Abstract
Nuclear medicine imaging is widely used in pain medicine. Low back pain is commonly encountered by physicians, with its prevalence from 49% to 70%. Computed tomography (CT) or magnetic resonance imaging (MRI) are usually used to evaluate the cause of low back pain, however, these findings from these scans could also be observed in asymptomatic patients. Bone scintigraphy has an additional value in patients with low back pain. Complex regional pain syndrome (CRPS) is defined as a painful disorder of the extremities, which is characterized by sensory, autonomic, vasomotor, and trophic disturbances. To assist the diagnosis of CRPS, three-phase bone scintigraphy is thought to be superior compared to other modalities, and could be used to rule out CRPS due to its high specificity. Studies regarding the effect of bone scintigraphy in patients with extremity pain have not been widely conducted. Ultrasound, CT and MRI are widely used imaging modalities for evaluating extremity pain. However, SPECT/CT has an additional role in assessing pain in the extremities.
Keywords: Complex regional pain syndrome, Diagnostic imaging, Extremity pain, Low back pain, Nuclear medicine, SPECT CT, Radionuclide imaging
INTRODUCTION
Low back pain is commonly defined as pain between the costal margin and the inferior gluteal folds [1]. The prevalence is known to vary from 49% to 70% [1]. Low back pain is categorized into specific and non-specific low back pain. Specific low back pain is derived from specific morphologic lesions and non-specific low back pain has no specific morphologic lesions [1].
Computed tomography (CT) or magnetic resonance imaging (MRI) are usually used to evaluate cause of low back pain and could reveal morphologic changes such as osteophytes, narrowing of the disk space, spondylolysis, Schmorl's nodes, or osteoporotic fractures. However, these findings could be observed in asymptomatic patients. Bone scintigraphy could show increased uptake at morphologic changes identified by CT or MRI imaging and the uptake of bone lesion could be assumed that it is related with clinical symptoms [2].
However, in some cases, diseases involving spine did not show increased uptake in bone scintigraphy. In one study, lumbar pain syndrome, herniated nucleus pulposus, post-surgery syndrome, and spinal stenosis did not show increased uptake, while tumor, Paget's disease, infection, pseudarthrosis, degenerative disk disease, spondylolysis, spondylolisthesis, and compression fracture showed often increased uptake [3]. Bone scintigraphy could be obtained either planar imaging or single photon emission computed tomography (SPECT). SPECT is known to have better resolution than planar images and helps to detect additional abnormal uptake in patients with low back pain [4].
Complex regional pain syndrome (CRPS) is a disorder showing continuous pain, which is characterized by sensory, vasomotor, sudomotor, or motor symptoms and signs [5,6]. It can be due to surgery, trauma, or minor injury [7] and nerve damage is the standard for classifying CRPS type I and II [5,8].
The diagnostic criteria of CRPS was developed by the International Association for the Study of Pain (IASP) through The Budapest Clinical Diagnostic Criteria for Complex Regional Pain Syndrome in 1994 [9]. However, the criteria of IASP had a tendency to over-diagnose CRPS and it has a difficulty in discriminating between CRPS and other neuropathic pain [10]. The criteria showed a sensitivity of 98%, and specificity of 36% [11]. In this context, refinement of the criteria was needed. In 2007, the revised criteria were introduced by IASP (Budapest Clinical Diagnostic Criteria) (Table 1) [12]. The revised criteria presented a sensitivity of 85% and a specificity of 69%, and reduced false positive diagnosis [12]. Although diagnostic procedures such as three-phase bone scintigraphy (TPBS), magnetic resonance imaging (MRI), X-ray, and skin temperature differences were not included in the revised criteria of CRPS, it could provide additional information to diagnose CRPS [13,14]. The usefulness of bone scintigraphy for extremity pain has not been well studied. However, as the study of single photon emission computed tomography/computed tomography (SPECT/CT) has increasing, studies regarding SPECT/CT in patients with extremity pain has been performed.
Table 1. The Revised Criteria of Complex Regional Pain Syndrome (Budapest Clinical Diagnostic Criteria).
In this review, we will discuss and illustrate the usefulness of bone scintigraphy and SPECT in pain medicine as follows: low back pain, complex regional pain syndrome, and pain on extremities and joints.
MAIN BODY
1. Low back pain
1) Metastatic bone disease
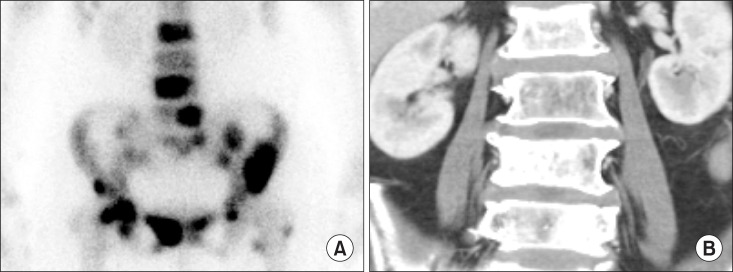
Bone metastases usually demonstrated as multiple foci with random distribution [15]. A 73-year-old female complained dyspnea and low back pain. CT chest was performed and the result reported lung cancer. Percutaneous needle aspirational biopsy revealed adenocarcinoma of lung. Subsequently, bone scintigraphy was performed and showed typical pattern of multiple bone metastases (Fig. 1A). CT showed sclerotic bone lesions consistent with bone scintigraphy (Fig. 1B). However, neuroblastoma, renal cell carcinoma, thyroid carcinoma, and anaplastic tumors are known to show ‘cold lesion’ in metastatic bone lesions due to aggressive tumor, disruption of the blood supply to the bone, or significant marrow involvement [16].
Fig. 1. Multiple bone metastases of lung cancer patient: (A) Bone scintigraphy shows increased uptake at L2, L4, L5, sacrum, both pelvic bones, and left proximal femur. (B) Computed tomography shows sclerotic bone lesions at L2, L4, and L5, corresponding with bone scintigraphy.
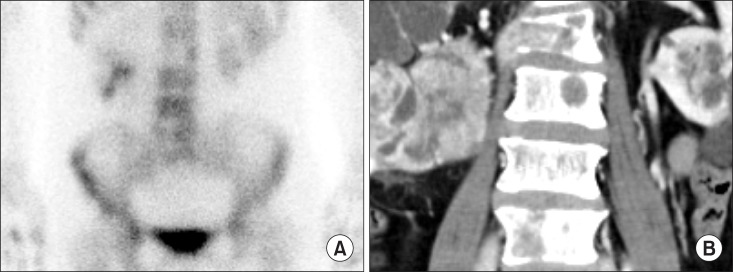
Fig. 2A showed ‘cold lesions’ at L2 and L5 spine in bone scintigraphy. CT showed osteolytic bone lesions at ‘cold lesion’ (Fig. 2B). Biopsy of L2 spine reported metastatic renal cell carcinoma. Bone scintigraphy is widely used to detect bone metastasis because of its effectiveness, low cost, widespread availability and favorable dosimetry [17]. The sensitivity and specificity of bone scintigraphy for detection of bone metastasis is 78% and 48% [17]. SPECT shows axial slices through the body, providing better localization of abnormal radionuclide uptake. The sensitivity and specificity of SPECT for detection of bone metastasis is 87% and 91% [17].
Fig. 2. Multiple bone metastases of renal cell carcinoma patient: (A) Bone sincintigraphy show ‘cold lesion’; at L2 and L5. (B) Computed tomography shows osteolytic bone lesions at L2, L3, and L5.
2) Benign bone tumor
Osteoid osteoma, osteoblastoma, giant cell tumor, and aneurysmal bone cyst are known to common primary benign spine tumors [18] and the uptake of these tumors is well shown in bone scintigraphy [16]. In case of chondroblastoma and enchondroma, bone scintigraphy show moderate uptake [16]. Hemangioma shows variable uptake [16].
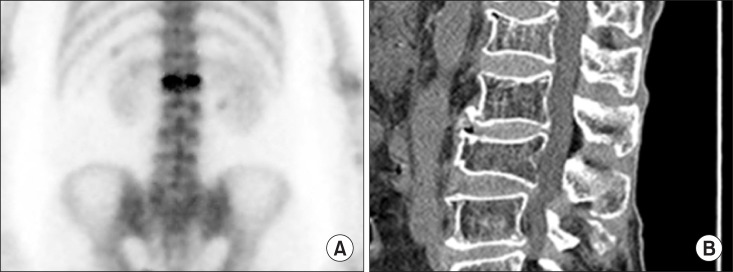
Fig. 3A showed mildly increased uptake at T11 and Fig. 3B showed osteolytic bone lesion with sclerotic rim. The lesion confirmed hemangioma.
Fig. 3. Hemangioma at T11: (A) Bone scintiraphy shows mildly increased uptake at T11. (B) Computed tomography shows osteolytic bone lesion with sclerotic margin.
3) Osteoporotic vertebral compression fracture
Osteoporotic vertebral compression fracture is a common cause of back pain in the elderly [19]. To diagnose symptomatic vertebra accurately before treatment is important. In one study, the treatment of osteoporotic vertebral fracture was performed in vertebrae that showed increased uptake on bone scintigraphy [20]. Ninety-three percent of patients reported pain relief after treatment [20]. As the uptake of vertebral fracture gradually decreases during the period of 6 to 24 months, bone scintigraphy can be used to discriminate between an acute fracture and an old fracture [21]. In addition, as bone scintigraphy can assess whole skeleton, it can detect additional traumatic bone lesions such as rib fracture during bed rest for treatment of vertebral fracture [22].
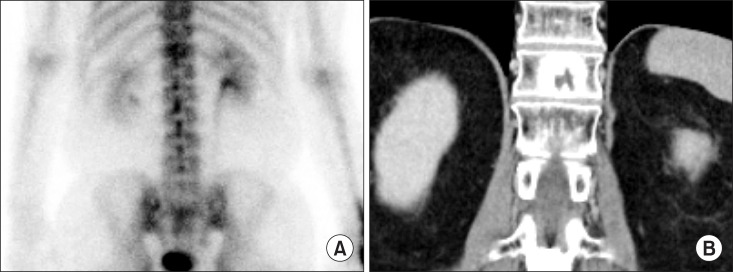
Fig. 4A showed horizontal uptake of L1. However, L1 and L3 were considered as a compression fracture on CT (Fig. 4B). Consequently, L1 was thought to recent compression fracture and L3 was thought to old compression fracture.
Fig. 4. Patient with compression fracture: (A) Bone scintigraphy showed horizontal uptake of L1 which means recent compression fracture. (B) Computed tomography showed compression fracture of L1 and L3. Consequently, compression fracture of L3 was thought to old fracture.
4) Vertebral osteomyelitis
Although bone is known to resistant to infection, however, trauma, bacteremia, surgery, or foreign bodies could cause osteomyelitis [23]. Vertebral osteomyelitis is increasing due to increased life expectancies, chronic disease, use of indwelling catheters, and immunosuppressive therapies [24,25]. The lumbar spine is the most affected region of vertebral osteomyelitis [26]. Early diagnosis is often difficult and this may result in permanent neurological damage or even death [26]. Bone scintigraphy can detect osteomyelitis 10 to 14 days prior to plain radiographs [23].
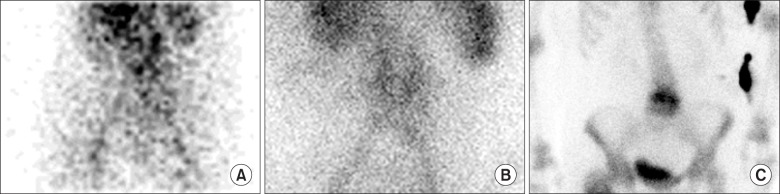
The typical pattern of vertebral osteomyelitis shows hyper-perfusion, hyperemia, and increased bone uptake in bone scan three phase. However, bone scintigraphy is sensitive, but not specific. Ga-67 scintigraphy can be used to supplement the low specificity of the study [27]. Fig. 5 shows typical pattern of vertebral osteomyelitis in 3 phase bone scintigraphy.
Fig. 5. Patient with vertebral osteomyelitis: (A) Flow, (B) blood pool, and (C) delayed phases showed increased uptake.
5) Facet joints arthropathy
The prevalence of symptomatic facet joint arthropathy varies from 5% to 15% [18]. The L4-L5 facet joints are the most commonly and severely implicated. Repetitive stress and low-grade trauma are major cause of facet joint arthropathy.
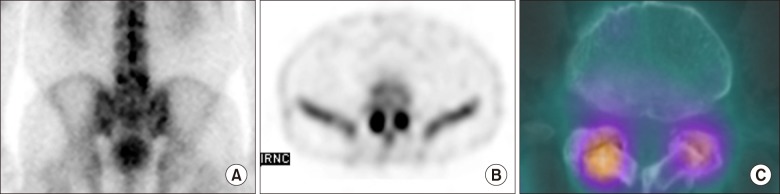
CT has high contrast between bony structures and soft tissues and show good delineation of osteophytosis, subchondral sclerosis and erosions, and capsular calcification. MRI has a superior delineation of soft tissues compared to other imaging modality. However, both modalities were not significant predictor of clinical outcome in the treatment of facet joint block while bone SPECT was able to detect appropriate patients to be treated [28]. Fig. 6A showed focal uptake at L5 on planar image. SPECT and SPECT/CT showed focal uptake at both facet joints (Fig. 6B and 6C).
Fig. 6. Patient with facet joint arthritis: (A) Planar image showed focal uptake at L5 (B) Single photon emission computed tomography, and (C) Single photon emission computed tomography/Computed tomography showed focal uptake at facet joints.
6) Spondylolysis
Spondylolysis is a disease showing bony defect in the pars interarticularis of the vertebral arch [29] and it is thought to be one of the most common causes of low back pain with incidence of 3-6% [30,31]. The major site of spondylolysis is a L5 level, and spondylolisthesis is commonly accompanied in patients with bilateral spondylolysis [29].
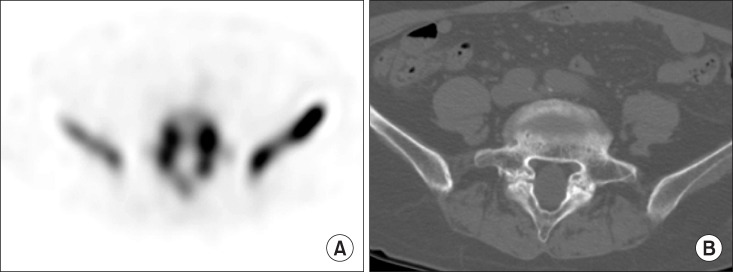
MRI is usually performed in the evaluation of children and adolescents with back pain [30]. However, MRI missed a spondylolysis in over half of the adolescents [30]. In one study, bone scintigraphy with SPECT showed superior diagnostic performance than MRI in detecting spondylolysis [32]. Fig. 7A shows spondylolysis of L5 on SPECT. Fig. 7B showed a bony defect in the pars interarticularis on CT.
Fig. 7. Patient with spondylolysis: (A) Single photon emission computed tomography showed increased uptake at pars interarticularis of L5 and (B) Computed tomography showed a bony defect in the pars interarticularis.
2. Complex regional pain syndrome (CRPS)
1) Three-phase bone scintigraphy (TPBS)
The radiopharmaceuticals used in TPBS were technetium-99m-labeled methylene diphosphonate, hydroxyl methylene diphosphonate, and dicarboxypropane diphosphonate, which did not show the significant difference between them [33]. These radiopharmaceuticals are absorbed to hydroxyapatite and calcium pyrophosphate of bones [34]. The uptake of radiopharmaceuticals in bones is associated with blood flow and osteoblastic activity, seen in fractures, osteomyelitis, and bone metastasis [35]. “Cold defect”, absent of uptake, could also be seen in bone scintigraphy by the following situations: 1) aggressive processes, 2) indolent processes that induce little healing reaction, 3) disruption of blood flow [35].
TPBS consists of flow, blood pool, and delayed phases [36]. The flow phase consists of serial 2- to 5-second dynamic images acquired for 60 seconds and reflects the relative amount of blood flow to the area of interest. The blood pool phase is obtained by static images for 5 minutes immediate after flow phase reflecting the amount of activity that has extravasated into the tissues around the area of interest. Finally, the delayed phase is obtained at 2-4 hours after radiopharmaceutical injection reflecting the rate of bone turnover [36,37]. Until now, the mechanism of uptake in TPBS in patients of CRPS is not fully understood. It might be due to decreased sympathetic activity, abolished sympathetic activity, or neurogenic inflammation [38].
2) Diagnostic performance of TPBS in CRPS
Diagnostic performance of TPBS in CRPS ranged widely as a sensitivity between 14% and 100% and a specificity between 50% and 100% [39]. Ringer et al. [40] showed the pooled sensitivity of 87% and the pooled specificity of 69% in a meta-analysis. False positive TPBS might be due to other clinical conditions mimicking similar biological processes such as posttraumatic or post-operative bone affections according to Ringer et al. [40].
Recently, Wertli et al. [41] reported diagnostic accuracy for TPBS according to diagnostic criteria. They reported the pooled sensitivity of 93.3% and the pooled specificity of 82.9% without diagnostic criteria, 90.6% and 94.6% with Kozin criteria, 60.8% and 89.7% with IASP criteria, and 55.1% and 93.5% with Budapest criteria. Wüppenhorst et al. [38] reported that diagnostic performance was limited by duration of CRPS, and the optimum time to use TPBS is within the first 5 months after onset of symptoms [38].
Schürmann et al. [42] compared the diagnostic performance of TPBS with thermography, and MRI. The sensitivity of TPBS, and MRI were 19%, and 43% at 8 weeks, and 14%, and 13% at 16 weeks after trauma, showing the poor sensitivity of both modalities. However, the specificity of TPBS, and MRI were 96%, and 78% at 8 weeks, and 100%, and 98% at 16 weeks after trauma, which might have a potential to rule out CRPS. According to a meta-analysis by Cappello et al. [13], TPBS had a significantly higher sensitivity and negative predictive value than MRI.
3) Findings of CRPS on TPBS
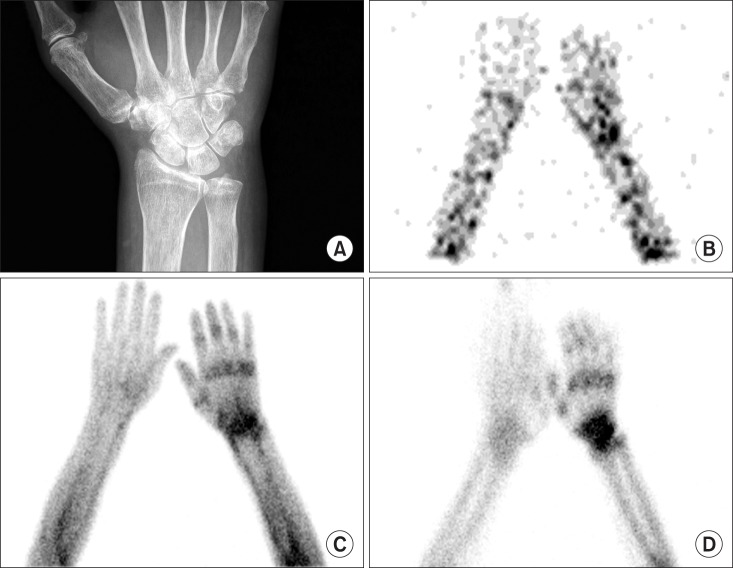
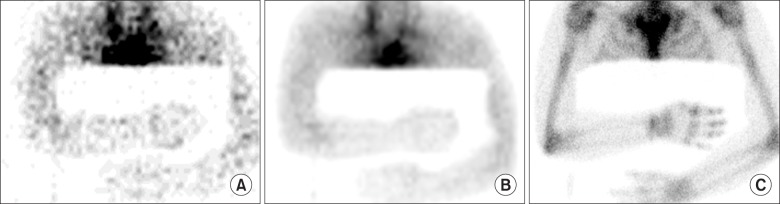
Although TPBS findings are variable depending on the duration of disease, the typical pattern of TPBS are increase of the uptake in all of blood flow, blood pool, and delayed phases [36,43]. Fig. 8 demonstrated a typical finding of CRPS on TPBS. Sixty-three years old female fell down and the styloid process of left radius fractured. Long arm cast was applied as a treatment. After 2 months, she complained of pain and swelling of left hand. X-ray showed osteopenia and styloid process fracture of left radius. TPBS showed increased uptake at left hand and wrist in all three phase. This pattern is commonly demonstrated in the early phase of CRPS [43].
Fig. 8. Sixty-three years old female patient with CRPS of left wrist and hand. TPBS showed increased uptake at left hand and wrist in all three phase: (A) a wrist X-ray, (B) flow, (C) blood pool, and (D) delayed phases.
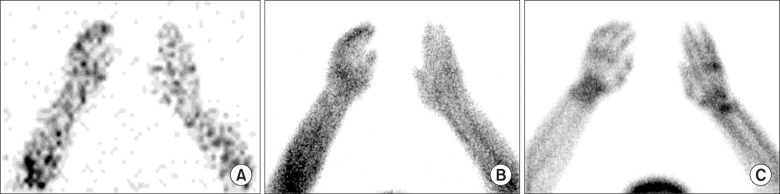
Fig. 9 presented an atypical finding of CRPS on TPBS, which is increased uptake only on delayed image without abnormalities on flow and blood pool phases. Forty-six years old female complained of pain of right shoulder and wrist after fell down. After 5 months, she underwent surgery due to carpal tunnel syndrome of right wrist. After surgery, she appealed sustained pain at right shoulder, right arm, right elbow, and right wrist and hypoesthesia of right 1st to 3rd fingers. TPBS was done at 6 years after initial trauma. Images of flow phase and blood pool phase did not show the abnormalities, however, increased periarticular uptake was seen on a delayed image.
Fig. 9. Forty-six years old female patient with CRPS of right wrist and hand. Increased uptake is shown only on delayed image at right wrist and hand without abnormalities on flow and blood pool phases: (A) flow, (B) blood pool, and (C) delayed phases.
Fig. 10 demonstrated the other type of atypical findings of CRPS on TPBS. A 44-year-old male complained of sustained left hand pain after median nerve decompression surgery. With suspicion of CRPS, TPBS was performed 15 months after initial trauma. The uptake of flow phase and blood pool phase decreased comparing with contralateral side, while increased uptake was seen on a delayed image. This pattern of TPBS in CRPS was known rare in adults, however, young patients with CRPS usually demonstrated this atypical finding [44]. Low et al. [45] reported that 7 of 14 young patients with CRPS showed the decreased uptake in the flow phase of TPBS. In case of paralysis and immobilization, TPBS could exhibit the similar finding [43].
Fig. 10. Forty-four years old male patient with CRPS of left wrist and hand. Decreased uptake on flow and blood pool phase, while increased uptake on delayed phase at left wrist and hand: (A) flow, (B) blood pool, and (C) delayed phases.
Fig. 11 showed another pattern in CRPS. A 54-year-old male complained of left wrist pain after trauma. Three years after trauma, TPBS was done to evaluate the possibility of CRPS. All three phases showed decreased uptake at the affected limb. However, including decreased uptake on a delayed image as a positive finding of CRPS is controversial. Decreased uptake on a delayed image might be a supportive finding for CRPS [39], however, it could be caused by disuse of affected limb [46].
Fig. 11. Fifty-four years old male patient with CRPS of left wrist and hand. All three phases showed decreased uptake at left wrist and hand: (A) flow, (B) blood pool, and (C) delayed phases.
3. Pain on extremities
1) Upper extremity
Few studies regarding usefulness of bone scintigraphy in patients with upper extremity pain are published [47]. Hirschmann et al. [48] reported that SPECT/CT in patient with shoulder pain could differentiate between infection and humeral head necrosis, identify prosthesis loosening, and localize exact sites of pain. The study for diagnosing acromioclavicular joint pain showed that bone scintigraphy had better diagnostic performance than MRI [49]. In patients with hand and wrist pain, MRI showed higher sensitivity (86%) than SPECT/CT (71%), however, the specificity (20%) was lower than SPECT/CT (100%). Accuracy of both MRI and SPECT/CT were 73% and 77%, respectively [50].
2) Lower extremity
Total hip replacement is commonly undertaken to relieve pain and functional disability of hip osteoarthritis [51]. Aseptic prosthesis loosening is known to most common cause of prosthetic failure [52]. MRI and CT are limited by metal artifacts to evaluate loosening of prosthesis [53]. In one meta-analysis, bone scintigraphy showed a pooled sensitivity of 85% and a pooled specificity of 72% [54]. In a study regarding diagnostic performance between planar/SPECT and SPECT/CT, SPECT/CT showed higher sensitivity (93.7%) than planar/SPECT (77.08%), especially in loosening of acetabular component [53].
Anterior knee pain, also known as patellofemoral pain syndrome, is one of common causes of knee pain [55]. It could be due to patellar pad lesions, patellar tendinopathy, patellofemoral malalignment, and chondral lesions of the patellofemoral joint [55]. However, these lesions are not always correlated with symptoms [55]. SPECT/CT showed that patella uptake was associated with treatment response and severity of patellar condral lesions [55]. Similar to painful hip prosthesis, In patients with painful knee prostheses, SPECT/CT could diagnose accurately infection and prosthesis loosening and had clinical impact on 85.5% of patients [56].
X-ray, US, CT, and MRI have been used to evaluate pain on foot and ankles [57]. The diagnosis of foot and ankle lesions is difficult due to complex anatomy and functions [57]. Singh et al. [57] reported that SPECT/CT had a high diagnostic accuracy in diagnosing foot and ankle lesions. The sensitivity and specificity were 94% and 95.45%m respectively [57]. Compared with MRI, the diagnostic performance was comparable to MRI [58]. The sensitivity, specificity, positive predictive value, and negative predictive value of SPECT/CT were 93%, 48%, 56%, 91%, and that of MRI were 98%, 24%, 48%, 95% [58]. Moreover, SPCET/CT had a high clinical impact on treatment decision. 68.6% of patients were changed diagnosis and treatment by SPECT/CT [59].
3) Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of joint [60]. Several studies reported that bone scintigraphy did not discriminate active inflammatory joint from inactive joint in RA patients [61,62] and was inferior to ultrasonography to detect early RA [63]. However, recent study showed that TPBS had better diagnostic performance than conventional bone scintigraphy and was helpful in the diagnosis of RA in patients with insufficient evidence of RA [64,65].
CONCLUSIONS
In patients with low back pain, CRPS, and extremity pain, nuclear medicine imaging is an essential imaging modality. Bone scintigraphy is superior to other imaging modalities in evaluate low back pain. CRPS, and extremity pain. Further studies are needed to investigate the underlying mechanisms of uptake in CRPS.
References
- 1.Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–1434. doi: 10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Maeseneer M, Lenchik L, Everaert H, Marcelis S, Bossuyt A, Osteaux M, et al. Evaluation of lower back pain with bone scintigraphy and SPECT. Radiographics. 1999;19:901–912. doi: 10.1148/radiographics.19.4.g99jl03901. [DOI] [PubMed] [Google Scholar]
- 3.Valdez DC, Johnson RG. Role of technetium-99m planar bone scanning in the evaluation of low back pain. Skeletal Radiol. 1994;23:91–97. doi: 10.1007/BF00563199. [DOI] [PubMed] [Google Scholar]
- 4.Matesan M, Behnia F, Bermo M, Vesselle H. SPECT/CT bone scintigraphy to evaluate low back pain in young athletes: common and uncommon etiologies. J Orthop Surg Res. 2016;11:76. doi: 10.1186/s13018-016-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 6.Fischer SG, Perez RS. Complex regional pain syndrome-diagnosis, treatment and future perspectives. Eur Neurol Rev. 2011;6:270–275. [Google Scholar]
- 7.Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi: 10.1186/s41038-016-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birklein F, O'Neill D, Schlereth T. Complex regional pain syndrome: an optimistic perspective. Neurology. 2015;84:89–96. doi: 10.1212/WNL.0000000000001095. [DOI] [PubMed] [Google Scholar]
- 9.Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–133. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 10.Galer BS, Bruehl S, Harden RN. IASP diagnostic criteria for complex regional pain syndrome: a preliminary empirical validation study. International Association for the Study of Pain. Clin J Pain. 1998;14:48–54. doi: 10.1097/00002508-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147–154. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 12.Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326–331. doi: 10.1111/j.1526-4637.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 13.Cappello ZJ, Kasdan ML, Louis DS. Meta-analysis of imaging techniques for the diagnosis of complex regional pain syndrome type I. J Hand Surg Am. 2012;37:288–296. doi: 10.1016/j.jhsa.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Wasner G, Schattschneider J, Binder A, Baron R. Complex regional pain syndrome--diagnostic, mechanisms, CNS involvement and therapy. Spinal Cord. 2003;41:61–75. doi: 10.1038/sj.sc.3101404. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys SC, Eck JC, Hodges SD. Neuroimaging in low back pain. Am Fam Physician. 2002;65:2299–2306. [PubMed] [Google Scholar]
- 16.Mettler FA, Guiberteau MJ. Essentials of nuclear medicine imaging. 6th ed. Philiadelphia (PA): Elsevier/Saunders; 2012. p. 280. [Google Scholar]
- 17.O'Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: an update. World J Radiol. 2015;7:202–211. doi: 10.4329/wjr.v7.i8.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World J Orthop. 2016;7:109–116. doi: 10.5312/wjo.v7.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kim JI, Jang BH, Seo JG, Kim JH. The comparison of bone scan and MRI in osteoporotic compression fractures. Asian Spine J. 2010;4:89–95. doi: 10.4184/asj.2010.4.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol. 2000;21:1807–1812. [PMC free article] [PubMed] [Google Scholar]
- 21.Vali R, Bajno L, Kousha M, Martin C. The potential role of radionuclide imaging in osteoporotic vertebral fracture and sacral fracture. Spine Res. 2016;2:11. [Google Scholar]
- 22.Jun DS, An BK, Yu CH, Hwang KH, Paik JW. Practical use of bone scan in patients with an osteoporotic vertebral compression fracture. J Korean Med Sci. 2015;30:194–198. doi: 10.3346/jkms.2015.30.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg. 2009;23:80–89. doi: 10.1055/s-0029-1214160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doutchi M, Seng P, Menard A, Meddeb L, Adetchessi T, Fuentes S, et al. Changing trends in the epidemiology of vertebral osteomyelitis in Marseille, France. New Microbes New Infect. 2015;7:1–7. doi: 10.1016/j.nmni.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong SJ, Choi SW, Youm JY, Kim HW, Ha HG, Yi JS. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc. 2014;56:21–27. doi: 10.3340/jkns.2014.56.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotaru N, Pripa V, Punga J, Condrea E, Seu V, Frumusachi O, et al. Nuclear medicine imaging for evaluation of low back pain [Internet] Dover (DE): SM Group; 2016. [cited 2017 Jun 25]. Available at: http://www.smgebooks.com/neuroimaging/chapters/NI-1 6-07.pdf. [Google Scholar]
- 27.Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25:963–977. doi: 10.1097/00003072-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Shur N, Corrigan A, Agrawal K, Desai A, Gnanasegaran G. Radiological and radionuclide imaging of degenerative disease of the facet joints. Indian J Nucl Med. 2015;30:191–198. doi: 10.4103/0972-3919.158526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrmou E, Tsitsopoulos PP, Marinopoulos D, Tsonidis C, Anagnostopoulos I, Tsitsopoulos PD. Spondylolysis: a review and reappraisal. Hippokratia. 2010;14:17–21. [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi KT, Jr, Skaggs DL, Acevedo DC, Myung KS, Choi P, Andras L. Spondylolysis is frequently missed by MRI in adolescents with back pain. J Child Orthop. 2012;6:237–240. doi: 10.1007/s11832-012-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Standaert CJ, Herring SA. Spondylolysis: a critical review. Br J Sports Med. 2000;34:415–422. doi: 10.1136/bjsm.34.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40:940–946. doi: 10.1136/bjsm.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frühling J, Verbist A, Balikdjian D. Which diphosphonate for routine bone scintigraphy (MDP, HDP or DPD)? Nucl Med Commun. 1986;7:415–425. doi: 10.1097/00006231-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto Y. Accumulation of technetium-99m methylene diphosphonate. Conditions affecting adsorption to hydroxyapatite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:115–119. doi: 10.1016/s1079-2104(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 35.Brenner AI, Koshy J, Morey J, Lin C, DiPoce J. The bone scan. Semin Nucl Med. 2012;42:11–26. doi: 10.1053/j.semnuclmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Ziessman HA, O'Malley JP, Thrall JH, Fahey H. Nuclear medicine: the requisites. 4th ed. Philadelphia (PA): Elsevier Sunders; 2014. p. 100. [Google Scholar]
- 37.Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23:341–358. doi: 10.1148/rg.232025103. [DOI] [PubMed] [Google Scholar]
- 38.Wüppenhorst N, Maier C, Frettlöh J, Pennekamp W, Nicolas V. Sensitivity and specificity of 3-phase bone scintigraphy in the diagnosis of complex regional pain syndrome of the upper extremity. Clin J Pain. 2010;26:182–189. doi: 10.1097/AJP.0b013e3181c20207. [DOI] [PubMed] [Google Scholar]
- 39.Kwon HW, Paeng JC, Nahm FS, Kim SG, Zehra T, Oh SW, et al. Diagnostic performance of three-phase bone scan for complex regional pain syndrome type 1 with optimally modified image criteria. Nucl Med Mol Imaging. 2011;45:261–267. doi: 10.1007/s13139-011-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringer R, Wertli M, Bachmann LM, Buck FM, Brunner F. Concordance of qualitative bone scintigraphy results with presence of clinical complex regional pain syndrome 1: meta-analysis of test accuracy studies. Eur J Pain. 2012;16:1347–1356. doi: 10.1002/j.1532-2149.2012.00137.x. [DOI] [PubMed] [Google Scholar]
- 41.Wertli MM, Brunner F, Steurer J, Held U. Usefulness of bone scintigraphy for the diagnosis of Complex Regional Pain Syndrome 1: a systematic review and Bayesian meta-analysis. PLoS One. 2017;12:e0173688. doi: 10.1371/journal.pone.0173688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schürmann M, Zaspel J, Löhr P, Wizgall I, Tutic M, Manthey N, et al. Imaging in early posttraumatic complex regional pain syndrome: a comparison of diagnostic methods. Clin J Pain. 2007;23:449–457. doi: 10.1097/AJP.0b013e31805c9e66. [DOI] [PubMed] [Google Scholar]
- 43.Elgazzar AH. Orthopedic nuclear medicine. New York (NY): Springer; 2004. pp. 88–91. [Google Scholar]
- 44.Turpin S, Taillefer R, Lambert R, Leveille J. “Cold” reflex sympathetic dystrophy in an adult. Clin Nucl Med. 1996;21:94–97. doi: 10.1097/00003072-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27:567–572. doi: 10.1097/BPO.0b013e318070cc4d. [DOI] [PubMed] [Google Scholar]
- 46.Park JM, Kim SJ, Chung SH, Lee YT. Study for reliability of interpretation of the three phase bone scintigraphy in patients with post-traumatic complex regional pain syndrome. Nucl Med Mol Imaging. 2008;42:44–51. [Google Scholar]
- 47.Saha S, Burke C, Desai A, Vijayanathan S, Gnanasegaran G. SPECT-CT: applications in musculoskeletal radiology. Br J Radiol. 2013;86:20120519. doi: 10.1259/bjr.20120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirschmann MT, Schmid R, Dhawan R, Skarvan J, Rasch H, Friederich NF, et al. Combined single photon emission computerized tomography and conventional computerized tomography: clinical value for the shoulder surgeons? Int J Shoulder Surg. 2011;5:72–76. doi: 10.4103/0973-6042.86242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walton J, Mahajan S, Paxinos A, Marshall J, Bryant C, Shnier R, et al. Diagnostic values of tests for acromioclavicular joint pain. J Bone Joint Surg Am. 2004;86-A:807–812. doi: 10.2106/00004623-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Huellner MW, Bürkert A, Schleich FS, Schürch M, Hug U, von Wartburg U, et al. SPECT/CT versus MRI in patients with nonspecific pain of the hand and wrist - a pilot study. Eur J Nucl Med Mol Imaging. 2012;39:750–759. doi: 10.1007/s00259-011-2034-3. [DOI] [PubMed] [Google Scholar]
- 51.Dreinhöfer KE, Dieppe P, Stürmer T, Gröber-Grätz D, Flören M, Günther KP, et al. Indications for total hip replacement: comparison of assessments of orthopaedic surgeons and referring physicians. Ann Rheum Dis. 2006;65:1346–1350. doi: 10.1136/ard.2005.047811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palestro CJ. Nuclear medicine and the failed joint replacement: past, present, and future. World J Radiol. 2014;6:446–458. doi: 10.4329/wjr.v6.i7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arıcan P, Okudan Tekin B, Şefizade R, Naldöken S, Baştuğ A, Özkurt B. The role of bone SPECT/CT in the evaluation of painful joint prostheses. Nucl Med Commun. 2015;36:931–940. doi: 10.1097/MNM.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 54.Temmerman OP, Raijmakers PG, Berkhof J, Hoekstra OS, Teule GJ, Heyligers IC. Accuracy of diagnostic imaging techniques in the diagnosis of aseptic loosening of the femoral component of a hip prosthesis: a meta-analysis. J Bone Joint Surg Br. 2005;87:781–785. doi: 10.1302/0301-620X.87B6.15625. [DOI] [PubMed] [Google Scholar]
- 55.Ro DH, Lee HY, Chang CB, Kang SB. Value of SPECT-CT imaging for middle-aged patients with chronic anterior knee pain. BMC Musculoskelet Disord. 2015;16:169. doi: 10.1186/s12891-015-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Nabhani K, Michopoulou S, Allie R, Alkalbani J, Saad Z, Sajjan R, et al. Painful knee prosthesis: can we help with bone SPECT/CT? Nucl Med Commun. 2014;35:182–188. doi: 10.1097/MNM.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 57.Singh VK, Javed S, Parthipun A, Sott AH. The diagnostic value of single photon-emission computed tomography bone scans combined with CT (SPECT-CT) in diseases of the foot and ankle. Foot Ankle Surg. 2013;19:80–83. doi: 10.1016/j.fas.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Ha S, Hong SH, Paeng JC, Lee DY, Cheon GJ, Arya A, et al. Comparison of SPECT/CT and MRI in diagnosing symptomatic lesions in ankle and foot pain patients: diagnostic performance and relation to lesion type. PLoS One. 2015;10:e0117583. doi: 10.1371/journal.pone.0117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claassen L, Uden T, Ettinger M, Daniilidis K, Stukenborg-Colsman C, Plaass C. Influence on therapeutic decision making of SPECT-CT for different regions of the foot and ankle. Biomed Res Int. 2014;2014:927576. doi: 10.1155/2014/927576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosado-de-Castro PH, Lopes de Souza SA, Alexandre D, Barbosa da, Gutfilen B. Rheumatoid arthritis: Nuclear Medicine state-of-the-art imaging. World J Orthop. 2014;5:312–318. doi: 10.5312/wjo.v5.i3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahin M, Bernay I, Basoglu T, Canturk F. Comparison of Tc-99m MDP, Tc-99m HSA and Tc-99m HIG uptake in rheumatoid arthritis and its variants. Ann Nucl Med. 1999;13:389–395. doi: 10.1007/BF03164932. [DOI] [PubMed] [Google Scholar]
- 62.Berná L, Torres G, Diez C, Estorch M, Martínez-Duncker D, Carrió I. Technetium-99m human polyclonal immunoglobulin G studies and conventional bone scans to detect active joint inflammation in chronic rheumatoid arthritis. Eur J Nucl Med. 1992;19:173–176. doi: 10.1007/BF00173277. [DOI] [PubMed] [Google Scholar]
- 63.Ozgul A, Yasar E, Arslan N, Balaban B, Taskaynatan MA, Tezel K, et al. The comparison of ultrasonographic and scintigraphic findings of early arthritis in revealing rheumatoid arthritis according to criteria of American College of Rheumatology. Rheumatol Int. 2009;29:765–768. doi: 10.1007/s00296-008-0765-7. [DOI] [PubMed] [Google Scholar]
- 64.Kim JY, Cho SK, Han M, Choi YY, Bae SC, Sung YK. The role of bone scintigraphy in the diagnosis of rheumatoid arthritis according to the 2010 ACR/EULAR classification criteria. J Korean Med Sci. 2014;29:204–209. doi: 10.3346/jkms.2014.29.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JY, Choi YY, Kim CW, Sung YK, Yoo DH. Bone Scintigraphy in the diagnosis of rheumatoid arthritis: is there additional value of bone scintigraphy with blood pool phase over conventional bone scintigraphy? J Korean Med Sci. 2016;31:502–509. doi: 10.3346/jkms.2016.31.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]