Abstract
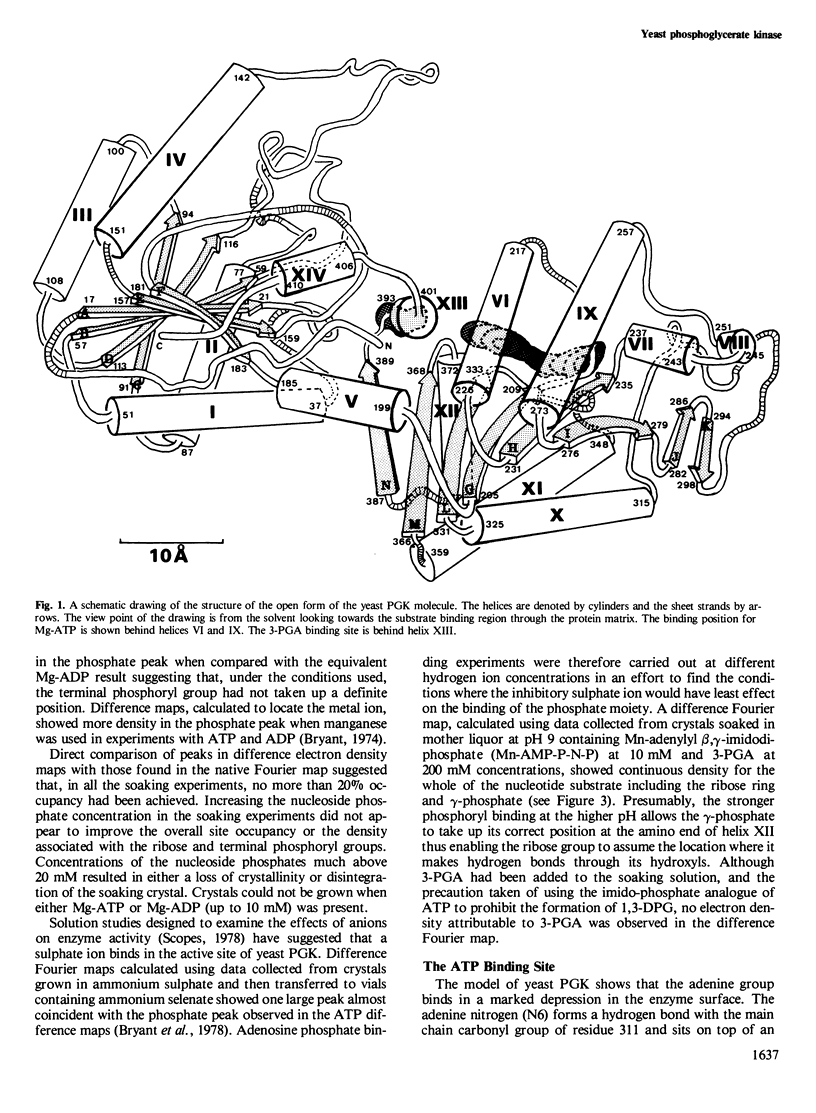
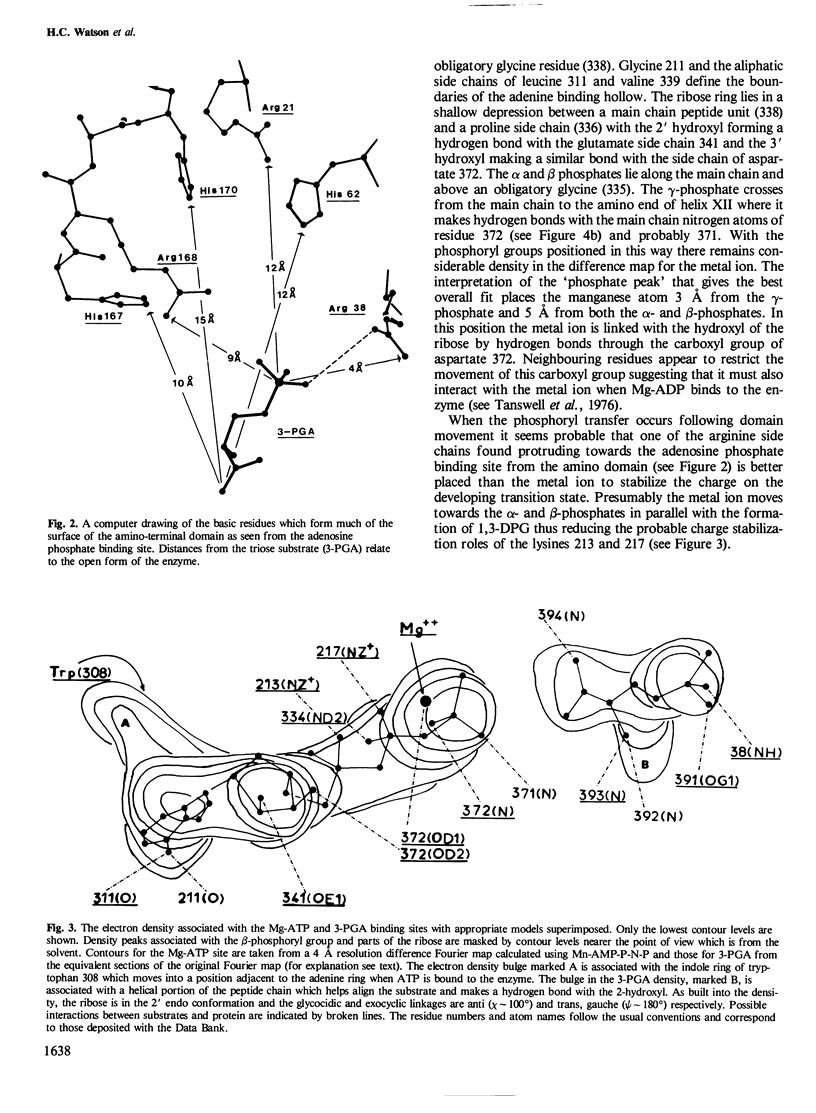
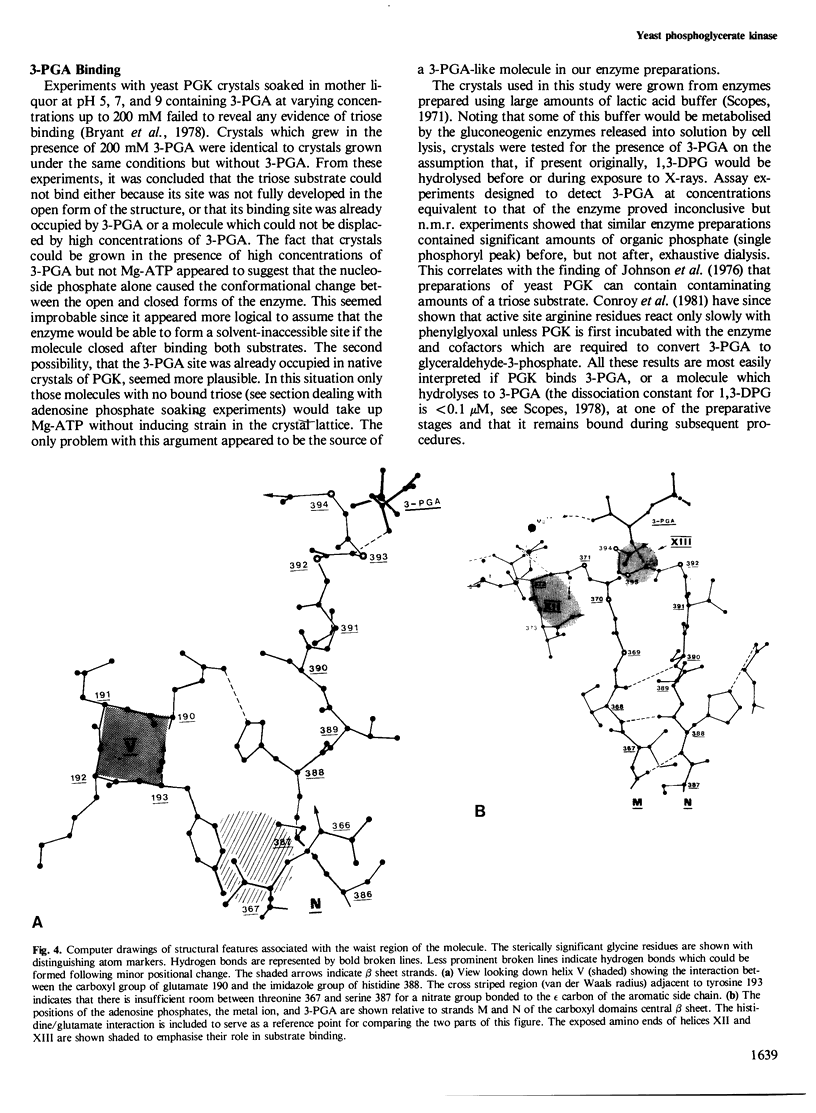
The structure of yeast phosphoglycerate kinase has been determined with data obtained from amino acid sequence, nucleotide sequence, and X-ray crystallographic studies. The substrate binding sites, as deduced from electron density maps, are compatible with known substrate specificity and the stereochemical requirements for the enzymic reaction. A carboxyl-imidazole interaction appears to be involved in controlling the transition between the open and closed forms of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacharach A. D., Markland F. S., Pellino A., Weber B. H. Modification of yeast 3-phosphoglycerate kinase: isolation and sequence determination of a nitrated active-site peptide and isolation of a carboxyl modified active-site peptide. Biochem Biophys Res Commun. 1977 Jan 10;74(1):165–171. doi: 10.1016/0006-291x(77)91389-4. [DOI] [PubMed] [Google Scholar]
- Banks R. D., Blake C. C., Evans P. R., Haser R., Rice D. W., Hardy G. W., Merrett M., Phillips A. W. Sequence, structure and activity of phosphoglycerate kinase: a possible hinge-bending enzyme. Nature. 1979 Jun 28;279(5716):773–777. doi: 10.1038/279773a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Evans P. R. Structure of horse muscle phosphoglycerate kinase. Some results on the chain conformation, substrate binding and evolution of the molecule from a 3 angstrom Fourier map. J Mol Biol. 1974 Apr 25;84(4):585–601. doi: 10.1016/0022-2836(74)90118-1. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Rice D. W. Phosphoglycerate kinase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):93–104. doi: 10.1098/rstb.1981.0063. [DOI] [PubMed] [Google Scholar]
- Bryant T. N., Watson H. C., Wendell P. L. Structure of yeast phosphoglycerate kinase. Nature. 1974 Jan 4;247(5435):14–17. doi: 10.1038/247014a0. [DOI] [PubMed] [Google Scholar]
- Conroy S. C., Adams B., Pain R. H., Fothergill L. A. Yeast phosphoglycerate kinase purified by affinity elution has tightly bound 3-phosphoglycerate. FEBS Lett. 1981 Jun 15;128(2):353–355. doi: 10.1016/0014-5793(81)80115-9. [DOI] [PubMed] [Google Scholar]
- Dobson M. J., Tuite M. F., Roberts N. A., Kingsman A. J., Kingsman S. M., Perkins R. E., Conroy S. C., Fothergill L. A. Conservation of high efficiency promoter sequences in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Apr 24;10(8):2625–2637. doi: 10.1093/nar/10.8.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I. Y., Welch C. D., Yoshida A. Complete amino acid sequence of human phosphoglycerate kinase. Cyanogen bromide peptides and complete amino acid sequence. J Biol Chem. 1980 Jul 10;255(13):6412–6420. [PubMed] [Google Scholar]
- Johnson P. E., Abbott S. J., Knowles J. R. The "phosphoryl-enzyme" from phosphoglycerate kinase. Biochemistry. 1976 Jun 29;15(13):2893–2898. [PubMed] [Google Scholar]
- Larsson-Raźnikiewicz M. Electrophoretic purification as well as some physical and chemical characterizations of phosphoglycerate kinase from yeast. Eur J Biochem. 1970 Sep;15(3):574–580. doi: 10.1111/j.1432-1033.1970.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Meyer M. C., Westhead E. W. Interaction of sulfate ion with a critical tyrosine residue in yeast phosphoglycerate kinase detected through the tetranitromethane reaction. FEBS Lett. 1976 Nov 15;72(1):25–28. doi: 10.1016/0014-5793(76)80890-3. [DOI] [PubMed] [Google Scholar]
- Pickover C. A., McKay D. B., Engelman D. M., Steitz T. A. Substrate binding closes the cleft between the domains of yeast phosphoglycerate kinase. J Biol Chem. 1979 Nov 25;254(22):11323–11329. [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Rose Z. B. The enzymology of 2,3-bisphosphoglycerate. Adv Enzymol Relat Areas Mol Biol. 1980;51:211–253. doi: 10.1002/9780470122969.ch5. [DOI] [PubMed] [Google Scholar]
- Roustan C., Fattoum A., Jeanneau R., Pradel L. A. Yeast 3-phosphoglycerate kinase: sulfate and substrate binding, their effect on the conformational state of the enzyme. Biochemistry. 1980 Nov 11;19(23):5168–5175. doi: 10.1021/bi00564a003. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. An improved procedure for the isolation of 3-phosphoglycerate kinase from yeast. Biochem J. 1971 Mar;122(1):89–92. doi: 10.1042/bj1220089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K. The steady-state kinetics of yeast phosphoglycerate kinase. Anomalous kinetic plots and the effects of salts on activity. Eur J Biochem. 1978 Apr 17;85(2):503–516. doi: 10.1111/j.1432-1033.1978.tb12266.x. [DOI] [PubMed] [Google Scholar]
- Tanswell P., Westhead E. W., Williams R. J. Nuclear-magnetic-resonance study of the active-site structure of yeast phosphoglycerate kinase. Eur J Biochem. 1976 Mar 16;63(1):249–262. doi: 10.1111/j.1432-1033.1976.tb10227.x. [DOI] [PubMed] [Google Scholar]
- Watson H. C., Wendell P. L., Scopes R. K. Crystallographic study of yeast phosophoglycerate kinase. J Mol Biol. 1971 May 14;57(3):623–625. doi: 10.1016/0022-2836(71)90114-8. [DOI] [PubMed] [Google Scholar]
- Webb M. R., Trentham D. R. Analysis of chiral inorganic [16O, 17O, 18O]thiophosphate and the stereochemistry of the 3-phosphoglycerate kinase reaction. J Biol Chem. 1980 Mar 10;255(5):1775–1778. [PubMed] [Google Scholar]
- Wendell P. L., Bryant T. N., Watson H. C. Low resolution structure of yeast phosphoglycerate kinase. Nat New Biol. 1972 Nov 29;240(100):134–136. doi: 10.1038/newbio240134a0. [DOI] [PubMed] [Google Scholar]
- Winn S. I., Watson H. C., Harkins R. N., Fothergill L. A. Structure and activity of phosphoglycerate mutase. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):121–130. doi: 10.1098/rstb.1981.0066. [DOI] [PubMed] [Google Scholar]