Summary
Background
About half of patients with papillary thyroid cancer have tumours with activating BRAFV600E mutations. Vemurafenib, an oncogenic BRAF kinase inhibitor approved for BRAF-positive melanoma, showed clinical benefit in three patients with BRAFV600E-positive papillary thyroid cancer in a phase 1 trial. We aimed to establish the activity of vemurafenib in patients with BRAFV600E-positive papillary thyroid cancer.
Methods
We did an open-label, non-randomised, phase 2 trial at ten academic centres and hospitals worldwide in patients aged 18 years or older with histologically confirmed recurrent or metastatic papillary thyroid cancer refractory to radioactive iodine and positive for the BRAFV600E mutation. Participants either had never received a multikinase inhibitor targeting VEGFR (cohort 1) or had been treated previously with a VEGFR multikinase inhibitor (cohort 2). Patients received vemurafenib 960 mg orally twice daily. The primary endpoint was investigator-assessed best overall response in cohort 1 (confirmed on two assessments 4 weeks or longer apart). Analyses were planned to have a minimum median follow-up of 15 months (data cutoff April 18, 2014) and were done in safety, intention-to-treat, and per-protocol populations. This trial is closed and is registered at ClinicalTrials.gov, number NCT01286753.
Findings
Between June 23, 2011, and Jan 15, 2013, 51 patients were enrolled to the study, 26 in cohort 1 and 25 in cohort 2. Median duration of follow-up was 18·8 months (IQR 14·2–26·0) in cohort 1 and 12·0 months (6·7–20·3) in cohort 2. Partial responses were recorded in ten of 26 patients in cohort 1 (best overall response 38·5%, 95% CI 20·2–59·4). Grade 3 or 4 adverse events were recorded in 17 (65%) of 26 patients in cohort 1 and 17 (68%) of 25 patients in cohort 2; the most common grade 3 and 4 adverse events were squamous cell carcinoma of the skin (seven [27% ] in cohort 1, five [20% ] in cohort 2), lymphopenia (two [8% ] in each cohort), and increased γ-glutamyltransferase (one [4% ] in cohort 1, three [12% ] in cohort 2). Two individuals in cohort 2 died due to adverse events, one from dyspnoea and one from multiorgan failure, but neither was treatment related. Serious adverse events were reported for 16 (62%) of 26 patients in cohort 1 and 17 (68%) of 25 patients in cohort 2.
Interpretation
Vemurafenib showed antitumour activity in patients with progressive, BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine who had never been treated with a multikinase inhibitor. As such, this agent represents a potential new treatment option for these patients.
Introduction
Worldwide, thyroid cancer is the sixteenth most common malignant disease.1 About 298 000 new cases were diagnosed globally in 2012, with the highest incidence reported in North America.1 In the USA, an estimated 62 450 cases of differentiated thyroid cancer were diagnosed in 2015.2 Standard treatments include surgery, suppression of thyroid-stimulating hormone (TSH), and selective treatment with radioactive iodine.3,4 However, 25–50% of patients with locally advanced or metastatic disease will become refractory to radioactive iodine.3
For patients who are not cured of their disease with surgery and radioactive iodine, few treatment options exist. Previously, the only agent approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), doxorubicin, showed little effectiveness and was associated with clinically significant toxic effects.5 In the past several years, clinical activity has been recorded with multikinase inhibitors for progressive differentiated thyroid cancer refractory to radioactive iodine.6–11 Within this group of agents, sorafenib and lenvatinib have been approved by the FDA and the EMA based on results of pivotal phase 3 trials.12,13 However, disease ultimately progresses in patients treated with either drug, and some individuals do not tolerate the side-effects (eg, hand-foot skin reaction and rash with sorafenib, and hypertension and asthenia with lenvatinib). Therefore, an unmet need remains: to develop additional molecularly targeted agents for treatment of these patients.
Most patients with differentiated thyroid cancer have papillary thyroid cancer. The somatic BRAFV600E mutation is found in 37–50% of patients with papillary thyroid cancer, mainly in classic or tall-cell variants.14–16 Presence of this mutation at diagnosis of papillary thyroid cancer correlates with aggressive tumour characteristics, including extrathyroidal extension, advanced tumour stage at presentation, lymph node or disease metastasis, and, possibly, increased cancer-related mortality.17,18 Furthermore, tumours harbouring the BRAFV600E mutation have a decreased ability to incorporate radioactive iodine, resulting in treatment failure or recurrent disease.15,17 The decrease in radioactive iodine uptake might, in part, be a result of dysregulation of the sodium iodide symporter that correlates with the presence of BRAFV600E.15 As a result, patients whose tumours harbour the BRAFV600E mutation might be more likely to need additional systemic treatment for recurrent and metastatic disease.
Vemurafenib is a kinase inhibitor specific for mutated BRAF kinase and is approved for the management of BRAFV600E-positive melanoma.19 Data from preliminary studies suggest that vemurafenib might have activity in BRAFV600E-positive papillary thyroid cancer.20,21 Therefore, we aimed to assess the activity and safety of vemurafenib in patients with progressive papillary thyroid cancer refractory to radioactive iodine.
Methods
Study design and participants
We did an open-label, non-randomised, phase 2 trial at ten academic centres and hospitals worldwide (seven in the USA, two in Italy, and one in the Netherlands; appendix). We included patients aged 18 years or older with histologically confirmed recurrent or metastatic papillary thyroid cancer refractory to radioactive iodine and positive for the BRAFV600E mutation (using the most recent tumour sample obtained). We defined refractoriness to radioactive iodine as: absence of uptake of radioactive iodine on either a low-dose diagnostic test or a post-treatment radioactive iodine scan in measurable lesions; radiographic progression of disease within 18 months of the last course of radioactive iodine treatment, despite the recorded uptake of radioactive iodine with that previous therapy; or having a cumulative lifetime administered dose of greater than 600 mCi of radioactive iodine and positive for the BRAFV600E mutation (because further exposure to radioactive iodine is unlikely to be beneficial and is associated with increased risk). We also required patients to have measurable disease, according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and evidence of progression by RECIST 1.1 within the preceding 14 months. Additional inclusion criteria were: Eastern Cooperative Oncology Group (ECOG) performance status 0–1; life expectancy longer than 3 months; TSH less than 0·5 mIU/L; haemoglobin concentration 9 g/dL or higher; platelet count 100 × 109 cells per L or higher; absolute neutrophil count 1·5 × 109 cells per L or higher; serum creatinine 1·5 times the upper limit of normal (ULN) or less; alanine aminotransferase and aspartate amino-transferase 2·5 times ULN or less (≤5 × ULN for patients with concurrent liver metastases); bilirubin 1·5 times ULN or less; alkaline phosphatase 2·5 times ULN or less (≤5 × ULN for patients with concurrent liver metastases); and recovery from a toxic effect associated with any previous treatment (which helps to discern whether an adverse event is attributable to study treatment).
We excluded patients who had previously received specific BRAF or MEK inhibitor agents; however, we allowed previous use of non-specifi c BRAF inhibitors—eg, sorafenib. Further exclusion criteria were: active or unstable brain metastases; other active malignant diseases (including squamous cell carcinoma); history of carcinomatous meningitis; other anticipated or ongoing anticancer treatment; pregnancy or lactation; refractory nausea and vomiting, malabsorption, external biliary shunt, or substantial bowel resection that would preclude adequate absorption; congenital prolonged QTc syndrome or QTc interval greater than 450 ms; grade 3 or worse haemorrhage within 28 days before initiation of study treatment; myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident or transient ischaemic attack, or active pulmonary embolism within 6 months of initiation of study treatment; clinically significant active infection; organ transplant or allogeneic bone marrow or stem cell transplant; known HIV positivity or AIDS-related illness, hepatitis B virus, or active hepatitis C virus; and social, familial, or psychological factors that preclude the required follow-up schedule.
We stratified participants into two cohorts. Cohort 1 comprised patients who had never received a multikinase inhibitor targeting VEGFR, and cohort 2 consisted of patients who had previously received treatment with a VEGFR multikinase inhibitor. We enrolled participants into cohort 1 or cohort 2 on the basis of previous treatment.
The study protocol is included in the appendix and was approved by the institutional review boards or independent ethics committees of the participating study centres, and the study was undertaken in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Procedures
To identify the BRAFV600E mutation, we tested the most recent tumour sample obtained from patients at a central laboratory with the cobas 4800 BRAFV600E mutation test (Roche Molecular Diagnostics, Pleasanton, CA, USA). All eligible patients started open-label vemurafenib (960 mg orally) twice a day in cycles of 28 days. To manage symptomatic adverse events, we permitted dose interruptions of up to 15 days and dose reductions (in increments of 240 mg twice daily) to 720 mg twice daily and 480 mg twice daily. Treatment was discontinued either because of disease progression or intolerable toxic effects (in accordance with dose adjustment recommendations in the prescribing information). Intolerable toxic effects with the 480 mg twice a day dosing schedule were also cause for discontinuation.
Tumours were assessed by local investigators with CT or MRI every 8 weeks using RECIST 1.1. Of note, according to RECIST 1.1, bone lesions were not judged target lesions but were followed up as non-target lesions. Safety assessments included monitoring of laboratory variables (haematology, chemistry, and urinalysis), vital signs, ECOG performance status, and physical examinations. We did these assessments monthly for the first 9 months, then every 2 months for 6 months, and every 3 months thereafter. In addition to physical examinations, we did dermatological, head and neck, and gynaecological examinations to detect development of squamous cell carcinoma. We followed up patients who discontinued treatment for 1 month for treatment-related adverse events and for up to 12 months for possible secondary malignant diseases. Adverse events were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.
We did not assess RAS mutations routinely because they are generally mutually exclusive from RAF mutations in patients not previously treated with BRAF inhibitors. Specimens from patients with secondary malignant diseases could be assessed for RAS mutations, according to local standard methodology and submitted for central laboratory analysis by multiplex sequencing.
Outcomes
The primary endpoint of the study was best overall response in patients in cohort 1 (ie, those never treated with a multikinase inhibitor targeting VEGFR) confirmed on two assessments a minimum of 4 weeks apart. Best overall response was defined as the proportion of patients with a complete or partial response, according to RECIST 1.1, as assessed by the investigator.
A secondary endpoint was best overall response according to RECIST 1.1 in cohort 2 (ie, those previously treated with a VEGFR multikinase inhibitor). Additional secondary endpoints in both cohorts were: safety; duration of response, defined as the time from the first recorded response to progressive disease or death from any cause; disease control, defined as a confirmed complete response, partial response, or stable disease for at least 6 months; progression-free survival, defined as the time from the first dose of study treatment to first recorded progressive disease or death from any cause; and overall survival, defined as the time from first dose of study treatment to date of death from any cause. Pharmacokinetics were also assessed as a secondary endpoint and will be reported elsewhere.
Statistical analysis
We judged the planned sample size of 25 assessable patients in cohort 1 sufficient to detect major safety or tolerability problems. If seven of 25 patients achieved a response (best overall response 28%), the anticipated 90% CI was 14–46%. Cohort 2 used a two-stage design. If two or more responses were recorded in the first 15 assessable patients, the lower bound (2·4%) of the 95% CI for the minimum activity target of 13% best overall response would be exceeded, indicating minimum expected activity in cohort 2 and justifying expansion of the cohort to include up to 25 assessable patients. We report 95% CIs in the Results, however, because these are more stringent than 90% CIs and if the threshold boundary is not crossed the interpretation is still valid.
We estimated progression-free survival and overall survival in both cohorts with the Kaplan-Meier method. We calculated 95% CIs with the Clopper-Pearson method for best overall response and disease control, and with the Brookmeyer and Crowley method for duration of response, progression-free survival, and overall survival.
We summarised all safety data using descriptive statistics. For study treatment, we reported duration, starting dose, and cumulative dose. We presented adverse event data in frequency tables (overall and by intensity) by body system. When reporting the overall incidence of adverse events, we included patients who had the same event on more than one occasion only once, and we recorded the highest grade for the calculation of event frequency.
Analyses were planned to have a minimum median follow-up of 15 months (data cut-off April 18, 2014). To address the primary endpoint of best overall response, which requires confirmation of response, we defined the assessable per-protocol population as patients who underwent at least two post-baseline tumour assessments, or who died or discontinued study treatment because of progressive disease or an adverse event before undergoing two post-baseline tumour assessments, and excluded patients with major protocol violations. Best overall response, disease control, duration of response, progression-free survival, and overall survival were assessed in the per-protocol population. We summarised demographic data and deaths for the intention-to-treat population (ie, all enrolled patients). We defined the safety population as all enrolled patients who received at least one dose of study treatment. We did all statistical analyses with SAS version 9.2.
This trial is registered with ClinicalTrials.gov number, NCT01286753. The trial was terminated early by decision of the funder; patients still receiving benefit from treatment were offered enrolment for treatment with continued vemurafenib in an extension trial (NCT01739764). Patients will continue to be followed up for safety and time-to-event outcomes in the extension trial.
Role of the funding source
The funder administered and sponsored the study, which was designed by the authors in conjunction with representatives of the funder. Data were collected by the funder and analysed and interpreted in collaboration with the authors. Editorial support that did not involve writing was supported by the funder. MSB, TR, and HY had access to raw data. The authors vouch for the accuracy and completeness of the data. The manuscript outline was prepared by MSB and TR, but all authors contributed to subsequent drafts and made the decision to submit the report for publication.
Results
Between June 23, 2011, and Jan 15, 2013, 116 patients were screened for eligibility. 105 (91%) tumour specimens were submitted to the central laboratory for mutation testing; samples from the remaining 11 (9%) patients were deemed inadequate for testing. 67 (64%) of 105 specimens were positive for the BRAFV600E mutation. 16 (24%) of these 67 patients failed eligibility criteria; therefore, 51 patients were enrolled into the study. 26 (51%) patients who had not previously received a multikinase inhibitor targeting VEGFR were treated in cohort 1 and 25 (49%) individuals who had previously received a VEGFR multikinase inhibitor were treated in cohort 2. Initially, 15 patients were enrolled into cohort 2, but this cohort was expanded to 25 patients according to the protocol specification of observation of at least two confirmed responders and tolerable safety profile in the first 15 patients.
Baseline characteristics are presented in table 1. Three (12%) patients in cohort 1 and seven (28%) in cohort 2 had previously received systemic chemotherapy with either a taxane or anthracycline. 21 (84%) patients in cohort 2 had previously received treatment with sorafenib, and ten (40%) had received other small-molecule agents, including cediranib, pazopanib, sunitinib, a VEGF inhibitor (PTC299; PTC Therapeutics, NJ, USA), and a selective ATP competitive inhibitor of p70 S6 kinase (LY2584702; Eli Lilly, IN, USA).
Table 1. Baseline characteristics.
| Cohort 1 (n=26) | Cohort 2 (n=25) | |
|---|---|---|
| Sex | ||
| Male | 15 (58%) | 13 (52%) |
| Female | 11 (42%) | 12 (48%) |
|
| ||
| Race | ||
| White | 24 (92%) | 20 (80%) |
| Other | 2 (8%) | 5 (20%) |
|
| ||
| Ethnic origin | ||
| Hispanic or Latino | 4 (15%) | 3 (12%) |
| Other | 22 (85%) | 22 (88%) |
|
| ||
| Age (years) | 67 (55–74) | 65 (58–71) |
| <65 | 12 (46%) | 12 (48%) |
| ≥65 | 14 (54%) | 13 (52%) |
|
| ||
| Local recurrent or unresectable cancer | 3 (12%) | 2 (8%) |
|
| ||
| Metastatic cancer | 23 (88%) | 23 (92%) |
|
| ||
| ECOG performance score | ||
| 0 | 15 (58%) | 13 (52%) |
| 1 | 11 (42%) | 12 (48%) |
|
| ||
| Previous treatments | ||
| 0 | 19 (73%) | 0 |
| 1 | 4 (15%) | 7 (28%) |
| 2 | 2 (8%) | 9 (36%) |
| ≥3 | 1 (4%) | 9 (36%) |
Data are number of patients (%) or median (IQR). Cohort 1 includes patients who had not previously received a multikinase inhibitor and cohort 2 includes those previously treated with a multikinase inhibitor. ECOG=Eastern Cooperative Oncology Group.
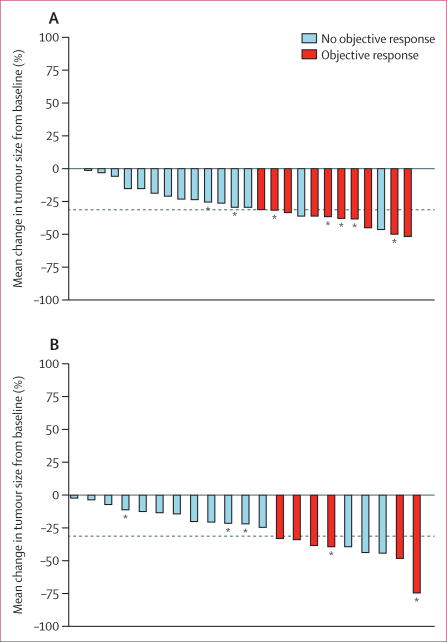
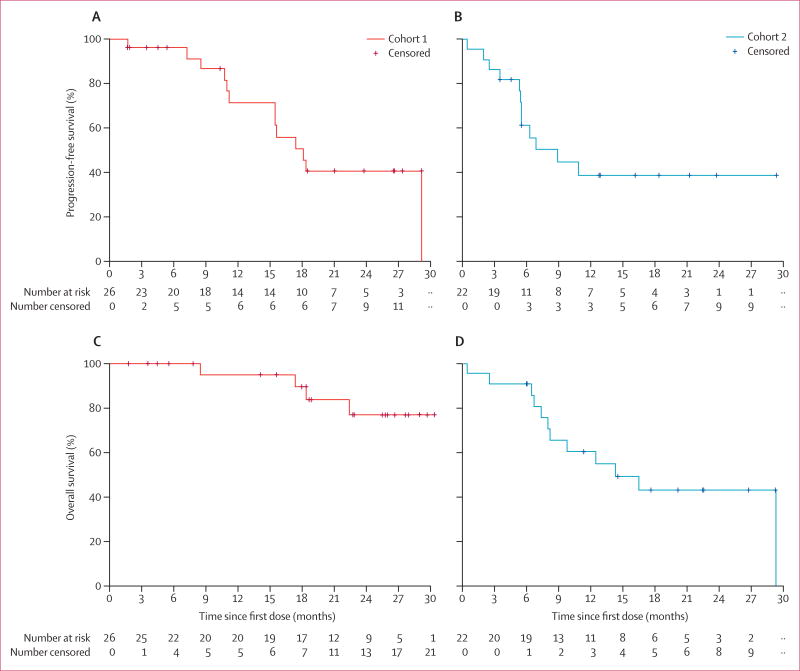
In cohort 1, the median duration of treatment was 63·6 weeks (IQR 24·6–99·1). All patients in cohort 1 were included in the per-protocol analysis. A best overall response of partial response was confirmed in ten (38·5%; 95% CI 20·2–59·4) of 26 patients (figure 1, table 2). Nine (35%) patients achieved stable disease for at least 6 months; therefore, 19 (73%; 95% CI 52–88) patients achieved disease control. After a median follow-up of 18·8 months (IQR 14·2–26·0), 13 (50%) patients in cohort 1 had a progression-free survival event, and four (15%) patients had died. Median progression-free survival in cohort 1 was 18·2 months (95% CI 15·5–29·3; figure 2), the median duration of response was 16·5 months (5·7–not estimable [NE]), and median overall survival was not yet reached (NE–NE; figure 2).
Figure 1. Waterfall plots showing mean change from baseline in tumour size in the per-protocol population.
Change in tumour size recorded as smallest sum of diameters. Objective response was either a complete or partial response. Dotted line represents the threshold for partial response. *Patients who are still on treatment as of data cutoff (April 18, 2014). (A) Patients who have never received a multikinase inhibitor (cohort 1). (B) Patients previously treated with a multikinase inhibitor (cohort 2). One patient in cohort 2 did not have a post-baseline tumour assessment because they died within the first two cycles of treatment.
Table 2. Activity of vemurafenib.
| Cohort 1 (n=26) | Cohort 2 (n=22) | |
|---|---|---|
| Best overall response (confirmed) | ||
| Complete response | 0 | 0 |
| Partial response | 10 (38·5%, 20·2-59·4) | 6 (27·3%, 10·7-50·2) |
| Stable disease | 15 (57·7%, 36·9-76·7) | 14 (63·6%, 40·7-82·8) |
| Progressive disease | 1 (3·8%, 0·1-19·6) | 1 (4·5%, 0·1-22·8) |
| Unknown | 0 | 1 (5%) |
Data are number of responses (%, 95% CI). Cohort 1 includes patients who have never received a multikinase inhibitor and cohort 2 includes those previously treated with a multikinase inhibitor. The analysis population included patients with at least two post-baseline tumour scans or progressive disease, worsening disease, death, or an adverse event within the first two cycles.
Figure 2. Kaplan-Meier curves of progression-free survival and overall survival.
Progression-free survival in (A) patients who have never received a multikinase inhibitor (cohort 1) and (B) patients previously treated with a multikinase inhibitor (cohort 2). Overall survival in (C) cohort 1 and (D) cohort 2.
In cohort 2, the median duration of treatment was 27·6 weeks (IQR 18·6–60·0). Two patients in cohort 2 were later deemed ineligible for the study because of previous treatment with a RAF inhibitor in one (XL281) and a MEK inhibitor in the other (selumetinib), and one patient withdrew consent before undergoing two post-baseline tumour assessments; these patients were included in the safety analysis but were excluded from per-protocol analysis. Six (27·3%; 95% CI 10·7–50·2) of 22 patients achieved a partial response as a best overall response (figure 1, table 2), and six (27·3%) patients had stable disease for at least 6 months. Therefore, 12 (55%; 95% CI 32–76) patients achieved disease control. After a median follow-up of 12·0 months (IQR 6·7–20·3), 12 (55%) of 22 patients had died. Median progression-free survival in cohort 2 was 8·9 months (95% CI 5·5–NE; figure 2), the median duration of response was 7·4 months (3·7–NE), and median overall survival was 14·4 months (8·2–29·5; figure 2).
All patients enrolled in cohorts 1 and 2 were included in the safety analysis. The starting dose of vemurafenib for most patients was 960 mg twice daily (26 [100% ] patients in cohort 1 and 24 [96% ] patients in cohort 2). The remaining patient in cohort 2 started on 480 mg twice daily as a result of a dispensing error by the clinical staff at that centre. The median cumulative dose of vemurafenib was 619·3 g (IQR 247·4–1132·8) in cohort 1 and 293·8 g (179·0–647·8) in cohort 2.
At the time of this analysis (April 18, 2014), in cohort 1, 19 (73%) patients had discontinued treatment: 11 (42%) because of disease progression, seven (27%) because of an adverse event, and one (4%) who withdrew consent and refused further treatment. In cohort 2, 20 (80%) patients had discontinued treatment: 11 (44%) because of disease progression, seven (28%) because of an adverse event, and two (8%) who withdrew consent.
Grade 1–2 adverse events occurring in 15% or more patients and all grade 3–5 adverse events are listed in table 3; all adverse events recorded in the study are shown in the appendix. Grade 1 increases in TSH were reported in two (8%) patients in cohort 1 and one (4%) patient in cohort 2, although adequate TSH suppression was noted before treatment.
Table 3. Adverse events.
| Cohort 1 (n=26) | Cohort 2 (n=25) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | ||
| Rash* | 19 (73%) | 0 | 0 | 0 | 16 (64%) | 1 (4%) | 0 | 0 | |
| Fatigue, asthenia, or malaise† | 18 (69%) | 2 (8%) | 0 | 0 | 16 (64%) | 0 | 0 | 0 | |
| Alopecia | 14 (54%) | 0 | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Dysgeusia | 14 (54%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Blood creatinine increase | 13 (50%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Weight decrease | 13 (50%) | 1 (4%) | 0 | 0 | 13 (52%) | 0 | 0 | 0 | |
| Arthralgia | 12 (46%) | 0 | 0 | 0 | 9 (36%) | 0 | 0 | 0 | |
| Decreased appetite | 12 (46%) | 1 (4%) | 0 | 0 | 11 (44%) | 0 | 0 | 0 | |
| Nausea | 12 (46%) | 1 (4%) | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Skin papilloma | 12 (46%) | 1 (4%) | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Diarrhoea | 11 (42%) | 1 (4%) | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Hyperkeratosis | 11 (42%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Photosensitivity or sunburn‡ | 11 (42%) | 0 | 0 | 0 | 10 (40%) | 0 | 0 | 0 | |
| Blood bilirubin increase | 10 (38%) | 0 | 0 | 0 | 9 (36%) | 1 (4%) | 0 | 0 | |
| Anaemia | 9 (35%) | 0 | 0 | 0 | 11 (44%) | 2 (8%) | 0 | 0 | |
| Myalgia | 8 (31%) | 1 (4%) | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Blood alkaline phosphatase increase | 7 (27%) | 0 | 0 | 0 | 5 (20%) | 1 (4%) | 0 | 0 | |
| Headache | 7 (27%) | 0 | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Palmar-plantar erythrodysaesthesia | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 2 (8%) | 0 | 0 | |
| Actinic keratosis | 6 (23%) | 1 (4%) | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Dry skin | 6 (23%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| γ-glutamyltransferase increase | 6 (23%) | 1 (4%) | 0 | 0 | 4 (16%) | 2 (8%) | 1 (4%) | 0 | |
| Melanocytic naevus | 6 (23%) | 0 | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Proteinuria | 6 (23%) | 0 | 0 | 0 | 4 (16%) | 0 | 0 | 0 | |
| Skin induration | 6 (23%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Vomiting | 6 (23%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Acrochordon | 5 (19%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Aspartate aminotransferase increase | 5 (19%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Cyst | 5 (19%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dysphagia | 5 (19%) | 0 | 0 | 0 | 2 (8%) | 1 (4%) | 0 | 0 | |
| Hypertension | 5 (19%) | 1 (4%) | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Hypocalcaemia | 5 (19%) | 0 | 0 | 0 | 4 (16%) | 0 | 0 | 0 | |
| Paraesthesia | 5 (19%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Skin lesion | 5 (19%) | 0 | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Alanine aminotransferase increase | 4 (15%) | 0 | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Conjunctivitis | 4 (15%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Constipation | 4 (15%) | 0 | 0 | 0 | 2 (8%) | 0 | 0 | 0 | |
| Dermal cyst | 4 (15%) | 0 | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Dermatitis acneiform | 4 (15%) | 0 | 0 | 0 | 2 (8%) | 0 | 0 | 0 | |
| Dyspnoea | 3 (12%) | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 1 (4%) | 1 (4%) | |
| Hyperglycaemia | 3 (12%) | 1 (4%) | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Insomnia | 4 (15%) | 0 | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Keratosis pilaris | 4 (15%) | 0 | 0 | 0 | 3 (12%) | 0 | 0 | 0 | |
| Neck pain | 4 (15%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Oedema peripheral | 4 (15%) | 0 | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Pain in extremity | 4 (15%) | 0 | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Back pain | 3 (12%) | 0 | 0 | 0 | 2 (8%) | 1 (4%) | 0 | 0 | |
| Blood uric acid increase | 3 (12%) | 0 | 1 (4%) | 0 | 1 (4%) | 0 | 1 (4%) | 0 | |
| Electrocardiograph QT prolonged | 3 (12%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hyponatraemia | 3 (12%) | 1 (4%) | 0 | 0 | 7 (28%) | 0 | 0 | 0 | |
| Hypokalaemia | 3 (12%) | 0 | 0 | 0 | 5 (20%) | 1 (4%) | 0 | 0 | |
| Lymphopenia | 3 (12%) | 2 (8%) | 0 | 0 | 3 (12%) | 2 (8%) | 0 | 0 | |
| Musculoskeletal pain | 3 (12%) | 0 | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Urinary tract infection | 3 (12%) | 0 | 0 | 0 | 4 (16%) | 0 | 0 | 0 | |
| Dehydration | 2 (8%) | 1 (4%) | 0 | 0 | 4 (16%) | 1 (4%) | 0 | 0 | |
| Dizziness | 2 (8%) | 0 | 0 | 0 | 8 (32%) | 0 | 0 | 0 | |
| Hyperbilirubinaemia | 2 (8%) | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Hypoalbuminaemia | 2 (8%) | 0 | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Pruritus | 2 (8%) | 0 | 0 | 0 | 4 (16%) | 0 | 0 | 0 | |
| White blood cell count decrease | 2 (8%) | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Atrial fibrillation | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Cough | 1 (4%) | 0 | 0 | 0 | 5 (20%) | 0 | 0 | 0 | |
| Hyperuricaemia | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | |
| Hypotension | 1 (4%) | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | |
| Keratoacanthoma | 1 (4%) | 2 (8%) | 0 | 0 | 2 (8%) | 1 (4%) | 0 | 0 | |
| Pleuritic pain | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pneumonia | 1 (4%) | 0 | 0 | 0 | 1 (4%) | 2 (8%) | 0 | 0 | |
| Pulmonary haemorrhage | 1 (4%) | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Pyrexia | 1 (4%) | 0 | 0 | 0 | 6 (24%) | 0 | 0 | 0 | |
| Anal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Angina pectoris | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Basal cell carcinoma | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Blood pressure increase | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Cardiac failure congestive | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cardiomegaly | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Convulsion | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Diverticulitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Food poisoning | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hepatotoxicity | 0 | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Large intestine perforation | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Laryngitis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymphocyte count decrease | 0 | 2 (8%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Laryngeal oedema | 0 | 1 (4%) | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Left ventricular dysfunction | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | |
| Malignant melanoma | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Multiorgan failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | |
| Musculoskeletal chest pain | 0 | 0 | 0 | 0 | 2 (8%) | 1 (4%) | 0 | 0 | |
| Myocardial infarction | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | |
| Oesophageal haemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | |
| Oral lichen planus | 0 | 1 (4%) | 0 | 0 | 1 (4%) | 0 | 0 | 0 | |
| Performance status decreased | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Pathological fracture | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Radiation necrosis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rectal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Right ventricular failure | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 0 | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 1 (4%) | 0 | |
| Thermal burn | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour pain | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Viral labyrinthitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Radiation necrosis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rectal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Right ventricular failure | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 0 | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 1 (4%) | 0 | |
| Thermal burn | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour pain | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Viral labyrinthitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Radiation necrosis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rectal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Right ventricular failure | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 0 | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 1 (4%) | 0 | |
| Thermal burn | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour pain | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Viral labyrinthitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Radiation necrosis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rectal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Right ventricular failure | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 0 | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 1 (4%) | 0 | |
| Thermal burn | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour pain | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Viral labyrinthitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Radiation necrosis | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rectal haemorrhage | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
| Right ventricular failure | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 0 | 7 (27%) | 0 | 0 | 0 | 4 (16%) | 1 (4%) | 0 | |
| Thermal burn | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tumour pain | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Viral labyrinthitis | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 0 | 0 | |
Data are number of adverse events (%). Table shows grade 1–2 adverse events occurring in 15% or more of patients in either cohort and all grade 3–5 adverse events, irrespective of whether it was treatment related. Cohort 1 includes patients who have never received a multikinase inhibitor and cohort 2 included those previously treated with a multikinase inhibitor.
Roche/Genentech basket adverse event term: rash (all)—adverse event preferred term of rash papular, drug eruption rash, dermatitis, folliculitis, rash pruritic, rash vesicular, toxic skin eruption, rash erythematosus, rash maculopapular, rash morbilliform, dermatitis bullous, eyelid rash, rash macular, rash follicular, erythema, generalised erythema, rash generalised, rash papulosquamous, rash pustular, dermatitis exfoliative, exfoliative rash, rash maculovesicular, or dermatitis allergic.
Roche/Genentech basket adverse event term: adverse event higher level term of asthenic conditions or adverse event preferred term of asthenia, autonomic nervous system imbalance, cachexia, chronic fatigue syndrome, decreased activity, fatigue, lethargy, listless, malaise, or sluggishness.
Roche/Genentech basket adverse event term: adverse event preferred term of photosensitivity reaction or sunburn.
Grade 3 or 4 adverse events were recorded in 17 (65%) patients in cohort 1 and 17 (68%) patients in cohort 2 (table 3). 15 (58%) patients in cohort 1 needed a dose reduction, to either 720 mg orally twice daily (n=10) or 480 mg orally twice daily (n=5) and 22 (85%) required a dose interruption. 12 (48%) patients in cohort 2 needed a dose reduction, to either 720 mg orally twice daily (n=5) or 480 mg orally twice daily (n=7), and 16 (64%) required a dose interruption because of an adverse event. Adverse events requiring discontinuation of treatment were similar between cohorts (seven [27% ] vs seven [28% ]). Adverse events leading to discontinuation were dysphagia (n=1, cohort 2), oesophogeal haemorrhage (n=1, cohort 2), oral lichen planus (n=1, cohort 1), arthralgia (n=2, one per cohort), pathological fracture (n=1, cohort 1), fatigue (n=1, cohort 1), gait disturbance (n=1, cohort 2), dyspnoea (n=2, both cohort 2), angina pectoris (n=1, cohort 1), hepatotoxicity (n=1, cohort 1), γ-glutamyltransferase increase (n=1, cohort 2), and palmar plantar erythrodysaesthesia (n=1, cohort 1).
Serious adverse events were reported for 16 (62%) patients in cohort 1 and 17 (68%) patients in cohort 2. Serious adverse events reported in two or more patients in either cohort were cutaneous squamous cell carcinoma (seven [27% ] in cohort 1, five [20% ] in cohort 2), keratoacanthoma (two [8% ] in cohort 1, three [12% ] in cohort 2), dyspnoea (two [8% ] in each cohort), pneumonia (two [8% ] in cohort 2), hypotension (two [8% ] in cohort 2), cerebrovascular accident (two [8% ] in cohort 2), and squamous cell carcinoma (two [8% ] in cohort 1). One (4%) patient in each cohort had a cutaneous pigmented lesion.
Three (6%) of the 51 patients had a non-cutaneous second malignant disease reported as an adverse event, according to the investigators: squamous cell carcinoma of the distal trachea (n=1, cohort 1), squamous cell carcinoma of the head and neck (n=1, cohort 1), and gastric adenocarcinoma (n=1, cohort 2). All three patients discontinued treatment at the time of diagnosis of the second malignant disease (separate to the number of discontinuations attributed to disease progression or need for treatment other than vemurafenib). The gastric adenocarcinoma was deemed unrelated to vemurafenib by the investigator. In the two patients with non-cutaneous squamous cell carcinoma (which were deemed possibly related to vemurafenib), the tumour arose at sites of thyroid cancer and was initially reported as progressive disease (and included as events for progression-free survival). These cases could represent dedifferentiation of the primary tumour with squamoid features. None of the three cases showed a RAS mutation on molecular analysis.
In cohort 1, four (15%) of 26 patients had died at the time of analysis; the cause of death was disease progression in two (8%), stroke in one (4%), and an unknown cause in one (4%). In cohort 2, 12 (48%) of 25 patients had died at the time of analysis; the cause of death was disease progression in eight (32%) and two (4%) patients died due to adverse events (one patient died from dyspnoea a symptom of simultaneously diagnosed progression of disease] and one patient died of multiorgan failure secondary to placement of a chest tube to treat pre-existing, disease-related, pleural effusion). None of the deaths in either cohort were judged related to vemurafenib by the investigators.
Discussion
In our open-label, non-randomised, phase 2 trial in patients with BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine, vemurafenib showed antitumour activity both in individuals who had never received a multikinase inhibitor (cohort 1) and in those previously treated with a multikinase inhibitor (cohort 2). The overall toxicity profile was generally consistent with that reported in patients with melanoma treated with vemurafenib. To our knowledge, our trial represents the first prospective clinical trial and the largest dataset to assess treatment with vemurafenib for this patient population. Although 67 (64%) of the 105 tissue specimens submitted to the central laboratory were positive for BRAFV600E mutations, some centres routinely prescreened patients for BRAF mutations with other methods before enrolment in this trial. Thus, our screening population was probably enriched with individuals harbouring the mutation. Moreover, BRAF mutations have been noted to occur more frequently with increasing age;22,23 consistent with this observation, the population of patients in our study was older than those enrolled in other phase 2 and phase 3 studies of multikinase inhibitors.
In our study, 38·5% (95% CI 20·2–59·4) of patients in cohort 1 achieved a partial response as best overall response, which met the primary endpoint (planned target 28%). The proportion of patients who achieved a best overall response was similar to that recorded with other kinase inhibitors studied in phase 2 and phase 3 trials, with responses of 8–65% reported for vandetanib, sorafenib, motesanib, axitinib, pazopanib, cabozantinib, and lenvatinib.6–13 However, direct comparison of these proportions is not possible because of differences in patients' characteristics and enrolment criteria across studies. Median progression-free survival in cohort 1 was 18·2 months (95% CI 15·2–29·3), which is also similar to values reported with other kinase inhibitors (median progression-free survival of 9·2–18·7 months for motesanib, sorafenib, vandetanib, pazopanib, axitinib, and lenvatinib).6–13 The median progression-free survival for cohort 1 suggests that targeting the BRAFV600E mutation might provide additional treatment options in patients with papillary thyroid cancer who harbour this mutation and have never been treated with a multikinase inhibitor.
A greater proportion of patients in cohort 1 achieved a response and had longer progression-free survival compared with those in cohort 2. However, most patients in cohort 2 were heavily pretreated and had been exposed to many other agents in addition to a VEGFR multikinase inhibitor. Therefore, a best overall response of 27·3% (95% CI 10·7–50·2) and median progression-free survival of 8·9 months (95% CI 5·5–NE) in cohort 2 might still suggest a clinical benefit. Data for use of a VEGFR multikinase inhibitor after failure of previous VEGFR multikinase inhibitors are available only for lenvatinib, for which the median progression-free survival was 15·1 months among patients with differentiated thyroid cancer who had received only one previous line of VEGFR multikinase inhibitor therapy.13 Therefore, the extent to which patients previously exposed to only one VEGFR inhibitor would benefit from vemurafenib treatment in the second-line setting is not clear, and because of the few patients in this study who had received only one previous line of therapy, this question warrants further investigation.
The most common adverse events observed in our study included rash, fatigue, weight loss, taste alteration, and alopecia, which were similar to those noted in patients with melanoma treated with vemurafenib.19 These adverse events were managed with supportive care and dose adjustments. Rash and fatigue occurred at a higher frequency in our trial than in previous melanoma studies,19,24 perhaps because of the longer duration of treatment. A greater proportion of patients in cohort 1 than in cohort 2 needed dose adjustments for adverse events (85% vs 64%), perhaps because of the longer duration of treatment (63·6 weeks vs 27·6 weeks) or the lack of previous exposure and clinician experience in controlling toxic effects associated with kinase inhibitors. Additionally, the proportion of patients discontinuing treatment because of adverse events was somewhat higher in the current study (roughly 27% in both cohorts) than was reported with other multikinase inhibitors,8,12 possibly because of the number of previous treatments received by patients in cohort 2. The frequency of non-cutaneous squamous cell carcinoma as a second malignant disease (6%) was consistent with that reported in melanoma patients (<5%).20 Although data suggest that vemurafenib might potentiate the growth of pre-existing lesions harbouring RAS mutations,25 none of the second malignant diseases reported in our study harboured RAS mutations, which suggests the presence of an alternative mechanism.
Our study is limited by the absence of data for direct historical comparison of overall survival for patients with thyroid cancer refractory to radioactive iodine and harbouring a BRAFV600E mutation. Furthermore, a good comparator is not available for the heavily pretreated population in cohort 2, making conclusions about the activity of vemurafenib in these patients difficult.
Although the clinical usefulness of screening all patients with papillary thyroid cancer for BRAFV600E mutations at the time of diagnosis remains to be elucidated, our data suggest that screening for these mutations in the setting of disease refractory to radioactive iodine could identify potential treatment options offering clinical benefit for these patients. The activity of vemurafenib is comparable with that of currently available multikinase inhibitors for the treatment of patients with progressive, recurrent, or metastatic disease that is refractory to radioactive iodine who have never been treated with multikinase inhibitors. The response to vemurafenib in patients previously treated with multikinase inhibitors might support use of this agent in this setting as well. The adverse event profile in these patients was similar to that of vemurafenib in individuals with melanoma, and no new safety signals were identified. We have shown the activity of vemurafenib in patients with progressive, BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine, and further investigation is warranted.
Research in context.
Evidence before this study
BRAFV600E mutations occur in 37–50% of all patients with papillary thyroid cancer and correlate with aggressive tumour characteristics and decreased ability of tumours to incorporate radioactive iodine. We searched MEDLINE and Embase with the terms “thyroid cancer”, “BRAF inhibitor”, “vemurafenib”, and “dabrafenib” for peer-reviewed articles and abstracts published between Jan 1, 2010, and Dec 1, 2015, with no restriction by language. Vemurafenib, an oncogenic BRAF kinase inhibitor approved for BRAFV600E-positive melanoma, showed clinical benefit in three patients with BRAFV600E-positive papillary thyroid cancer in a phase 1 trial; one patient had a partial response lasting 7·6 months and two patients had stable disease for 11·4 months and 13·2 months. Four responses were also observed in patients with thyroid cancer as part of a phase 1 study of dabrafenib in BRAFV600E solid tumours.
Added value of this study
Our study is the first prospective phase 2 trial to assess vemurafenib in patients with papillary thyroid cancer refractory to radioactive iodine and harbouring the BRAFV600E mutation. Vemurafenib showed antitumour activity in two cohorts of patients: those who had never received a multikinase inhibitor targeting VEGFR and those previously treated with a VEGFR multikinase inhibitor. Best overall response, duration of response, and progression-free survival were encouraging in patients who had never received a VEGFR multikinase inhibitor. Best overall response was also promising in patients previously treated with a multikinase inhibitor targeting VEGFR. The overall toxicity profile was generally consistent with that reported in patients with melanoma treated with a BRAF inhibitor.
Implications of all the available evidence
Vemurafenib has shown activity in patients with progressive, BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine. Further investigation is warranted.
Acknowledgments
Funding: F Hoffmann-La Roche.
The study was funded by F Hoffmann-La Roche. Editorial support that did not entail writing was provided by Melanie Sweetlove (ApotheCom, San Francisco, CA, USA) and was funded by F Hoffmann-La Roche.
Footnotes
Contributors: MSB participated in study design and implementation, data collection, data interpretation, and writing and editing of the report. MEC participated in study design, data collection, enrolment and treatment of patients, and data interpretation. EEWC participated in protocol design, patients' enrolment, data review and analysis, and writing of the report, and was a member of the study's steering committee. LJW participated in study design, enrolment and treatment of patients, data collection, data analysis, data interpretation, and writing of the report. TR acted as Roche clinical oversight during the course of the study and participated in data cleaning, data analysis, data interpretation, and review of the report. HY participated in data analysis and data interpretation. SIS participated in study design, data interpretation, and writing and editing of the report. EJS participated in study design, patients' accrual, data analysis, and writing of the report.
Declaration of interests: MSB reports research grants, consultant fees, and honoraria from Bayer Healthcare Pharmaceuticals, Exelixis, and Eisai; consultant fees from Onyx Pharmaceuticals; and research grants from Genentech and Novartis; all outside the submitted work. MEC reports grants from Roche for the work under consideration; and grants and advisory board fees from Eisai and advisory board fees from Bayer outside the submitted work. LJW reports consulting fees for Eisai and Merck outside the submitted work. TR is an employee of Roche/Genentech and HY is an employee of Roche. SIS reports grants from Roche for the work under consideration; and grants and personal fees from Genzyme and personal fees from Eisai, Exelixis, Bayer, Onyx, and Amgen outside the submitted work. EEWC and EJS declare no competing interests.
Contributor Information
Marcia S Brose, Department of Otorhinolaryngology: Head and Neck Surgery, and Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA.
Maria E Cabanillas, Department of Endocrine Neoplasia and Hormonal Disorders, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ezra E W Cohen, Moores Cancer Center, University of California San Diego, La Jolla, CA, USA.
Lori J Wirth, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA.
Todd Riehl, Product Development Clinical Oncology.
Huibin Yue, Product Development Clinical Oncology and Biostatistics, Genentech Inc, South San Francisco, CA, USA; and Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Prof Steven I Sherman, Department of Endocrine Neoplasia and Hormonal Disorders, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Eric J Sherman, Product Development Clinical Oncology and Biostatistics, Genentech Inc, South San Francisco, CA, USA; and Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Hodak SP, Carty SE. Radioiodine-resistant differentiated thyroid cancer: hope for the future. Oncology. 2009;23:775–76. [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–99. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Mehra R, Cohen RB. New agents in the treatment for malignancies of the salivary and thyroid glands. Hematol Oncol Clin North Am. 2008;22:1279–95. doi: 10.1016/j.hoc.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 7.Bible KC, Suman VJ, Molina JR, et al. for the Endocrine Malignancies Disease Oriented Group ,Mayo Clinic Cancer Center, the Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–72. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabanillas ME, Schlumberger M, Jarzab B, et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer. 2015;121:2749–56. doi: 10.1002/cncr.29395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabanillas ME, Brose MS, Ramies DA, Lee Y, Miles D, Sherman SI. Antitumor activity of cabozantinib (XL184) in a cohort of patients(pts) with differentiated thyroid cancer (DTC) Proc Am Soc Clin Oncol. 2012;30 abstr 5547. [Google Scholar]
- 10.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 12.Brose MS, Nutting CM, Jarzab B, et al. on behalf of the DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 15.Fallahi P, Ferrari SM, Santini F, et al. Sorafenib and thyroid cancer. BioDrugs. 2013;27:615–28. doi: 10.1007/s40259-013-0049-y. [DOI] [PubMed] [Google Scholar]
- 16.Elisei R, Ugolini C, Viola D, et al. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–55. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 17.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–79. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 18.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23:1277–83. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadu R, Shah K, Busaidy NL, et al. Efficacy and tolerability of vemurafenib in patients with BRAF(V600E)-positive papillary thyroid cancer: MD Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100:E77–81. doi: 10.1210/jc.2014-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell N, Jeremiah S, Morishita M, et al. Frequency of BRAF T1796A mutation in papillary thyroid carcinoma relates to age of patient at diagnosis and not to radiation exposure. J Pathol. 2005;205:558–64. doi: 10.1002/path.1736. [DOI] [PubMed] [Google Scholar]
- 23.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–22. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–15. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]