Abstract
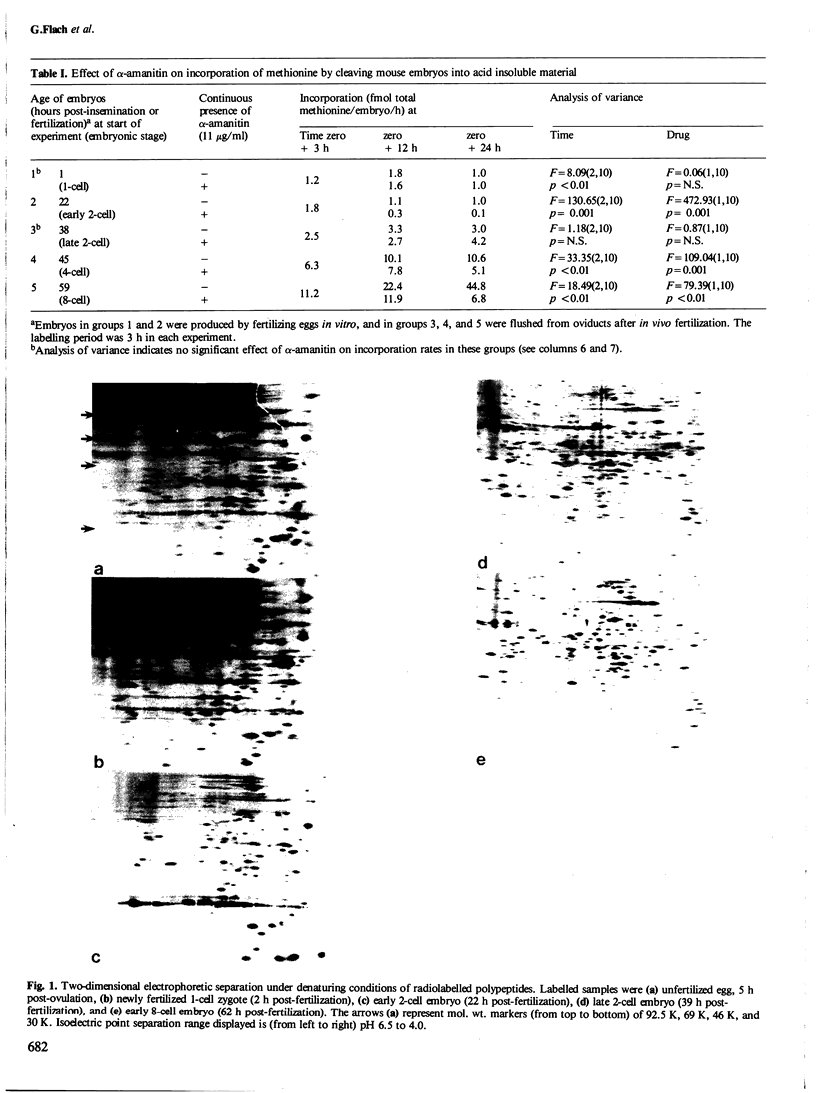
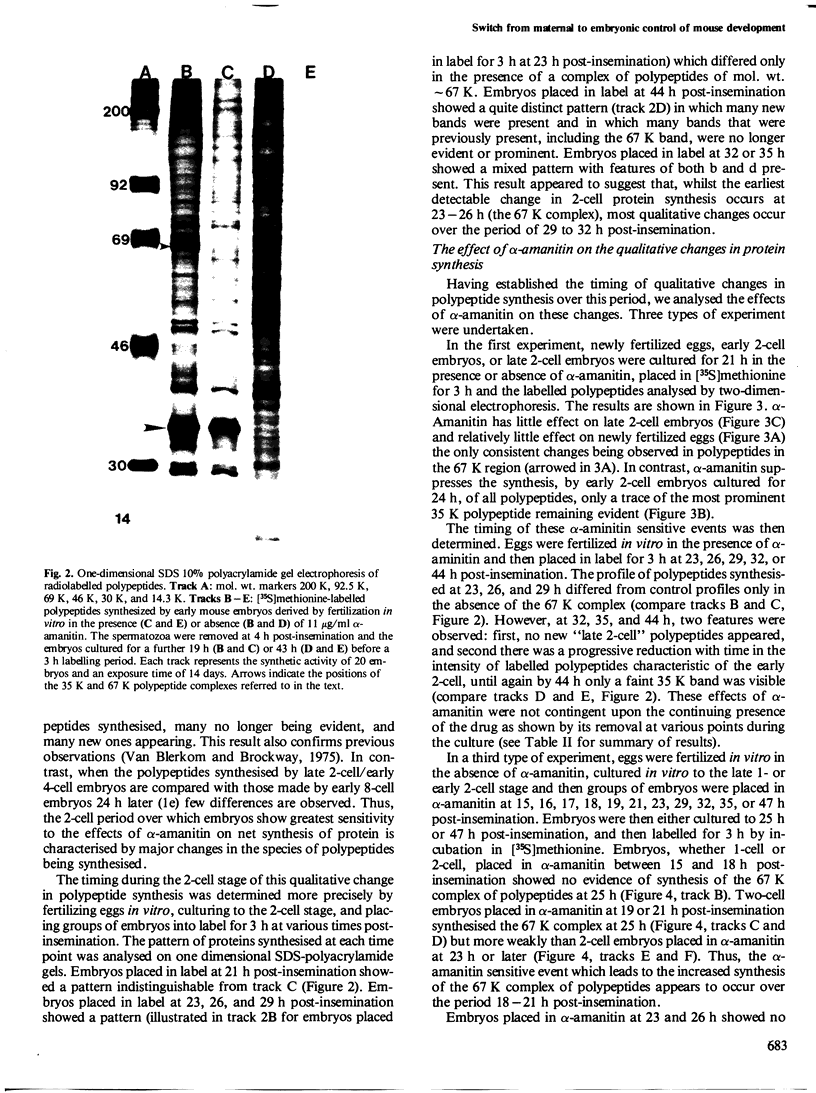
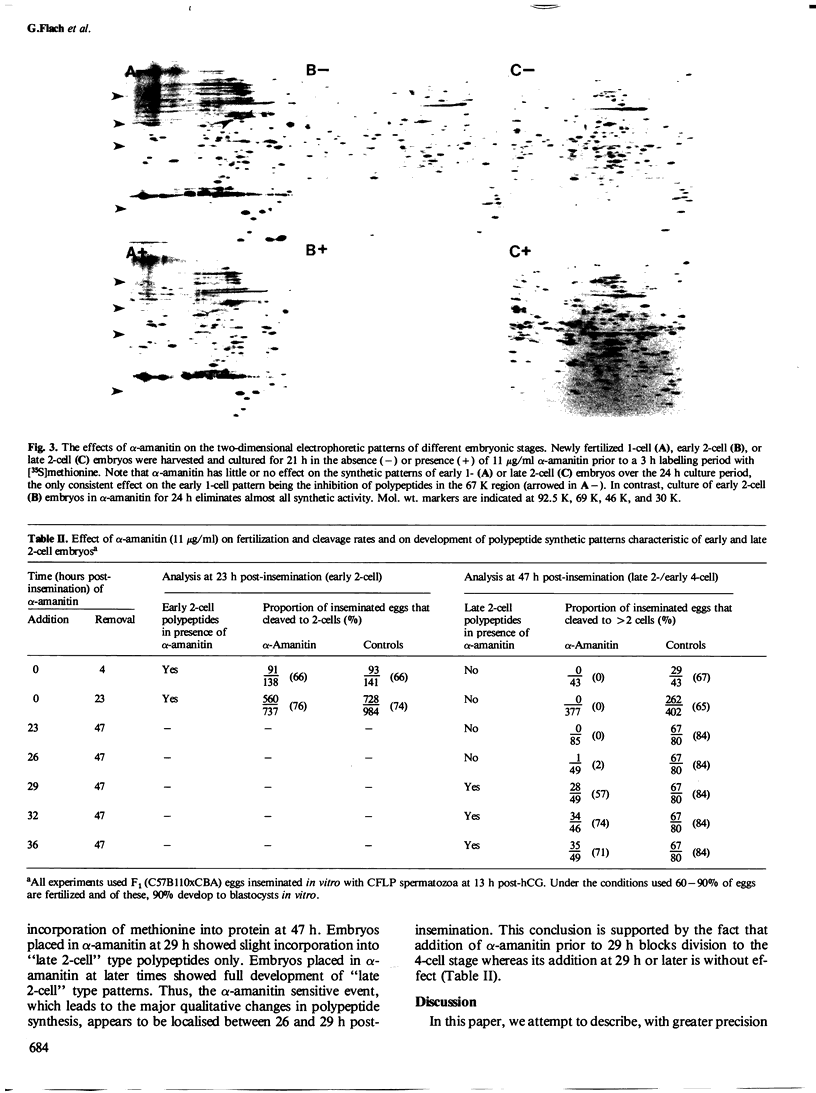
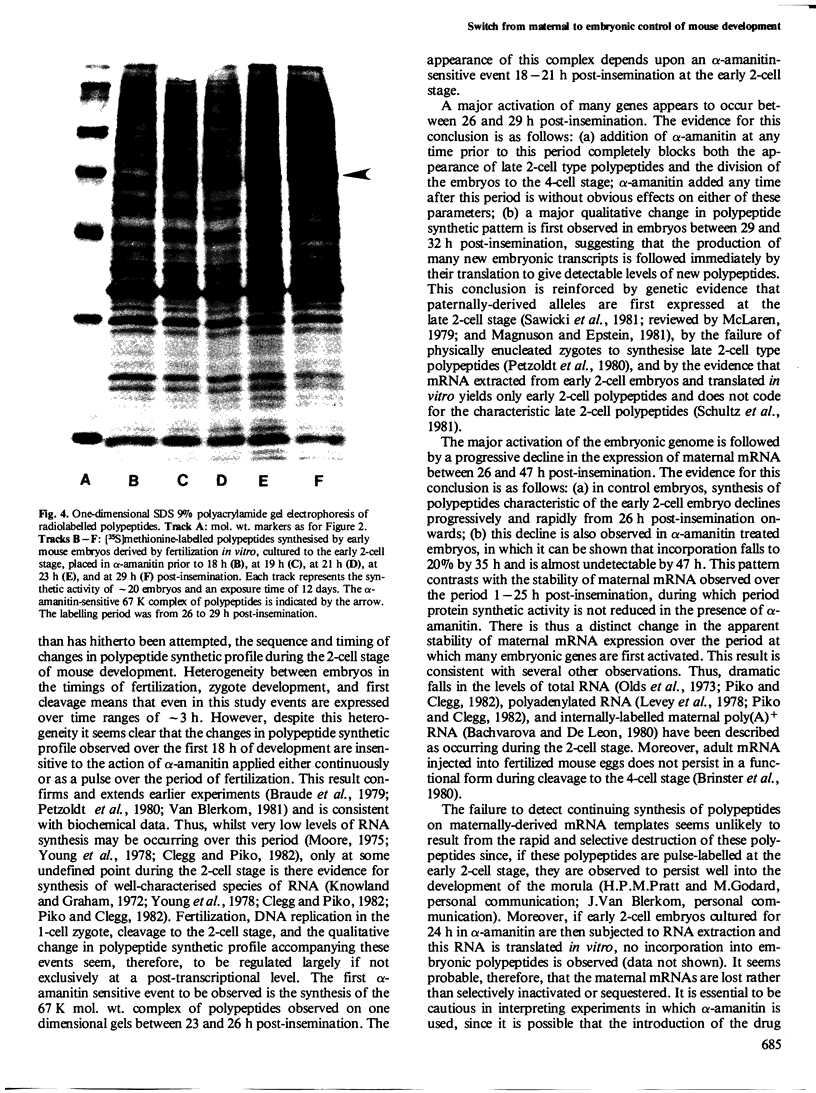
The development of the early 2-cell mouse embryo to the late 2-cell stage is marked by the appearance between 23 and 26 h post-insemination of a complex of polypeptides of mol. wt. approximately 67 K. Addition of alpha-amanitin between 18 and 21 h post-insemination prevents or reduces the subsequent appearance of these polypeptides. Addition of alpha-amanitin after 21 h does not obviously affect the appearance of the approximately 67 K polypeptides. A major change in synthetic profile occurs between 29 and 32 h post-insemination involving many polypeptides. Addition of alpha-amanitin to 2-cell embryos prior to 29 h post-insemination prevents the appearance of the new polypeptides observed during this major change but does not prevent the disappearance of the old polypeptides. In contrast, addition of alpha-amanitin after this time does not affect the appearance of the new polypeptides. This result, together with other evidence presented, suggests that during the 2-cell stage the embryonic genome shows transcriptional activity in two phases at 18-21 and 26-29 h post-insemination, that these transcripts are utilized soon after their synthesis, and that most maternal transcripts used before the second phase of embryonic transcription become ineffective soon afterwards.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRACHET J., FICQ A., TENCER R. AMINO ACID INCORPORATION INTO PROTEINS OF NUCLEATE AND ANUCLEATE FRAGMENTS OF SEA URCHIN EGGS: EFFECT OF PARTHENOGENETIC ACTIVATION. Exp Cell Res. 1963 Oct;32:168–170. doi: 10.1016/0014-4827(63)90081-8. [DOI] [PubMed] [Google Scholar]
- Bachvarova R., De Leon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev Biol. 1980 Jan;74(1):1–8. doi: 10.1016/0012-1606(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Ballantine J. E., Woodland H. R., Sturgess E. A. Changes in protein synthesis during the development of Xenopus laevis. J Embryol Exp Morphol. 1979 Jun;51:137–153. [PubMed] [Google Scholar]
- Braude P. R. Control of protein synthesis during blastocyst formation in the mouse. Dev Biol. 1979 Feb;68(2):440–452. doi: 10.1016/0012-1606(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Braude P., Pelham H., Flach G., Lobatto R. Post-transcriptional control in the early mouse embryo. Nature. 1979 Nov 1;282(5734):102–105. doi: 10.1038/282102a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Avarbock M. R. Translation of globin messenger RNA by the mouse ovum. Nature. 1980 Jan 31;283(5746):499–501. doi: 10.1038/283499a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio S. M., Wassarman P. M. Program of early development in the mammal: post-transcriptional control of a class of proteins synthesized by mouse oocytes and early embryos. Dev Biol. 1982 Feb;89(2):397–408. doi: 10.1016/0012-1606(82)90328-1. [DOI] [PubMed] [Google Scholar]
- Cullen B., Emigholz K., Monahan J. The transient appearance of specific proteins in one-cell mouse embryos. Dev Biol. 1980 Apr;76(1):215–221. doi: 10.1016/0012-1606(80)90373-5. [DOI] [PubMed] [Google Scholar]
- Denny P. C., Tyler A. Activation of protein biosynthesis in non-nucleate fragments of sea urchin eggs. Biochem Biophys Res Commun. 1964;14:245–249. doi: 10.1016/0006-291x(64)90443-7. [DOI] [PubMed] [Google Scholar]
- Fraser L. R., Drury L. M. The relationship between sperm concentration and fertilization in vitro of mouse eggs. Biol Reprod. 1975 Dec;13(5):513–518. doi: 10.1095/biolreprod13.5.513. [DOI] [PubMed] [Google Scholar]
- Holmberg S. R., Johnson M. H. Amino acid transport in the unfertilized and fertilized mouse egg. J Reprod Fertil. 1979 May;56(1):223–231. doi: 10.1530/jrf.0.0560223. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Kaumeyer J. F., Young E. M., Raff R. A. A test for masked message: the template activity of messenger ribonucleoprotein particles isolated from sea urchine eggs. Dev Biol. 1978 Apr;63(2):279–298. doi: 10.1016/0012-1606(78)90134-3. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Rossant J. Molecular studies on cells of the trophectodermal lineage of the postimplantation mouse embryo. J Embryol Exp Morphol. 1981 Feb;61:103–116. [PubMed] [Google Scholar]
- Johnson M. H. The molecular and cellular basis of preimplantation mouse development. Biol Rev Camb Philos Soc. 1981 Aug;56(3):463–498. doi: 10.1111/j.1469-185x.1981.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Knowland J., Graham C. RNA synthesis at the two-cell stage of mouse development. J Embryol Exp Morphol. 1972 Feb;27(1):167–176. [PubMed] [Google Scholar]
- Levey I. L., Brinster R. L. Effects of alpha-amanitin on RNA synthesis by mouse embryos in culture. J Exp Zool. 1978 Mar;203(3):351–360. doi: 10.1002/jez.1402030303. [DOI] [PubMed] [Google Scholar]
- Levey I. L., Stull G. B., Brinster R. L. Poly(A) and synthesis of polyadenylated RNA in the preimplantation mouse embryo. Dev Biol. 1978 May;64(1):140–148. doi: 10.1016/0012-1606(78)90066-0. [DOI] [PubMed] [Google Scholar]
- Luthardt F. W., Donahue R. P. DNA synthesis in developing two-cell mouse embryos. Dev Biol. 1975 May;44(1):210–216. doi: 10.1016/0012-1606(75)90389-9. [DOI] [PubMed] [Google Scholar]
- Magnuson T., Epstein C. J. Genetic control of very early mammalian development. Biol Rev Camb Philos Soc. 1981 Aug;56(3):369–408. doi: 10.1111/j.1469-185x.1981.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Moore G. P. The RNA polymerase activity of the preimplantation mouse embryo. J Embryol Exp Morphol. 1975 Oct;34(2):291–298. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olds P. J., Stern S., Biggers J. D. Chemical estimates of the RNA and DNA contents of the early mouse embryo. J Exp Zool. 1973 Oct;186(1):39–46. doi: 10.1002/jez.1401860107. [DOI] [PubMed] [Google Scholar]
- Pikó L., Clegg K. B. Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol. 1982 Feb;89(2):362–378. doi: 10.1016/0012-1606(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Pratt H. P., Chakraborty J., Surani M. A. Molecular and morphological differentiation of the mouse blastocyst after manipulations of compaction with cytochalasin D. Cell. 1981 Oct;26(2 Pt 2):279–292. doi: 10.1016/0092-8674(81)90310-x. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Sawicki W., Abramczuk J., Blaton O. DNA synthesis in the second and third cell cycles of mouse preimplantation development. A cytophotometric study. Exp Cell Res. 1978 Mar 1;112(1):199–205. doi: 10.1016/0014-4827(78)90540-2. [DOI] [PubMed] [Google Scholar]
- Schultz G. A., Clough J. R., Johnson M. H. Presence of cap structures in the messenger RNA of mouse eggs. J Embryol Exp Morphol. 1980 Apr;56:139–156. [PubMed] [Google Scholar]
- Van Blerkom J. Structural relationship and posttranslational modification of stage-specific proteins synthesized during early preimplantation development in the mouse. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7629–7633. doi: 10.1073/pnas.78.12.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Showman R. M., Klein W. H., Raff R. A. Delayed recruitment of maternal histone H3 mRNA in sea urchin embryos. Nature. 1981 Jul 30;292(5822):477–478. doi: 10.1038/292477a0. [DOI] [PubMed] [Google Scholar]
- Whittingham D. G. Culture of mouse ova. J Reprod Fertil Suppl. 1971 Jun;14:7–21. [PubMed] [Google Scholar]
- Woodland H. R., Ballantine J. E. Paternal gene expression in developing hybrid embryos of Xenopus laevis and Xenopus borealis. J Embryol Exp Morphol. 1980 Dec;60:359–372. [PubMed] [Google Scholar]
- Woodland H. R., Flynn J. M., Wyllie A. J. Utilization of stored mRNA in Xenopus embryos and its replacement by newly synthesized transcripts: histone H1 synthesis using interspecies hybrids. Cell. 1979 Sep;18(1):165–171. doi: 10.1016/0092-8674(79)90365-9. [DOI] [PubMed] [Google Scholar]
- Young R. J., Sweeney K., Bedford J. M. Uridine and guanosine incorporation by the mouse one-cell embryo. J Embryol Exp Morphol. 1978 Apr;44:133–148. [PubMed] [Google Scholar]
- van Blerkom J., Brockway G. O. Qualitative patterns of protein synthesis in the preimplantation mouse embryo. I. Normal pregnancy. Dev Biol. 1975 May;44(1):148–157. doi: 10.1016/0012-1606(75)90382-6. [DOI] [PubMed] [Google Scholar]