Abstract
Osteopontin (OPN) has been investigated in the field of tumor research for several years. However, the prognostic role of OPN overexpression in acute myeloid leukemia (AML) remains controversial. A meta-analysis of four studies, including a total of 492 patients, was performed to determine the association of OPN with overall survival (OS) in AML patients. The random-effects model of Der Simonian and Laird was used to synthesize data; hazard ratio (HR) with its 95% confidence interval (CI) was used as the effect size estimate. It was observed that serum-based OPN was inversely correlated with OS and the difference was statistically significant (HR=1.83; 95% CI: 1.43–2.35; P<0.001). Experimental findings indicate that OPN overexpression is associated with a poor prognosis in AML and may be of prognostic value for AML stage and metastasis.
Keywords: osteopontin, prognosis, acute myeloid leukemia, meta-analysis
Introduction
Acute myeloid leukemia (AML) is a unique type of malignancy, the main treatment for which is chemotherapy alone or chemotherapy combined with radiotherapy, as surgical intervention is not indicated, with certain exceptions (1). Although significant progress has been made in the diagnosis and treatment of AML, the prognosis of leukemia patients is dismal due to recurrence and poor response to adjunctive treatments in advanced stages. Thus, it is crucial to identify predictive factors for prognosis and metastasis/recurrence, which may help with regimen selection and improve patient survival.
Predictive factors are subdivided into host-related (e.g., age and performance status) and tumor-related (e.g., number of tumor cells, cytogenetic and molecular characteristics of the leukemic clone, growth characteristics and resistance to apoptosis). Hematological malignancies are not only affected by the molecular interactions within the malignant cell clone, but also by interactions with the microenvironment. The association between niche cells and malignant cells significantly affects the pathophysiological behavior and development of the disease. Leukemic cell localization in the bone marrow and the cross-talk with the bone niche trigger marked changes in the bone marrow microenvironment, which are critical for tumor progression, resistance to treatment and recurrence (2–5).
Osteopontin (OPN) is a candidate prognostic factor for the progression of various hematological malignancies. OPN was initially considered to be a transformation-associated protein in the epithelial cells (6) and has since been extensively investigated. OPN is a secreted glycophosphoprotein that may physiologically act as a cytokine as well as an extracellular matrix molecule. A number of studies recently demonstrated that overexpression of OPN is significantly associated with tumorigenesis and metastasis in several malignant tumors, such as breast, pulmonary, prostatic, thyroid, ovarian, bladder and gastrointestinal carcinomas (7–9). Furthermore, aberrant OPN expression has been correlated with poor prognosis of leukemia (10–12).
Although a number of studies have considered OPN as a marker in patient specimens, published data have not yet been fully analyzed. Moreover, conflicting results have been reported regarding the value of OPN as predictive factor for overall survival (OS) in hematological malignancies. Thus, a meta-analysis is required in order to systematically and comprehensively elucidate the prognostic value of OPN in hematological malignancies.
Data collection methods
The aim of the present study was to objectively evaluate the prognostic role of elevated OPN levels for OS in patients with AML using pooled results from the available data.
Criteria for study inclusion
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org/).
Identification of studies
A search was conducted through PubMed, China National Knowledge Infrastructure, Web of Science, Embase and the Cochrane Central Register of Controlled Trials from inception until December 25th, 2015, and the articles referenced in the identified studies were also manually searched for additional studies that met the inclusion criteria. No restriction on publication status was applied. The search terms included osteopontin (1–168) human or SPP1 protein human (13).
Data collection and analysis
Two reviewers (Drs Sidan Li and Yongbing Chen) independently screened the initially identified studies. The full-text articles of potentially eligible studies were independently assessed against the eligibility criteria. Differences were then compared and referred to the consultants (Drs Zhongli Du and Simei Ren) for resolution. When more than one article was derived from the same study, only the most eligible records were included, considering data integrity and availability.
Selection of studies
The inclusion criteria were as follows: i) Measurement of OPN expression in patients with leukemia, lymphoma or myeloma by reverse transcription quantitative polymerase chain reaction (RT-qPCR), enzyme-linked immunosorbent assay (ELISA), microarray analysis or immunohistochemistry (IHC); ii) investigation of the possible correlation between changes in OPN expression and clinical prognosis; and iii) information on survival.
Data extraction and management
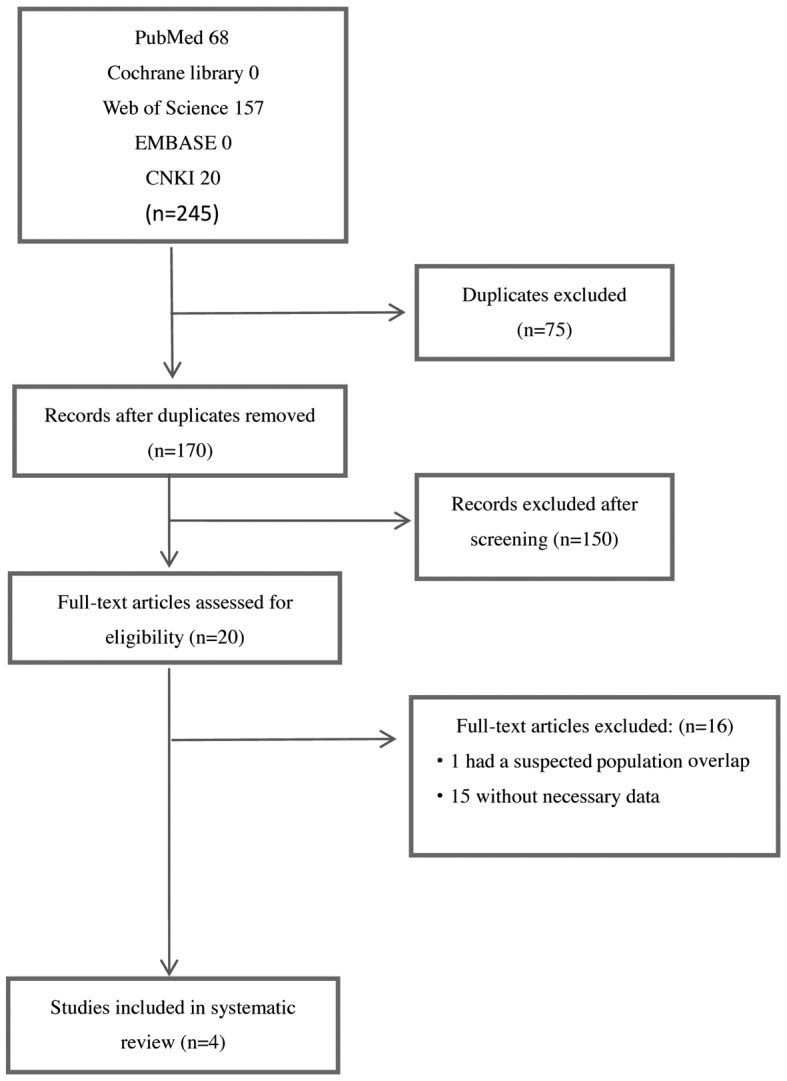
Two researchers (Dr Sidan Li and Dr Yongbing Chen) independently extracted data and selected the included studies. If there was discrepancy in data selection, the two researchers would review and discuss the study together, or resort to the consultants (Dr Zhongli Du and Dr Simei Ren) until a consensus was reached. When there more than one article was derived from the same study, only the most eligible records were included, considering data integrity and availability. The main reasons for exclusion of trials are described in Fig. 1.
Figure 1.
Flow diagram summarizing the identification process of relevant studies. CNKI, China National Knowledge Infrastructure.
Definitions
In the meta-analysis, OS was defined as the time from the diagnosis of hematological malignancies or initiation of different medical treatments to death or the date of the last follow-up.
Quality assessment
Quality assessment of available data included in this meta-analysis on the significance of OPN in hematological malignancies was performed independently by the two researchers in accordance with the Newcastle-Ottawa Scale (NOS) for cohort studies and case control studies, with mild modifications (13). This scale assesses patient population and selection, study comparability, follow-up and outcome of interest with scores ranging from 0 (lowest quality) to 9 (highest quality). The high-quality studies had scores of ≥6.
Statistical analysis
Survival outcome data were synthesized with the hazard ratio (HR) and its 95% confidence interval (CI) to analyze the effect of OPN expression on the survival of hematological malignancies. Studies providing HRs and 95% CIs were pooled directly. Otherwise, the HR and its 95% CI were calculated from available data or a Kaplan-Meier survival curve using Engauge Digitizer software, version 4.1 (http://sourceforge.net). The heterogeneity among the data was assessed by a Chi-squared-based Q statistic test, and the I2 value was used to quantify the heterogeneity (I2=0–50%, no or moderate heterogeneity; I2>50%, significant heterogeneity). If homogeneity was not significant (Q test P>0.10), the fixed-effects model was used; otherwise, the random-effects model was applied. Publication bias was assessed using the Begg's and Egger's tests. To adjust for multiple comparisons, the stepdown Bonferroni method was applied, which controls for family-wise error rate. Additionally, a sensitivity analysis was conducted to determine the stability of the pooled results. The statistical analyses were conducted using Stata 12.0 software (Stata Corp LP, College Station, TX, USA). All P-values were two-sided, and P<0.05 was considered to indicate statistically significant differences.
Results
Selection and characteristics of studies
A total of 170 studies on the association of OPN with hematological tumors were identified during the initial literature search; 150 studies were excluded after screening the titles or abstracts, as they were either review articles, abstracts, experimental researches, duplicate reports or studies irrelevant to the present analysis. A total of 20 studies were selected for detailed evaluation, 16 of which were excluded following full assessment (1 study was excluded due to a suspected population overlap and 15 studies lacked necessary data). Following careful evaluation based on the inclusion criteria, 4 eligible studies including a total of 492 patients were finally selected for the meta-analysis (11,12,14); the study performed by Liersch et al consisted of three independent researches. Two of the studies were performed in Germany (12), one in Chinese Taiwan (14) and one in Australia (11). The specimen size in each study varied from 52 to 261 patients and the median age of the patients ranged from 45 to 58 years. The percentage of the female population ranged from 37 to 45%. One study used ELISA to measure OPN expression, whereas the others used RT-qPCR, microarray analysis and IHC. OS was reported in each study. The grade of study quality as assessed by NOS ranked from 5 to 7 (Table I). The OPN cut-off value was determined using different methods in each study. The basic characteristics of the selected studies are summarized in Table II.
Table I.
Assessment of methodological quality according to Newcastle-Ottawa Scale quality assessment.
Table II.
Baseline characteristics of studies included in the meta-analysis.
| First author | Year | Country | Design | Study population | Median age (years) | Male gender (%) | Tumor vascular biomarker (yes/no) | Tumor stage (yes/no) | Osteopontin detection method | Survival analysis | Method for determining ‘high’ osteopontin cut.off level | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lierscha | 2012 | Germany | RC | 84 AML patients | 58 | 55 | NR | NR | IHC | OS | Recursive partitioning | (12) |
| Lierscha | 2012 | Germany | RC | 261 AML patients | NR | NR | NR | NR | Microarray analysis | OS | Recursive partitioning | (12) |
| Powell | 2009 | Australia | RC | 95 AML patients | 52 | 61 | NR | NR | RT-qPCR | OS | Recursive partitioning | (11) |
| Lee | 2008 | Taiwan | PC | 52 AML patients | 45 | 63 | Yes | NR | ELISA | OS | ROC curve | (14) |
Liersch et al performed three independent studies but only two were selected for inclusion. AML, acute myeloid leukemia; NR, not recorded; PC, prospective cohort study; RC, retrospective cohort study; IHC, immunohistochemistry; ELISA, enzyme-linked immunosorbent assay; RTqPCR, reverse transcription quantitative polymerase chain reaction; OS, overall survival; ROC, receiver operating characteristics.
OPN expression and OS in AML
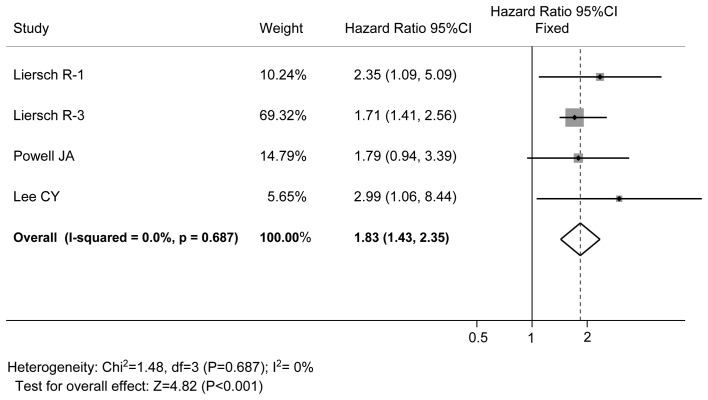
All the studies reported data on OPN expression and OS, as shown Table III. OPN expression was found to be inversely correlated with OS in all studies, and the difference was statistically significant, with a pooled HR estimate of 1.83 (95% CI: 1.43–2.35, P<0.001, fixed-effects model). There was no significant heterogeneity (I2=0%, P=0.687; Fig. 2).
Table III.
Characteristics of eligible studies in the meta-analysis on the prognostic value of osteopontin for overall survival in hematological malignancies.
| Study | Hazard ratio | 95% confidence interval | (Refs.) |
|---|---|---|---|
| Liersch et ala | 2.35 | 1.09–5.09 | (12) |
| Liersch et ala | 1.708 | .141-2.557 | (12) |
| Powell et al | 2.09 | 1.16–4.22 | (11) |
| Lee et al | 2.99 | 1.06–8.44 | (14) |
Liersch et al performed three independent studies but only two were selected for inclusion.
Figure 2.
Forest plot of the association between OPN elevation and OS of patients with hematological malignancies. OPN expression was found to be inversely correlated with OS in all studies, and the difference was statistically significant, with a pooled HR estimate of 1.83. There was no significant heterogeneity. OPN, osteopontin; OS, overall survival; HR, hazard ratio.
Publication bias
Publication bias assessment was used to evaluate the reliability of the meta-analysis results, particularly those that were found to be statistically significant (15). Publication bias was assessed using the Egger's test (16), with statistical significance set at P<0.05. There was no significant publication bias in the OS studies (P=0.687).
Discussion
The clinical outcome in patients with AML depends on various prognostic factors, including patient age and status, as well as the cytogenetic and molecular characteristics of the leukemic clone. Although there have been advances in the diagnosis and treatment, the overall prognosis of AMLs is dismal, due to the poor response to adjunctive treatments and high incidence of tumor recurrence in advanced stages (17). Thus, it is crucial to identify effective biomarkers of survival to help clinical decision-making for AML treatment and improve outcomes. OPN is considered to be one of the candidate markers of stage and prognosis for a number of malignant tumors and has been extensively investigated in that context; however, the available data have not been comprehensively analyzed to date. As meta-analysis is considered to be a valuable method for biomarker validation, the present meta-analysis was performed to investigate the correlation of OPN with AML prognosis.
In the present study, the correlation between OPN overexpression and OS in AML was initially assessed. The pooled results demonstrated that high OPN expression was significantly associated with a poor OS for AML (P<0.001); Although the analysis outcomes were considered significant, the cause of the differences in OS among AML patients with different OPN expression remain obscure. Several data demonstrated that OPN expression was inversely correlated with prognosis in AML patients (11,12,14). Whereas the majority of the publications had focused on solid tumors (18–21), there have been recent studies on the role of OPN in hematological malignancies (10,12). Increased serum OPN concentrations have been reported in chronic myeloid leukemia, multiple myeloma and AML (22). Lee et al suggested that higher bone marrow levels of OPN were significantly associated with shorter survival in a 1-year survival analysis of a small cohort of AML patients (14). Powell et al performed expression screening to identify targets in AML (11). These analyses identified OPN as a functionally relevant target. Liersch et al performed three independent cohort studies and identified OPN expression at the mRNA and protein level as a prognostic marker in AML (12). Furthermore, high OPN expression was found to be independently correlated with vascular invasion (14,23,24) and plays an important role in metastasis and stage of hematological malignances (23,25–27), possibly through activation of the mitogen-activated protein kinase and NF-κB pathways, as well as matrix metalloproteinase-2 (28). In summary, the pooled results of the present meta-analysis supported the hypothesis that OPN overexpression may promote AML progression through direct or indirect mechanisms, leading to a poor outcome of AML.
Although the prognostic role of OPN in AML was comprehensively evaluated, there were certain limitations that should be discussed. First, language and risk bias may be a factor in this meta-analysis, as positive results were more often published compared with negative results; furthermore, only reports written in English or Chinese were selected, which may affect the validity of the results to a certain degree. Second, despite the lack of significant heterogeneity or publication bias, only data from small databases were collected and the number and sample of included studies were relatively insufficient, which may also affect the reliability of the analysis results. Third, geographical bias was a concern. Fourth, as our meta-analysis was performed on the collected data, strong recommendations at an individual patient level could not be obtained. Due to these limitations, the results of the present meta-analysis should be interpreted carefully and conclusions should be drawn with caution.
In summary, OPN overexpression was found to be associated with poor prognosis of AML patients. Therefore, OPN, alone or combined with other markers, may provide important predictive information on AML invasion, metastasis and prognosis and it may also serve as a valuable molecular target for the clinical treatment of AML.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (no. 81641006) and Beijing City Youth Top-Notch Talents Project (no. 2016000021223ZK16).
References
- 1.Yanada M, Narimatsu H, Suzuki T, Matsuo K, Naoe T. Randomized controlled trials of treatments for hematologic malignancies: Study characteristics and outcomes. Cancer. 2007;110:334–339. doi: 10.1002/cncr.22776. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Peled A. CXCR4 antagonists: Targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 3.Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, Serve H, Berdel WE, Kienast J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 4.Schliemann C, Bieker R, Padro T, Kessler T, Hintelmann H, Buchner T, Berdel WE, Mesters RM. Expression of angiopoietins and their receptor Tie2 in the bone marrow of patients with acute myeloid leukemia. Haematologica. 2006;91:1203–1211. [PubMed] [Google Scholar]
- 5.Colombo M, Mirandola L, Platonova N, Apicella L, Basile A, Figueroa AJ, Cobos E, Chiriva-Internati M, Chiaramonte R. Notch-directed microenvironment reprogramming in myeloma: A single path to multiple outcomes. Leukemia. 2013;27:1009–1018. doi: 10.1038/leu.2013.6. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-X. [DOI] [PubMed] [Google Scholar]
- 7.Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF, Senger DR. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94:513–521. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- 9.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.CCR-1405-2. [DOI] [PubMed] [Google Scholar]
- 10.Chagan-Yasutan H, Tsukasaki K, Takahashi Y, Oguma S, Harigae H, Ishii N, Zhang J, Fukumoto M, Hattori T. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk Res. 2011;35:1484–1490. doi: 10.1016/j.leukres.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Powell JA, Thomas D, Barry EF, Kok CH, McClure BJ, Tsykin A, To LB, Brown A, Lewis ID, Herbert K, et al. Expression profiling of a hemopoietic cell survival transcriptome implicates osteopontin as a functional prognostic factor in AML. Blood. 2009;114:4859–4870. doi: 10.1182/blood-2009-02-204818. [DOI] [PubMed] [Google Scholar]
- 12.Liersch R, Gerss J, Schliemann C, Bayer M, Schwöppe C, Biermann C, Appelmann I, Kessler T, Löwenberg B, Büchner T, et al. Osteopontin is a prognostic factor for survival of acute myeloid leukemia patients. Blood. 2012;119:5215–5220. doi: 10.1182/blood-2011-11-389692. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Lee CY, Tien HF, Hou HA, Chou WC, Lin LI. Marrow osteopontin level as a prognostic factor in acute myeloid leukaemia. Br J Haematol. 2008;141:736–739. doi: 10.1111/j.1365-2141.2008.07082.x. [DOI] [PubMed] [Google Scholar]
- 15.E Y, He N, Wang Y, Fan H. Percutaneous transluminal angioplasty (PTA) alone versus PTA with balloon-expandable stent placement for short-segment femoropopliteal artery disease: A metaanalysis of randomized trials. J Vasc Interv Radiol. 2008;19:499–503. doi: 10.1016/j.jvir.2007.12.446. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Smith G Davey, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadia TM, Ravandi F, O'Brien S, Cortes J, Kantarjian HM. Progress in acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15:139–151. doi: 10.1016/j.clml.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Liang F, Zhang B, Yan W, Zhang J. The impact of osteopontin on prognosis and clinicopathology of colorectal cancer patients: A systematic meta-analysis. Sci Rep. 2015;5:12713. doi: 10.1038/srep12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Zou D, Li C, Meng H, Sui W, Feng S, Cheng T, Zhai Q, Qiu L. Targeting stem cell niche can protect hematopoietic stem cells from chemotherapy and G-CSF treatment. Stem Cell Res Ther. 2015;6:175. doi: 10.1186/s13287-015-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu ZD, Wei TT, Yang M, Ma N, Tang QQ, Qin BD, Fu HT, Zhong RQ. Diagnostic value of osteopontin in ovarian cancer: A meta-analysis and systematic review. PLoS One. 2015;10:e0126444. doi: 10.1371/journal.pone.0126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan HG, Xu H, Gu YM, Wang H, Xu W, Zu MH. Comparison osteopontin vs AFP for the diagnosis of HCC: A meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:706–714. doi: 10.1016/j.clinre.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Saeki Y, Mima T, Ishii T, Ogata A, Kobayashi H, Ohshima S, Ishida T, Tabunoki Y, Kitayama H, Mizuki M, et al. Enhanced production of osteopontin in multiple myeloma: Clinical and pathogenic implications. Br J Haematol. 2003;123:263–270. doi: 10.1046/j.1365-2141.2003.04589.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang HL, Ruan LH, Zhao XQ. Expression of osteopontin and VEGF in acute leukemia and their relationship with angiogenesis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:926–929. (In Chinese) [PubMed] [Google Scholar]
- 24.Colla S, Morandi F, Lazzaretti M, Rizzato R, Lunghi P, Bonomini S, Mancini C, Pedrazzoni M, Crugnola M, Rizzoli V, Giuliani N. Human myeloma cells express the bone regulating gene Runx2/Cbfa1 and produce osteopontin that is involved in angiogenesis in multiple myeloma patients. Leukemia. 2005;19:2166–2176. doi: 10.1038/sj.leu.2403976. [DOI] [PubMed] [Google Scholar]
- 25.Minarik J, Pika T, Bacovsky J, Petrova P, Langova K, Scudla V. Prognostic value of hepatocyte growth factor, syndecan-1, and osteopontin in multiple myeloma and monoclonal gammopathy of undetermined significance. ScientificWorldJournal. 2012;2012:356128. doi: 10.1100/2012/356128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sfiridaki A, Miyakis S, Pappa C, Tsirakis G, Alegakis A, Kotsis V, Stathopoulos E, Alexandrakis M. Circulating osteopontin: A dual marker of bone destruction and angiogenesis in patients with multiple myeloma. J Hematol Oncol. 2011;4:22. doi: 10.1186/1756-8722-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Z, Xiao B, Chen S, Li S, Liu Y, Liu J. Studies of WT1 gene expression in leukemia patients. Zhonghua Xue Ye Xue Za Zhi. 2002;23:367–369. [PubMed] [Google Scholar]
- 28.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]