 18.1 kg (mean ± SD). Pre‐menopausal females were tested in the follicular phase of the menstrual cycle or the placebo phase, if they were taking birth control pills. Subjects were not taking cardiovascular acting medications (aside from birth control pills), were non‐smokers, were free of any known cardiovascular, metabolic, or neurological diseases and refrained from alcohol, caffeine, and exercise for 24 h before the study.
Instrumentation and experimental protocol
Prior to instrumentation subject's height and weight were recorded. Arterial blood pressure was non‐invasively and continuously measured on a finger on the right hand using photoplethysmography (NexFin®, BMEYE, Amsterdam, Netherlands), corrected to the height of the heart and used to calculate mean arterial pressure (Bogert et al. 2010; Truijen et al. 2012; Ameloot et al. 2013). Heart rate was calculated from R‐R interval obtained from three lead electrocardiogram interfaced with a bioamp (ADInstruments, CO). Aortic pulse wave velocity (aPWV; m/sec) was estimated using an automated sphygmomanometer (Mobil‐O‐Graph®; I.E.M, Stolberg, Germany) (Weiss et al. 2012; Feistritzer et al. 2015) wrapped around the left arm. Applanation tonometry of the right radial artery was used to assess central pulse pressure and pulse wave reflection (SphygmoCor®, AtCor Medical, New South Wales, Australia). Central (ascending aortic) pressure waves were generated from the radial artery pressure waves using a validated and generalized transfer function (Pauca et al. 2001). Radial artery blood pressure waves were continuously stored in the data acquisition system and analyzed retrospectively via pulse wave analysis (SphygmoCor®) to estimate the central (aortic) blood pressure waveform. Using the integral software, central pulse pressure (CPP) was calculated as the difference between the systolic and diastolic aortic pressures, while central augmentation pressure (cAP) was calculated as the difference between the second and first aortic systolic pressure peaks. Augmentation index (Aix) was subsequently calculated as cAP expressed as a percentage of the CPP. Owing to alterations in heart rate throughout the cold pressor test (CPT), augmentation index was standardized to 75 bpm (AIx@75) (Wilkinson et al. 2000).
Following instrumentation, subjects rested in the supine position for 30 min to allow for the stabilization of fluid shifts. Baseline data was subsequently obtained. While remaining supine subjects completed the cold pressor test wherein the left hand was immersed up to the wrist in a bucket of water held between 0 and 4°C for 3 min resulting in robust increases in sympathetic nerve activity (Victor et al. 1987; Seals 1990). The water inside the bucket was carefully stirred throughout the cold pressor test.
Data analysis
Hemodynamic data were collected continuously via a data‐acquisition system (Lab Chart, ADInstruments, CO). Baseline values of peripheral pressure and heart rate represent the average across a 60‐sec period. Baseline aPWV, AIx75 and central blood pressure variables represent the average of at least two successive measurements. Increases in all hemodynamic variables during the CPT are referenced as the value obtained at the point of peak change during the CPT. Data were continuously collected throughout the CPT and averaged at 12‐sec intervals to enable identification of the peak change in hemodynamic variables throughout the cold pressor test relative to baseline. To examine the influence of arterial stiffness upon the pressor responses to the cold pressor test we split subjects into either lower or higher arterial stiffness groups based upon the median aPWV (6.05 m/sec).
Statistical analysis
Univariate regression was used to examine correlations between independent and dependent variables. Independent variables included age, height, weight, BMI and gender, as well as baseline hemodynamic variables (peripheral and central blood pressures, aPWV, AIx75 and heart rate). Dependent variables were the peak increases in peripheral and central blood pressures, AIx75 and heart rate during the CPT relative to baseline. Changes in hemodynamic variables during the CPT in all subjects and groups of lower and higher arterial stiffness were compared to baseline using a two‐way ANOVA (group × time). Non‐normally distributed variables were log transformed prior to analysis. A priori statistical significance set at P ≤ 0.05. Data are reported as mean ± SD. Statistical analysis was performed using SPSS v24 (IBM, NY).
Results
All subjects
Descriptive data and baseline hemodynamic values are reported in Table 1. During the CPT, values for all measured hemodynamic variables, including central and peripheral pulse pressures, increased relative to baseline (all P < 0.05; Table 1). In all subjects, univariate regression analysis indicated that neither height, weight, gender nor BMI were correlated with baseline aPWV, or any pressor variable either at baseline or at the point of greatest increase during the CPT (all P > 0.05).
Table 1.
Baseline and peak cardiovascular responses to the cold pressor test in all individuals and separated into low and high aPWV groups
| All (n = 22) | Lower Arterial Stiffness (n = 11) | Higher Arterial Stiffness (n = 11) | ||||
|---|---|---|---|---|---|---|
| Baseline | CPT | Baseline | CPT | Baseline | CPT | |
| Age (years) | 41 ± 19 (18–73) | — | 26 ± 7 (18–39) | — | 56 ± 15b (24–73) | — |
| Gender (Male/Female) | 14/8 | — | 8/3 | — | 6/5 | — |
| Height (cm) | 175 ± 9 | — | 176 ± 10 | — | 173 ± 8 | — |
| Weight (Kg) | 76.7 ± 18.1 | — | 77.3 ± 14.4 | — | 76.2 ± 21.8 | — |
| BMI (Kg/m2) | 24.98 ± 4.47 | — | 24.69 ± 2.97 | — | 25.28 ± 5.74 | — |
| aPWV (m/sec) | 6.62 ± 1.74 (4.4–10.3) | — | 5.25 ± 0.56 (4.4–6.0) | — | 7.98 ± 1.40b (6.1–10.3) | — |
| AIx75 (%) | 6 ± 16 | 26 ± 10a | −2 ± 16 | 20 ± 12a | 14 ± 13b | 31 ± 5a , c |
| Mean arterial pressure (mmHg) | 90 ± 9 | 114 ± 9a | 91 ± 10 | 114 ± 8a | 90 ± 9 | 113 ± 11a |
| Peripheral systolic pressure (mmHg) | 125 ± 10 | 152 ± 13a | 127 ± 9 | 151 ± 13a | 123 ± 11 | 153 ± 14a |
| Peripheral diastolic pressure (mmHg) | 71 ± 9 | 89 ± 8a | 72 ± 9 | 90 ± 7a | 70 ± 9 | 88 ± 10a |
| Peripheral pulse pressure (mmHg) | 54 ± 7 | 66 ± 11a | 55 ± 6 | 64 ± 10a | 53 ± 9 | 69 ± 12a |
| Central systolic pressure (mmHg) | 109 ± 9 | 134 ± 13a | 107 ± 10 | 135 ± 14a | 110 ± 9 | 142 ± 11a |
| Central diastolic pressure (mmHg) | 72 ± 9 | 91 ± 9a | 73 ± 9 | 92 ± 8a | 71 ± 9 | 90 ± 10a |
| Central pulse pressure (mmHg) | 36 ± 7 | 51 ± 12a | 34 ± 4 | 45 ± 9a | 39 ± 8b | 56 ± 12a , c |
| Heart rate (bpm) | 66 ± 9 | 77 ± 14a | 69 ± 10 | 81 ± 17a | 64 ± 7 | 77 ± 10a |
AIx75, aortic augmentation index relative to 75 bpm. Values are means ± SD for 22 subjects. Range is included in parenthesis for age and aPWV. CPT, cold pressor test.
Different from baseline within that respective group (P ≤ 0.05).
Different between lower and higher aPWV groups at baseline (P ≤ 0.05).
Different between lower and higher aPWV groups at peak increase during CPT (P ≤ 0.05).
Relative to baseline central pulse pressure increased during the CPT (36 ± 7 vs. 51 ± 12 mmHg; P < 0.05). Univariate regression analysis indicated that aPWV was the only independent variable correlated with the increase in central pulse pressure during the CPT (r 2 = 0.221, P = 0.027; Fig. 1, Table 2). The increase in central pulse pressure during the CPT was not correlated with baseline central pulse pressure (r 2 = 0.096, P = 0.160). The increase in peripheral pulse pressure during CPT from baseline (from: 54 ± 7 to: 66 ± 11; P < 0.05) was correlated with aPWV (r 2 = 0.423, P = 0.001) and age (r 2 = 0.274, P = 0.012).
Figure 1.
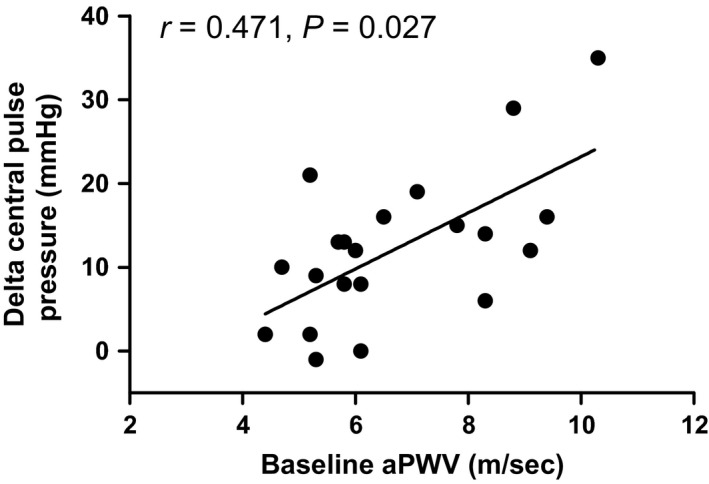
Correlation between baseline aortic pulse wave velocity and the increase in central pulse pressure during the cold pressor test in all subjects. The increase in central pulse pressure during the cold pressor test was independently predicted by aortic pulse wave velocity (r 2 = 0.221; P = 0.027) but not age (P > 0.05).
Table 2.
Univariate regression analysis results for the correlation between age, baseline aPWV and baseline AIx75 with the change in central and peripheral pressures during the CPT
| Age (years) | Baseline aPWV (m/sec) | Baseline AIx75 (%) | |
|---|---|---|---|
| All subjects | |||
| Peripheral pulse pressure (mmHg) | r = 0.523 | r = 0.650 | r = 0.317 |
| r 2 = 0.274 | r 2 = 0.423 | r 2 = 0.101 | |
| P = 0.012a | P = 0.001a | P = 0.150 | |
| Central pulse pressure (mmHg) | r = 0.369 | r = 0.470 | r = 0.125 |
| r 2 = 0.093 | r 2 = 0.221 | r 2 = 0.016 | |
| P = 0.091 | P = 0.027a | P = 0.581 | |
| Lower arterial stiffness | |||
| Peripheral pulse pressure (mmHg) | r = 0.184 | r = 0.446 | r = −0.148 |
| r 2 = 0.034 | r 2 = 0.199 | r 2 = 0.022 | |
| P = 0.588 | P = 0.169 | P = 0.663 | |
| Central pulse pressure (mmHg) | r = 0.243 | r = 0.350 | r = −0.225 |
| r 2 = 0.059 | r 2 = 0.122 | r 2 = 0.050 | |
| P = 0.472 | P = 0.292 | P = 0.507 | |
| Higher arterial stiffness | |||
| Peripheral pulse pressure (mmHg) | r = 0.514 | r = 0.644 | r = 0.385 |
| r 2 = 0.264 | r 2 = 0.415 | r 2 = 0.148 | |
| P = 0.106 | P = 0.032a | P = 0.243 | |
| Central pulse pressure (mmHg) | r = 0.180 | r = 0.385 | r = 0.135 |
| r 2 = 0.032 | r 2 = 0.149 | r 2 = 0.018 | |
| P = 0.597 | P = 0.242 | P = 0.691 | |
AIx75, aortic augmentation index relative to 75 bpm. Results are representative of data from 22 subjects.
Relationship statistically significant (P ≤ 0.05).
Increased arterial stiffness
Descriptive subject data, baseline and peak increases in hemodynamic as well as central and peripheral pressures during the CPT are reported in Table 1 for higher and lower arterial stiffness groups. Central systolic and diastolic pressures, peripheral pressure variables, heart rate, height, weight and BMI were not different at baseline between groups (all P > 0.05). However, in the higher arterial stiffness group, age (56 ± 15 years), aPWV (7.98 ± 1.40 m/sec), baseline AIx75 (14 ± 13%) and baseline central pulse pressure (39 ± 8 mmHg) were greater relative to the lower arterial stiffness group (26 ± 7 years, 5.25 ± 0.56 m/sec, −2 ± 16% and 34 ± 4 mmHg, respectively; all P < 0.05).
Peripheral and central pressures, heart rate and AIx75 increased during the CPT relative to baseline in both groups (all P < 0.05; Table 1; Fig. 2). In the higher arterial stiffness group the increase in AIx75 (to 31 ± 5%) and central pulse pressure (to 56 ± 12 mmHg) during the CPT was greater relative to the lower arterial stiffness group (to 20 ± 12% and to 45 ± 9 mmHg, respectively; both P < 0.05). In both groups, the increase in central and peripheral pressures during the CPT did not correlate with height, weight, BMI, gender or age (all P > 0.05). Of all measured independent variables, the increase in peripheral pulse pressure in the higher arterial stiffness group was only correlated with baseline aPWV (r 2 = 0.415, P = 0.032; Fig. 3; Table 2). In the higher arterial stiffness group, the increase in central pulse pressure during the CPT did not correlate with any of the independent variables measured.
Figure 2.
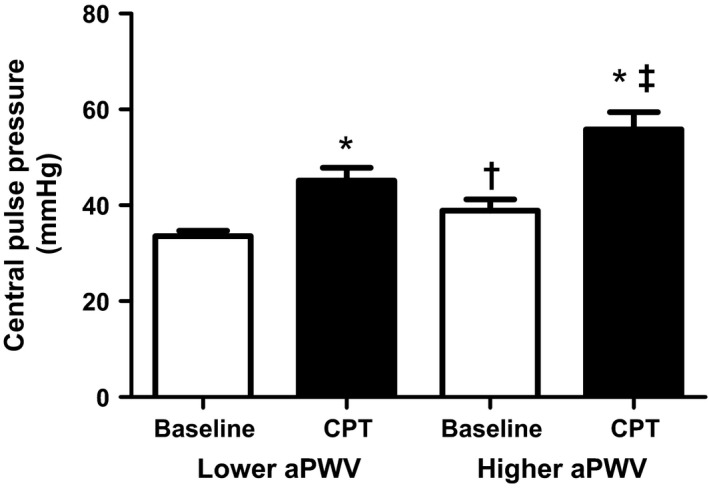
Peripheral and central pulse pressures at baseline and during the CPT in lower and higher arterial stiffness groups. Peripheral and central pulse pressures increased during the CPT in both groups. The central pulse pressure was greater during the CPT in the group with higher arterial stiffness. *Different from baseline within that respective group. †Different between lower and higher aPWV groups at baseline. ‡Different between lower and higher aPWV groups at peak increase during CPT. CPT, cold pressor test
Figure 3.
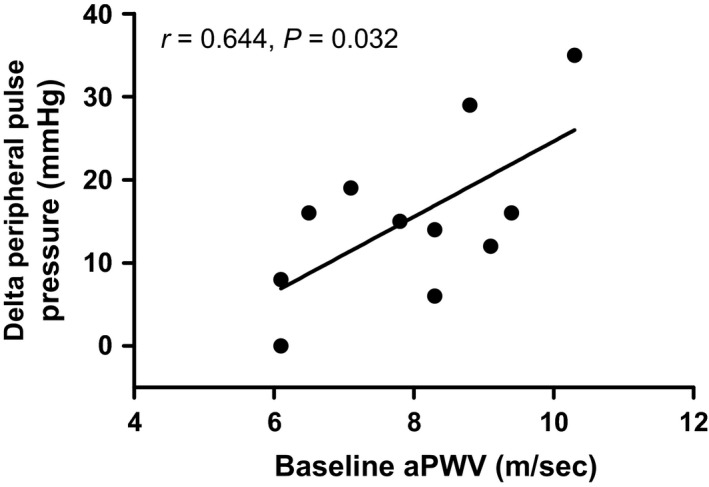
Correlation between baseline aortic pulse wave velocity and the increase in peripheral pulse pressure during the cold pressor test in subjects with higher arterial stiffness. In individuals with higher arterial stiffness the increase in peripheral pulse pressure during the cold pressor test was independently predicted by aortic pulse wave velocity (r 2 = 0.415; P = 0.032) but not age (P > 0.05).
Discussion
The aim of this study was to examine the influence of arterial stiffness upon the central and peripheral pressor responses to acute sympathetic activation via cold pressor test, in individuals spanning a wide range of age and arterial stiffness. Across this range, aortic pulse wave velocity correlated with the increase in central pulse pressure during the CPT while age did not. In individuals with an elevated arterial stiffness, aortic pulse wave velocity was correlated with the increase in peripheral pulse pressure during the CPT while age did not share a correlation with any pressor response. These data indicate that baseline arterial stiffness, as indexed here by aortic pulse wave velocity, is an independent predictor of the central and peripheral pressor responses to acute sympathetic activation via the cold pressor test.
We chose to examine the association between the acute central and peripheral pressor responses with baseline arterial stiffness during a cold pressor test as this perturbation increases sympathetic nerve activity and blood pressure (Greene et al. 1965; Victor et al. 1987). Increased sympathetic activity and blood pressure have been associated with the increased incidence of acute cardiovascular events occurring in aged individuals exposed to cold environmental temperatures (Marchant et al. 1993; Culic 2007). Moreover, central and peripheral pulse pressures are independent risk factors for incident cardiovascular events (Roman et al. 2007; Glasser et al. 2014). Previous studies have reported similar increases in central and peripheral pressor responses to cold induced stressors to those reported in this study (Geleris et al. 2004; Edwards et al. 2006, 2008; Hess et al. 2009; King et al. 2013; Hintsala et al. 2014; Lim et al. 2015). A relationship between resting arterial stiffness and the pressor response to whole body cold stress has been reported in a subject population comprised of young and aged individuals (Hess et al. 2009), which also displayed distinct differences in baseline arterial stiffness between these two groups. Here, we examined the association between baseline arterial stiffness and the acute central and peripheral pulse pressure responses to the cold pressor test across a wide range of baseline arterial stiffness and age. Our aim was to gain an insight into the influence of increased arterial stiffness through aging upon these cardiovascular risk factors during acute increases in sympathetic activation via the cold pressor test. These data indicate that increases in central pulse pressure during the cold pressor test were correlated with baseline aortic pulse wave velocity but not age in a group of individuals spanning a wide range of age and baseline arterial stiffness (Fig. 1). When individuals were split into either lower or higher arterial stiffness groups based upon aortic pulse wave velocity, the rise in central pulse pressure during the CPT was greater in the higher arterial stiffness group (Table 1), although it did not correlate with either age or arterial stiffness (Table 2). However, in the higher arterial stiffness group, aortic pulse wave velocity was correlated with the increase in peripheral pulse pressure during the CPT (Fig. 3; Table 2) while age was not correlated with any pressor response. Taken together, the acute central and peripheral pulse pressure responses to acute sympathetic activation are greater in individuals with a higher aortic pulse wave velocity and are independent of age.
It is important to note that the positive correlations between arterial stiffness and increased central and peripheral pulse pressures during the CPT were independent of chronological age. Arterial stiffness was indexed by aortic pulse wave velocity, a measure of central vascular aging. Acute increases in arterial stiffness during sympathetic activation are likely of particular importance to individuals with an already elevated central and peripheral pulse pressure given the association with incident cardiovascular events (Roman et al. 2007; Glasser et al. 2014). We chose to examine the relationship between baseline aortic pulse wave velocity and the pressor responses during CPT, rather than its increase during the CPT, because baseline aortic pulse wave velocity is an independent predictor of cardiovascular events (Sutton‐Tyrrell et al. 2005; Mattace‐Raso et al. 2006; Willum‐Hansen et al. 2006; Mitchell et al. 2010). Furthermore, while aortic pulse wave velocity increases through age (Mitchell et al. 2004; McEniery et al. 2005) it is lower in individuals with a history of lifelong habitual exercise training relative to sedentary counterparts (Vaitkevicius et al. 1993; Tanaka et al. 1998; Gates et al. 2003; Pierce et al. 2013, 2016) and can be reduced with acute exercise‐training even in aged, sedentary and diseased individuals (Tabara et al. 2007; Madden et al. 2009; Corrick et al. 2013; Tanaka et al. 2013; Vogel et al. 2013; Donley et al. 2014). Therefore, while chronological aging and increasing arterial stiffness generally share a close relationship there is likely between subject variations in this relationship. Within the context of the results of this study, a reduction in aortic pulse wave velocity would attenuate the rise in central and peripheral pulse pressures during acute sympathetic activation. Central aortic pressure is indicative of the pressure exerted upon central organs such as the heart (McEniery et al. 2014), and a greater central pulse pressure, for example, is likely reflective of an increased left ventricular afterload and coronary artery pressure (Roman et al. 2007), which may lead to impaired left ventricular diastolic function (Subherwal et al. 2010). In line with this, elevated central and peripheral pulse pressures are a risk factor for incident cardiovascular events, including myocardial infarction (Roman et al. 2007; Glasser et al. 2014). Therefore, a greater increase in pulse pressure upon acute sympathetic activation will likely exacerbate this incident cardiovascular risk. The influence of aortic pulse wave velocity upon the augmented central and peripheral pulse pressure responses to sympathetic activation found here, which was independent of chronological age, may help us better understand the increased incidence of acute cardiovascular events during cold temperature exposure in aged individuals (Danet et al. 1999; Abrignani et al. 2009; Wolf et al. 2009; Bhaskaran et al. 2010).
Aortic pulse wave velocity independently explained 22% of the peak increase in central pulse pressure during the CPT in all individuals, and 42% of the increase in peripheral pulse pressure during the CPT in individuals with a higher arterial stiffness. Therefore, aortic pulse wave velocity did not explain all the variance in the central and peripheral pressor responses to acute sympathetic activation via the CPT. Some of this additional variance may owe to sympathetic nerve activity, which is elevated in individuals with increased arterial stiffness (Boutouyrie et al. 1994; Swierblewska et al. 2010). If the increase in sympathetic activation was the same in all individuals during the CPT (Ng et al. 1994), sympathetic nerve activity may have been greater in individuals with higher versus lower aortic pulse wave velocity, owing to a higher baseline value. Additionally, aortic and carotid stiffening impair baroreceptor sensitivity (Monahan et al. 2001a,b; Mattace‐Raso et al. 2007). As central and peripheral pressures increased in all subjects during the CPT, it is possible that reduced baroreflex sensitivity impaired compensatory parasympathetic control in individuals with elevated arterial stiffness. Taken together, an augmented cardio acceleratory and/or vasoconstrictor effect during the CPT may have contributed to heightened central and peripheral pulse pressures during the CPT and blood pressure‐dependent rise in aortic pulse wave velocity (Maki‐Petaja et al. 2016; Harvey et al. 2017) in individuals with arterial stiffening. Nevertheless, while other factors undoubtedly contribute to the increased central and peripheral pulse pressures during acute sympathetic activation via the CPT, a significant proportion of this variability is associated with aortic pulse wave velocity and is independent of chronological age.
Limitations
We utilized a cold pressor test to cause acute increases in sympathetic nerve activity allowing examination of the consequent central and peripheral pressor responses and their relationship with baseline aortic pulse wave velocity. While the cold pressor test is a different perturbation to cold stress and not representative of natural seasonal cold exposure, we employed it in this study to create a profound and robust increase in sympathetic nerve activity (Victor et al. 1987; Seals 1990) which has been hypothesized to accompany increased cardiovascular events upon exposure to cold temperatures (Marchant et al. 1993; Culic 2007). However, we did not measure sympathetic nerve activity in this study and therefore, we were unable to quantify the influence of sympathetic nerve activity upon the central and peripheral pressor responses. However, central and peripheral pressures increased in all subjects during the CPT (Table 1 and Fig. 2) and therefore it is reasonable to possible that the CPT resulted in physiologically significant increases in sympathetic nerve activity in all individuals. Second, increases in sympathetic nerve activity may have increased more in aged relative to young individuals, as shown during whole body cold stress (Greaney et al. 2014), but not a cold pressor test (Ng et al. 1994). Although sympathetic nerve activity was not measured here, given that this study employed the cold pressor test it is reasonable to hypothesize that the increase was similar across all individuals. Interestingly, the increases in central and peripheral pulse pressures observed here during the CPT (Table 1) were similar to those observed during whole body skin surface cooling in older individuals (Hess et al. 2009). These similarities suggest that alterations in central and peripheral pulse pressures during increased sympathetic activity may be comparable between a cold pressor test and skin surface cooling although further studies are required to clarify this. Furthermore, physical fitness and habitual activity influence vascular health (Vaitkevicius et al. 1993; Tanaka et al. 1998, 2013; Gates et al. 2003; Tabara et al. 2007; Madden et al. 2009; Corrick et al. 2013; Pierce et al. 2013, 2016; Vogel et al. 2013; Donley et al. 2014) while baroreflex sensitivity also changes with age and arterial stiffening (Monahan et al. 2001a,b; Pierce et al. 2016). We did not measure these and differences in these variables within our subject pool may have influenced the pressor responses to the cold pressor test. Finally, after splitting the subject population into lower and higher arterial stiffness categories, the sample size was lower relative to other studies examining the association of arterial stiffness with cardiovascular disease events.
Perspectives and significance
These data indicate that aortic pulse wave velocity is a modest, but independent predictor of the central and peripheral responses during a cold pressor test and, presumably, activation of the sympathetic nervous system. Importantly the associations between aortic pulse wave velocity and pulse pressures were independent of age. Central and peripheral pulse pressures are independent risk factors for incident cardiovascular events in healthy individuals. Incident cardiovascular risk increases in aged individuals upon cold exposure via several mechanisms including cooling induced sympathetic nervous system activation and consequent vasoconstriction leading to elevated blood pressure and an increased cardiac afterload. These data suggest that in a healthy population spanning a wide age range, aortic pulse wave velocity is a stronger predictor of this increased risk relative to age. These data also suggest that a reduction of baseline aortic pulse wave velocity may attenuate the rise in central and peripheral pressor responses during any stressor, which presumably, elicits a sympatho‐excitatory response. Consequently, a lower baseline aortic pulse wave velocity and reduced pressor response during a stressor presumably eliciting an increased sympathetic activity may reduce the risk of acute incident cardiovascular events. Finally, aortic pulse wave velocity is one indices of arterial stiffness and the association reported here with central and peripheral pressor responses to the cold pressor test may vary depending upon the indices of arterial stiffness used (Lim et al. 2015).
Conflict of Interest
There are no conflicts of interest to report.
Acknowledgements
We would like to thank the subjects for participating in our study. We would also like to thank Jessi Champi B.S., Mitchell Sandefur B.S. and Brittany Lydon B.S. for their assistance with this study.
Borner A., Murray K., Trotter C., Pearson J.. Baseline aortic pulse wave velocity is associated with central and peripheral pressor responses during the cold pressor test in healthy subjects. Physiol Rep, 5 (14), 2017, e13357, https://doi.org/10.14814/phy2.13357
Funding Information
No funding information provided.
References
- Abrignani, M. G. , Corrao S., Biondo G. B., Renda N., Braschi A., Novo G., et al. 2009. Influence of climatic variables on acute myocardial infarction hospital admissions. Int. J. Cardiol. 137:123–129. [DOI] [PubMed] [Google Scholar]
- Ameloot, K. , van de Vijver K., Broch O., van Regenmortel N., De Laet I., Schoonheydt K., et al. 2013. Nexfin noninvasive continuous hemodynamic monitoring: validation against continuous pulse contour and intermittent transpulmonary thermodilution derived cardiac output in critically ill patients. Sci. World J. 2013:519080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran, K. , Hajat S., Haines A., Herrett E., Wilkinson P., and Smeeth L.. 2010. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ 341:c3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogert, L. W. , Wesseling K. H., Schraa O., Van Lieshout E. J., de Mol B. A., van Goudoever J., et al. 2010. Pulse contour cardiac output derived from non‐invasive arterial pressure in cardiovascular disease. Anaesthesia 65:1119–1125. [DOI] [PubMed] [Google Scholar]
- Boutouyrie, P. , Lacolley P., Girerd X., Beck L., Safar M., and Laurent S.. 1994. Sympathetic activation decreases medium‐sized arterial compliance in humans. Am. J. Physiol. 267:H1368–H1376. [DOI] [PubMed] [Google Scholar]
- Corrick, K. L. , Hunter G. R., Fisher G., and Glasser S. P.. 2013. Changes in vascular hemodynamics in older women following 16 weeks of combined aerobic and resistance training. J. Clin. Hypertens (Greenwich) 15:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culic, V. 2007. Acute risk factors for myocardial infarction. Int. J. Cardiol. 117:260–269. [DOI] [PubMed] [Google Scholar]
- Danet, S. , Richard F., Montaye M., Beauchant S., Lemaire B., Graux C., et al. 1999. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10‐year survey: the Lille‐World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 100:E1–E7. [DOI] [PubMed] [Google Scholar]
- Donley, D. A. , Fournier S. B., Reger B. L., DeVallance E., Bonner D. E., Olfert I. M., et al. 2014. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J. Appl. Physiol. (1985) 116:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, D. G. , Gauthier A. L., Hayman M. A., Lang J. T., and Kenefick R. W.. 2006. Acute effects of cold exposure on central aortic wave reflection. J. Appl. Physiol. (1985) 100:1210–1214. [DOI] [PubMed] [Google Scholar]
- Edwards, D. G. , Roy M. S., and Prasad R. Y.. 2008. Wave reflection augments central systolic and pulse pressures during facial cooling. Am. J. Physiol. Heart Circ. Physiol. 294:H2535–H2539. [DOI] [PubMed] [Google Scholar]
- Feistritzer, H.‐J. , Reinstadler S. J., Klug G., Kremser C., Seidner B., Esterhammer R., et al. 2015. Comparison of an oscillometric method with cardiac magnetic resonance for the analysis of aortic pulse wave velocity. PLoS ONE 10:e0116862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Z. , Wilson T. E., Drew R. C., Ettinger J., and Monahan K. D.. 2012. Altered coronary vascular control during cold stress in healthy older adults. Am. J. Physiol. Heart Circ. Physiol. 302:H312–H318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, P. E. , Tanaka H., Graves J., and Seals D. R.. 2003. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur. Heart J. 24:2213–2220. [DOI] [PubMed] [Google Scholar]
- Geleris, P. , Stavrati A., and Boudoulas H.. 2004. Effect of cold, isometric exercise, and combination of both on aortic pulse in healthy subjects. Am. J. Cardiol. 93:265–267. [DOI] [PubMed] [Google Scholar]
- Glasser, S. P. , Halberg D. L., Sands C., Gamboa C. M., Muntner P., and Safford M.. 2014. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? The REGARDS study Am. J. Hypertens. 27:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney, J. L. , Stanhewicz A. E., Kenney W. L., and Alexander L. M.. 2014. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J. Appl. Physiol. 117:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, M. A. , Boltax A. J., Lustig G. A., and Rogow E.. 1965. Circulatory dynamics during the cold pressor test. Am. J. Cardiol. 16:54–60. [DOI] [PubMed] [Google Scholar]
- Harvey, R. E. , Barnes J. N., Hart E. C., Nicholson W. T., Joyner M. J., and Casey D. P.. 2017. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am. J. Physiol. Heart Circ. Physiol. 312:H340–H346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, K. L. , Wilson T. E., Sauder C. L., Gao Z., Ray C. A., and Monahan K. D.. 2009. Aging affects the cardiovascular responses to cold stress in humans. J. Appl. Physiol. (1985) 107:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, E. A. Jr , and Brown G. E.. 1936. The cold pressor test for measuring the reactibility of the blood pressure: data concerning 571 normal and hypertensive subjects. Am. Heart J. 11:1–9. [Google Scholar]
- Hintsala, H. , Kandelberg A., Herzig K. H., Rintamaki H., Mantysaari M., Rantala A., et al. 2014. Central aortic blood pressure of hypertensive men during short‐term cold exposure. Am. J. Hypertens. 27:656–664. [DOI] [PubMed] [Google Scholar]
- Keatinge, W. R. , Coleshaw S. R., Cotter F., Mattock M., Murphy M., and Chelliah R.. 1984. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br. Med. J. (Clin Res Ed) 289:1405–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. G. , Ahuja K. D., Wass J., Shing C. M., Adams M. J., Davies J. E., et al. 2013. Effect of whole‐body mild‐cold exposure on arterial stiffness and central haemodynamics: a randomised, cross‐over trial in healthy men and women. Eur. J. Appl. Physiol. 113:1257–1269. [DOI] [PubMed] [Google Scholar]
- Lim, J. , Pearman M. E., Park W., Alkatan M., Machin D. R., and Tanaka H.. 2015. Impact of blood pressure perturbations on arterial stiffness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309:R1540–R1545. [DOI] [PubMed] [Google Scholar]
- Madden, K. M. , Lockhart C., Cuff D., Potter T. F., and Meneilly G. S.. 2009. Short‐term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 32:1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki‐Petaja, K. M. , Barrett S. M., Evans S. V., Cheriyan J., McEniery C. M., and Wilkinson I. B.. 2016. The role of the autonomic nervous system in the regulation of aortic stiffness. Hypertension 68:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, B. , Ranjadayalan K., Stevenson R., Wilkinson P., and Timmis A. D.. 1993. Circadian and seasonal factors in the pathogenesis of acute myocardial infarction: the influence of environmental temperature. Br. Heart. J. 69:385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace‐Raso, F. U. , van der Cammen T. J., Hofman A., van Popele N. M., Bos M. L., Schalekamp M. A., et al. 2006. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113:657–663. [DOI] [PubMed] [Google Scholar]
- Mattace‐Raso, F. U. , van den Meiracker A. H., Bos W. J., van der Cammen T. J., Westerhof B. E., Elias‐Smale S., et al. 2007. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J. Hypertens. 25:1421–1426. [DOI] [PubMed] [Google Scholar]
- McEniery, C. M. , Yasmin, Hall I. R., Qasem A., Wilkinson I. B., Cockcroft J. R.. 2005. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol. 46:1753–1760. [DOI] [PubMed] [Google Scholar]
- McEniery, C. M. , Cockcroft J. R., Roman M. J., Franklin S. S., and Wilkinson I. B.. 2014. Central blood pressure: current evidence and clinical importance. Eur. Heart J. 35:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer, J. B. , Osterud B., and Tveita T.. 1999. The effect of short‐term cold exposure on risk factors for cardiovascular disease. Thromb. Res. 95:93–104. [DOI] [PubMed] [Google Scholar]
- Mitchell, G. F. , Parise H., Benjamin E. J., Larson M. G., Keyes M. J., Vita J. A., et al. 2004. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the framingham heart study. Hypertension 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- Mitchell, G. F. , Hwang S. J., Vasan R. S., Larson M. G., Pencina M. J., Hamburg N. M., et al. 2010. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan, K. D. , Dinenno F. A., Seals D. R., Clevenger C. M., Desouza C. A., and Tanaka H.. 2001a. Age‐associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am. J. Physiol. Heart Circ. Physiol. 281:H284–H289. [DOI] [PubMed] [Google Scholar]
- Monahan, K. D. , Tanaka H., Dinenno F. A., and Seals D. R.. 2001b. Central arterial compliance is associated with age‐ and habitual exercise‐related differences in cardiovagal baroreflex sensitivity. Circulation 104:1627–1632. [DOI] [PubMed] [Google Scholar]
- Monahan, K. D. , Feehan R. P., Sinoway L. I., and Gao Z.. 2013. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: effect of ageing. J. Physiol. 591:2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama, K. , and Ifuku H.. 2010. Increased cardiovascular reactivity to the cold pressor test is not associated with increased reactivity to isometric handgrip exercise. Eur. J. Appl. Physiol. 108:837–843. [DOI] [PubMed] [Google Scholar]
- Ng, A. V. , Callister R., Johnson D. G., and Seals D. R.. 1994. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am. J. Physiol. 267:H344–H353. [DOI] [PubMed] [Google Scholar]
- Pauca, A. L. , O'Rourke M. F., and Kon N. D.. 2001. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38:932–937. [DOI] [PubMed] [Google Scholar]
- Pierce, G. L. , Casey D. P., Fiedorowicz J. G., Seals D. R., Curry T. B., Barnes J. N., et al. 2013. Aortic pulse wave velocity and reflecting distance estimation from peripheral waveforms in humans: detection of age‐ and exercise training‐related differences. Am. J. Physiol. Heart Circ. Physiol. 305:H135–H142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, G. L. , Harris S. A., Seals D. R., Casey D. P., Barlow P. B., and Stauss H. M.. 2016. Estimated aortic stiffness is independently associated with cardiac baroreflex sensitivity in humans: role of ageing and habitual endurance exercise. J. Hum. Hypertens. 30:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, M. J. , Devereux R. B., Kizer J. R., Lee E. T., Galloway J. M., Ali T., et al. 2007. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50:197–203. [DOI] [PubMed] [Google Scholar]
- Seals, D. R. 1990. Sympathetic activation during the cold pressor test: influence of stimulus area. Clin. Physiol. 10:123–129. [DOI] [PubMed] [Google Scholar]
- Subherwal, S. , de las Fuentes L., Waggoner A. D., Heuerman S., Spence K. E., and Davila‐Roman V. G.. 2010. Central aortic pressure is independently associated with diastolic function. Am. Heart J. 159: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton‐Tyrrell, K. , Najjar S. S., Boudreau R. M., Venkitachalam L., Kupelian V., Simonsick E. M., et al. 2005. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation 111:3384–3390. [DOI] [PubMed] [Google Scholar]
- Swierblewska, E. , Hering D., Kara T., Kunicka K., Kruszewski P., Bieniaszewski L., et al. 2010. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J. Hypertens. 28:979–984. [DOI] [PubMed] [Google Scholar]
- Tabara, Y. , Yuasa T., Oshiumi A., Kobayashi T., Miyawaki Y., Miki T., et al. 2007. Effect of acute and long‐term aerobic exercise on arterial stiffness in the elderly. Hypertens. Res. 30:895–902. [DOI] [PubMed] [Google Scholar]
- Tanaka, H. , DeSouza C. A., and Seals D. R.. 1998. Absence of age‐related increase in central arterial stiffness in physically active women. Arterioscler. Thromb. Vasc. Biol. 18:127–132. [DOI] [PubMed] [Google Scholar]
- Tanaka, M. , Sugawara M., Ogasawara Y., Izumi T., Niki K., and Kajiya F.. 2013. Intermittent, moderate‐intensity aerobic exercise for only eight weeks reduces arterial stiffness: evaluation by measurement of stiffness parameter and pressure‐strain elastic modulus by use of ultrasonic echo tracking. J. Med. Ultrason. (2001) 40:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truijen, J. , van Lieshout J. J., Wesselink W. A., and Westerhof B. E.. 2012. Noninvasive continuous hemodynamic monitoring. J. Clin. Monit. Comput. 26:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius, P. V. , Fleg J. L., Engel J. H., O'Connor F. C., Wright J. G., Lakatta L. E., et al. 1993. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88:1456–1462. [DOI] [PubMed] [Google Scholar]
- Victor, R. G. , Leimbach W. N. Jr, Seals D. R., Wallin B. G., and Mark A. L.. 1987. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9:429–436. [DOI] [PubMed] [Google Scholar]
- Vogel, T. , Lepretre P. M., Brechat P. H., Lonsdorfer‐Wolf E., Kaltenbach G., Lonsdorfer J., et al. 2013. Effect of a short‐term intermittent exercise‐training programme on the pulse wave velocity and arterial pressure: a prospective study among 71 healthy older subjects. Int. J. Clin. Pract. 67:420–426. [DOI] [PubMed] [Google Scholar]
- Weiss, W. , Gohlisch C., Harsch‐Gladisch C., Tolle M., Zidek W., and van der Giet M.. 2012. Oscillometric estimation of central blood pressure: validation of the Mobil‐O‐Graph in comparison with the SphygmoCor device. Blood Press. Monit. 17:128–131. [DOI] [PubMed] [Google Scholar]
- Wilkinson, I. B. , MacCallum H., Flint L., Cockcroft J. R., Newby D. E., and Webb D. J.. 2000. The influence of heart rate on augmentation index and central arterial pressure in humans. J. Physiol. 525(Pt 1):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willum‐Hansen, T. , Staessen J. A., Torp‐Pedersen C., Rasmussen S., Thijs L., Ibsen H., et al. 2006. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113:664–670. [DOI] [PubMed] [Google Scholar]
- Wilson, T. E. , Gao Z., Hess K. L., and Monahan K. D.. 2010. Effect of aging on cardiac function during cold stress in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298:R1627–R1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, K. , Schneider A., Breitner S., von Klot S., Meisinger C., Cyrys J., et al.; Cooperative Health Research in the Region of Augsburg Study G . 2009. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120:735–742. [DOI] [PubMed] [Google Scholar]


