Abstract
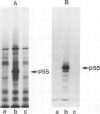
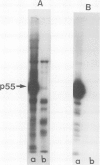
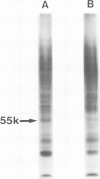
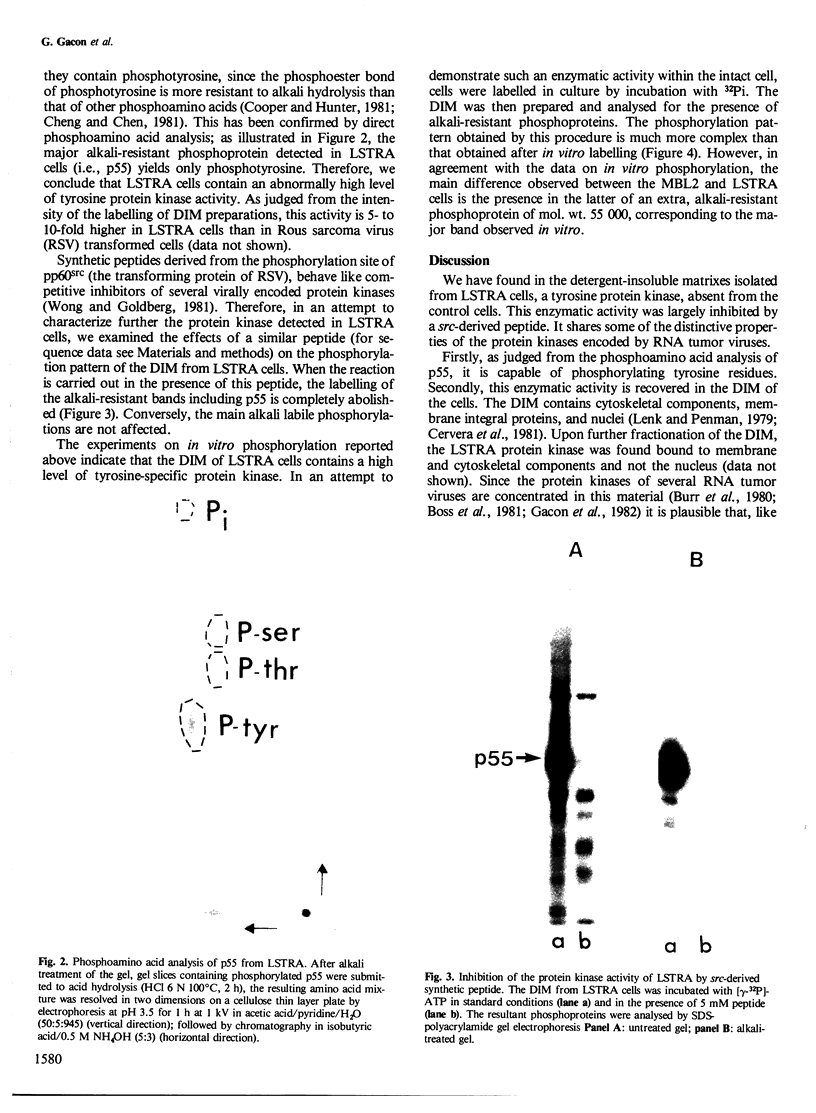
Several sarcoma-inducing viruses encode protein kinases that phosphorylate tyrosine residues. Such enzymatic activities can be detected within the detergent-insoluble matrix of transformed fibroblasts. We have analysed the protein kinase activities in two murine lymphoma cell lines ( MBL2 and LSTRA) induced by Moloney murine leukemia virus (Mo-MuLV). After incubation of the detergent-insoluble matrix of these cells with [gamma-32P]ATP, several alkali-resistant phosphoproteins, including a very heavily labelled 55 000 mol. wt. protein ( p55 ), have been detected in LSTRA, reflecting the activity of a protein kinase specific to this cell line. This protein kinase activity shares some of the distinctive properties of the protein kinases of transforming viruses, i.e., specificity for tyrosine residues, association with membranous and/or cytoskeletal structures, and inhibition by a synthetic peptide derived from the phosphorylation site of pp60src. In view of the absence of a transforming gene in MoMuLV , it is likely that the high level of protein kinase detected in the LSTRA cell line arises from the expression of a cellular gene.
Full text
PDF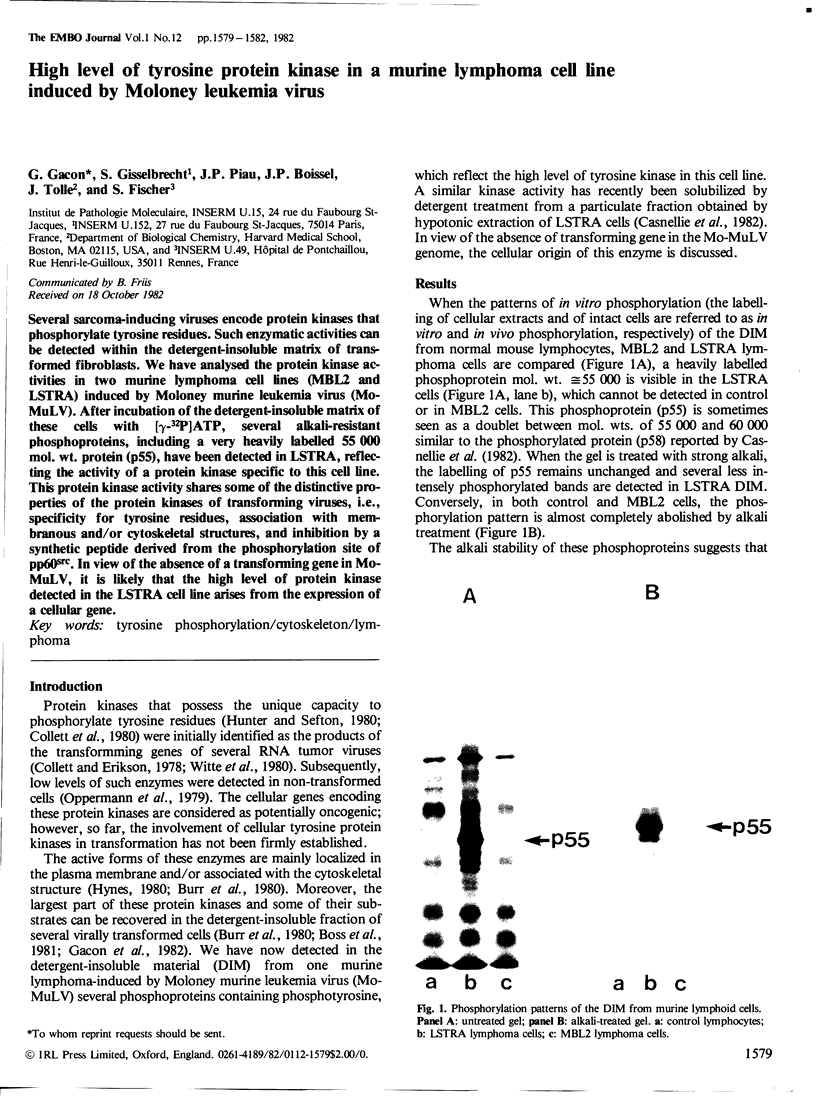


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss M. A., Dreyfuss G., Baltimore D. Localization of the Abelson murine leukemia virus protein in a detergent-insoluble subcellular matrix: architecture of the protein. J Virol. 1981 Nov;40(2):472–481. doi: 10.1128/jvi.40.2.472-481.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN J. P., BLANCO A. R., GOLDIN A. STUDIES ON INDUCED RESISTANCE AGAINST ISOTRANSPLANTS OF VIRUS-INDUCED LEUKEMIA. Cancer Res. 1964 Apr;24:502–508. [PubMed] [Google Scholar]
- Gacon G., Gisselbrecht S., Piau J. P., Fiszman M. Y., Fischer S. Phosphorylations of the subcellular matrix in cells transformed by Rous' sarcoma virus. Eur J Biochem. 1982 Jul;125(2):453–456. doi: 10.1111/j.1432-1033.1982.tb06704.x. [DOI] [PubMed] [Google Scholar]
- Gutte B., Merrifield R. B. The synthesis of ribonuclease A. J Biol Chem. 1971 Mar 25;246(6):1922–1941. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cellular location of viral transforming proteins. Cell. 1980 Oct;21(3):601–602. doi: 10.1016/0092-8674(80)90421-3. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R., Hanafusa H. Changes in amino-terminal sequences of pp60src lead to decreased membrane association and decreased in vivo tumorigenicity. Cell. 1982 Apr;28(4):889–896. doi: 10.1016/0092-8674(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. Abelson murine leukemia virus mutants with alterations in the virus-specific P120 molecule. J Virol. 1980 Jan;33(1):340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N. E., Baltimore D. A normal cell protein cross-reactive to the major Abelson murine leukaemia virus gene product. Nature. 1979 Oct 4;281(5730):396–398. doi: 10.1038/281396a0. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Synthetic peptide fragment of src gene product inhibits the src protein kinase and crossreacts immunologically with avian onc kinases and cellular phosphoproteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7412–7416. doi: 10.1073/pnas.78.12.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]