Abstract
Crizotinib is one of the molecularly-targeted agents targeted against anaplastic lymphoma kinase (ALK)-rearranged non-small-cell lung cancer (NSCLC). Although its effects appear to be promising, crizotinib may cause adverse effects in patients with ALK-rearranged NSCLC. Hepatic laboratory abnormalities are frequently observed with crizotinib and treatment discontinuation is occasionally required. We herein report the case of a 51-year-old woman diagnosed with relapsed ALK-rearranged NSCLC, who received crizotinib as second-line systemic chemotherapy. After 17 days of crizotinib therapy, the patient developed grade >3 hepatotoxicity. Treatment discontinuation improved the laboratory abnormalities and fifth-line oral desensitization with crizotinib achieved successful response without hepatotoxicity. Therefore, oral desensitization with crizotinib may be a viable option following crizotinib-induced hepatitis.
Keywords: desensitization, anaplastic lymphoma kinase, crizotinib, non-small-cell lung cancer, hepatitis
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. Anaplastic lymphoma kinase (ALK) rearrangement mutation was observed in 3–5% of lung adenocarcinomas from patients in East Asia (1). Crizotinib, which is the first agent against lung cancer with ALK rearrangement mutation, has been found to be superior to standard first-line cytotoxic chemotherapy in the first-line setting (2). Crizotinib is occasionally associated with drug-induced hepatitis. In the present study, a case of successful oral desensitization with crizotinib following development of crizotinib_induced hepatitis is presented.
Case report
A 51-year-old Japanese woman with no history of smoking received crizotinib (250 mg twice daily) as second-line treatment for relapse of resected lung adenocarcinoma following progression while on first-line chemotherapy with carboplatin/pemetrexed. On day 2, crizotinib was discontinued due to abdominal pain, nausea and grade 3 diarrhea. The symptoms improved in 3 days and crizotinib was re-introduced at 250 mg once daily on day 7, with dexamethasone 3 mg daily for nausea. Although there was no abdominal pain or diarrhea, the patient developed fever and nausea on day 15. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels increased and crizotinib was again discontinued on day 16. The peak of AST was 437 IU/l on day 18 and that of ALT 1,096 IU/l on day 19 (Fig. 1). The level of alkaline phosphatase did not increase, whereas γ-glutamyltransferase and total bilirubin increased to grade 2 toxicity levels. Hepatocellular injury was suspected and contrast-enhanced computed tomography and ultrasonography did not reveal other causes, whereas hepatitis A, B or C was excluded. The patient's serum tested negative for polymerase chain reaction of Epstein-Barr virus and cytomegalovirus. The symptoms and blood test abnormalities completely resolved without increase of steroids after 1 month.
Figure 1.
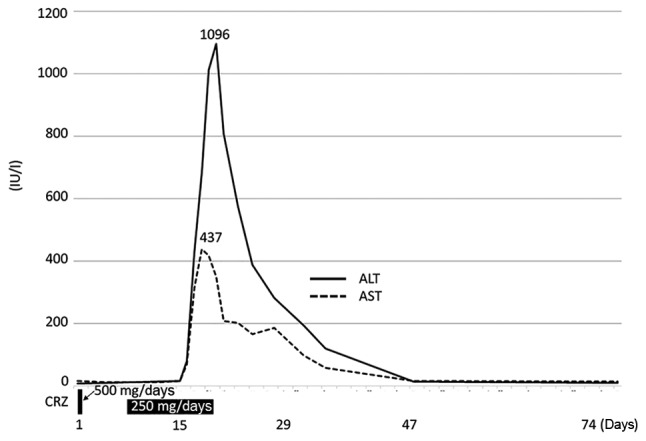
Clinical course of the patient treated with crizotinib (CRZ) as second-line therapy. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
After carboplatin/pemetrexed rechallenge as third-line treatment and docetaxel as fourth-line treatment, the disease progressed and the patient was started on crizotinib at 50 mg daily, as there was no alternative treatment for lung cancer. The contents of crizotinib capsules were dissolved in sterile water and diluted to the desired concentrations. The patient was also administered 2 mg of betamethasone for nausea, as she developed brain metastasis and was treated with whole-brain radiotherapy. Crizotinib was gradually increased and grade 1 hepatotoxicity developed on day 12. Ursodeoxycholic acid and glycyrrhizic acid were started thereafter, and no hepatotoxicity was observed (Fig. 2). All the metastatic lymph nodes shrank and the patient achieved partial response (Fig. 3). There was disease progression after 5 months of treatment, and crizotinib was continued beyond progression. However, the treatment beyond progression did not improve her condition and the patient succumbed to respiratory failure with carcinomatous pleurisy.
Figure 2.
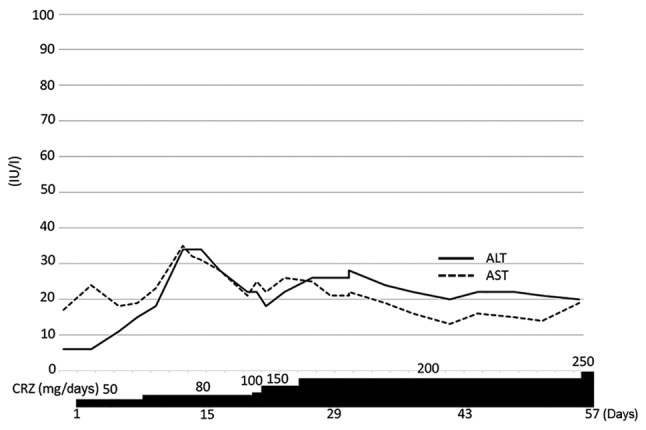
Clinical course of the patient undergoing crizotinib (CRZ) desensitization as fifth-line therapy. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
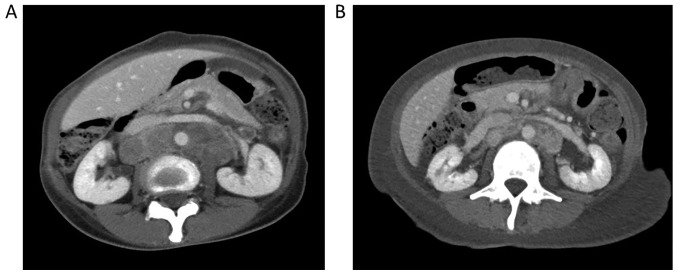
Figure 3.
(A) Computed tomography (CT) scan prior to desensitization with crizotinib treatment and (B) CT scan 1 month after starting crizotinib treatment, showing partial response of the tumor.
Discussion
The detection of ALK rearrangement in non-small-cell lung cancer (NSCLC) prompted the development of ALK inhibitors. Crizotinib is the first inhibitor of ALK, ROS1 and MET. Although there are several ALK inhibitors, one of which, alectinib, appears to be more efficacious and less toxic compared with crizotinib (3), crizotinib is not only an ALK inhibitor, but also acts against ROS1 (4). However, is important to establish a method of re-administration following the development of crizotinib-induced adverse effects.
One of the most common adverse effects of crizotinib is hepatotoxicity. Grade 3–4 ALT elevations occur in ~15% of NSCLC patients with ALK rearrangement mutations (2,5). Three cases of crizotinib-induced hepatitis have been reported, all of which occurred within a few months after the introduction of crizotinib (6–8). In one of those cases, rechallenge with half-dose crizotinib failed due to recurrence of hepatitis (6). Similarly, in the present case, dose-independent hepatotoxicity suggested that the underlying mechanism was partly an allergic reaction to crizotinib or its metabolites; however, the precise mechanism underlying crizotinib-induced liver toxicity has not been fully elucidated. Although successful desensitization with crizotinib following crizotinib-induced urticarial rash was reported (9), there was no report of desensitization to ALK inhibitors following drug-induced liver injury. In the present case, oral steroids were used together during oral desensitization, which may have contributed to the treatment success. However, common dose reduction with oral steroids following crizotinib-induced hepatitis did not improve the hepatitis; therefore, oral desensitization may be considered as a viable option following crizotinib-induced hepatitis. Drug-induced liver injury is generally associated with type 4 hypersensitivity reactions; the fact that crizotinib desensitization overcame hepatitis suggested the involvement of type 1 hypersensitivity.
Ceritinib and alectinib are also approved ALK inhibitors; however, since they were not approved at that time, crizotinib desensitization was attempted instead. A previous case report reported successful treatment with ceritinib following crizotinib-induced hepatitis (8). Therefore, these novel ALK inhibitors may be additional options for the management of crizotinib-induced hepatitis.
While the progression-free survival (PFS) of crizotinib as first-line treatment is ~11 months, its PFS as second-line treatment is ~8 months (2,5). The PFS in the present case was shorter compared with these previously reported results. The later crizotinib is administered, or the lower its dose, the shorter the PFS. The efficacy of crizotinib is optimal at a dose of 250 mg twice daily. A recent study on crizotinib pharmacokinetics revealed that crizotinib at a starting dose appeared to be effective and tolerable, regardless of race, age, sex, hepatic function, or mild to moderate renal impairment (10). Since the hepatitis in the present case was not dose-dependent, the crizotinib dose was increased to the greater extent possible.
In conclusion, our case suggests that oral desensitization with crizotinib may be a viable alternative option in cases of crizotinib-induced hepatitis.
References
- 1.Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, Goto K. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–164. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 3.Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, de Pas T, Besse B, Solomon BJ, Blackhall F, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Ripault MP, Pinzani V, Fayolle V, Pageaux GP, Larrey D. Crizotinib-induced acute hepatitis: First case with relapse after reintroduction with reduced dose. Clin Res Hepatol Gastroenterol. 2013;37:e21–e23. doi: 10.1016/j.clinre.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Fujimoto D, Shibata Y, Seo R, Suginoshita Y, Imai Y, Tomii K. Fulminant hepatitis following crizotinib administration for ALK-positive non-small-cell lung carcinoma. Jpn J Clin Oncol. 2014;44:872–875. doi: 10.1093/jjco/hyu086. [DOI] [PubMed] [Google Scholar]
- 8.Sassier M, Mennecier B, Gschwend A, Rein M, Coquerel A, Humbert X, Alexandre J, Fedrizzi S, Gervais R. Successful treatment with ceritinib after crizotinib induced hepatitis. Lung Cancer. 2016;95:15–16. doi: 10.1016/j.lungcan.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Awad MM, Lax TP, Slawski BR, Shaw AT. Successful desensitization of two patients with ALK-positive lung cancer and hypersensitivity to crizotinib. J Thorac Oncol. 2014;9:1726–1728. doi: 10.1097/JTO.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 10.Wang E, Nickens DJ, Bello A, Khosravan R, Amantea M, Wilner KD, Parivar K, Tan W. Clinical implications of the pharmacokinetics of crizotinib in populations of patients with non-small cell lung cancer. Clin Cancer Res. 2016;22:5722–5728. doi: 10.1158/1078-0432.CCR-16-0536. [DOI] [PubMed] [Google Scholar]