Abstract
Esophageal cancer is the eighth most common malignant tumor worldwide, and the number of incidences of esophageal adenocarcinoma is increasing in the Western world. Despite improvements in perioperative treatment, the overall survival rate of patients with esophageal adenocarcinoma remains poor. Breast cancer type 1 susceptibility protein (BRCA1)-associated protein (BAP1) is located on chromosome 3p21, and it is an enzyme with ubiquitin carboxyl hydrolase activity that regulates cell growth. It interacts with BRCA1, and the nuclear localization of BAP1 is required for its tumor suppressor function. BAP1 is frequently mutated in uveal melanomas, malignant mesothelioma and several carcinomas, including a subtype of renal cell carcinoma, intrahepatic cholangiocarcinoma and squamous cell carcinoma of the esophagus. Furthermore, several germline-associated mutations of tumors have been described (BAP1 hereditary cancer syndrome). However, the importance and frequency of BAP1 alterations in adenocarcinoma of the esophagus remain to be elucidated. In the present study, tissue microarrays of 332 resected adenocarcinomas (including a few cases of concomitant Barrett dysplasia) of the esophagus were constructed. The tumor tissue was analyzed using immunohistochemistry to investigate the levels of BAP1 expression. Fibroblasts or inflammatory cells served as an internal positive control. Three adenocarcinomas revealed nuclear loss of BAP1 (0.9%). One case with concomitant Barrett dysplasia also exhibited a loss of BAP1. Of the resected adenocarcinomas, 329 of them exhibited an intact and uniform strong nuclear staining pattern. To the best of our knowledge, this is the first description of BAP1 deficiency in adenocarcinomas of the esophagus. Furthermore, it has been demonstrated that BAP1 loss is possibly an early event in esophageal adenocarcinoma. These results warrant further functional and clinical evaluation.
Keywords: esophageal adenocarcinoma, breast cancer type 1 susceptibility protein, BRCA1-associated protein, tumor suppressor, mutation, immunohistochemistry
Introduction
Esophageal cancer is the eighth most common malignant tumor worldwide, and the number of incidences of esophageal adenocarcinoma is increasing in the Western world (see http://www.wcrf.org). The majority of adenocarcinomas arise from Barrett metaplasia due to chronic reflux disease, followed subsequently by an accumulation of different mutations causing genetic instability (Barrett multistep carcinogenesis) (1,2). Frequently patients present with a locally advanced tumor stage. Despite improvements in perioperative treatments, the overall survival rates of patients with esophageal adenocarcinoma remain poor.
Breast cancer type 1 susceptibility protein (BRCA1)-associated protein (BAP1) is located on chromosome 3p21, and is an enzyme with ubiquitin carboxyl hydrolase activity that is involved in regulation of cell cycle and transcription, as well as in double-stranded DNA repair (3–5). It binds BRCA1 and acts as a tumor suppressor by forming a complex with BRCA1 (6). Missense or truncating mutations lead to loss of nuclear localization and deubiquitinating activity, which are essential for BAP1 tumor suppressor function. BAP1 analysis using immunohistochemistry (IHC) offers a cost-effective, fast and reliable method for the evaluation of BAP1 status, as a loss of nuclear expression correlates very well with biallelic inactivation of BAP1 (7–9). BAP1 frequently exhibits inactivating mutations in uveal melanoma with high metastatic risk, malignant mesothelioma and other carcinoma types, including a subtype of renal cell carcinoma and intrahepatic cholangiocarcinoma (4,10–15). In squamous cell carcinoma of the esophagus, BAP1 nuclear expression was shown to be reduced in 44% of cases (16). Germline mutations in BAP1 have been demonstrated to cause a tumor predisposition syndrome termed BAP1 hereditary cancer syndrome (17), a syndrome that predisposes to the development of uveal melanoma, cutaneous melanoma, renal cell carcinoma, and malignant mesothelioma (18–21).
The importance and frequency of BAP1 loss in adenocarcinoma of the esophagus have yet to be elucidated. The aim of the present study was to investigate the loss of BAP1 in adenocarcinomas of the esophagus, and it is demonstrated that BAP1 loss is possibly an early event in esophageal adenocarcinoma, a result that warrants further functional and clinical evaluation.
Patients and methods
Patients and tumor samples
In this retrospective study, 332 esophageal adenocarcinomas, including several cases with concomitant Barrett dysplasia, that underwent primary surgical resection or resection following neoadjuvant therapy were analyzed. The patient characteristics are presented in Table I. For tissue microarrays (TMAs), two tissue cores from different areas of each tumor were punched out and transferred to a TMA recipient block. TMA construction was performed as previously described (22,23). In brief, tissue cylinders, each with a diameter of 1.2 mm, were punched from selected tumor tissue blocks using a self-constructed semi-automated precision instrument and embedded in empty recipient paraffin blocks. Sections (4 µm-thick) of the resulting TMA blocks were transferred to an adhesive coated slide system (Instrumedics, Inc., Hackensack, NJ, USA) for IHC analysis.
Table I.
Clinicopathological features of the 322 arrayed esophageal adenocarcinomas.
| Adenocarcinoma | ||
|---|---|---|
| Parameter | Number | Percentage |
| Total | 322 | |
| Sex | ||
| Female | 32 | 9.9 |
| Male | 290 | 90.1 |
| Average age (years) | 62 | |
| Underwent primary surgery | 126 | 39.1 |
| pT stage | ||
| pT1 | 51 | 40.5 |
| pT2 | 18 | 14.3 |
| pT3 | 56 | 44.4 |
| pT4 | 11 | 0.8 |
| Neoadjuvant treated tumors | 196 | 60.9 |
pT, primary tumor.
IHC analysis
IHC was performed on TMA slides using the primary mouse anti-BAP1 monoclonal antibody (cat. no. SC-28383; dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). IHC stainings were performed using the Ventana BenchMark stainer (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol and an additional amplification kit (OptiView; Ventana, Roche Diagnostics GmbH, Mannheim, Germany) to increase the staining intensity of the antibody. BAP1 staining was evaluated by two pathologists independently (A.Q. and H.L.).
Procedures were followed as outlined in accordance with ethical standards formulated in the Helsinki Declaration 1975 (and revised in 1983), with pre-approval granted by the Ethics Committee at the University Hospital, University of Cologne, Cologne (ref. no.: 09-232).
Results
IHC analysis
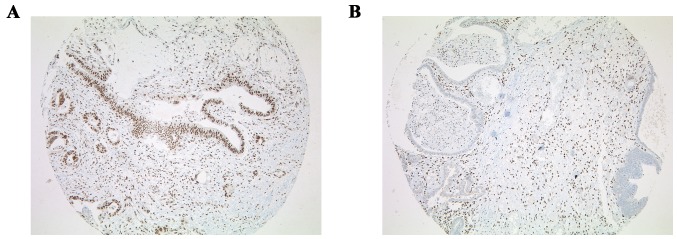
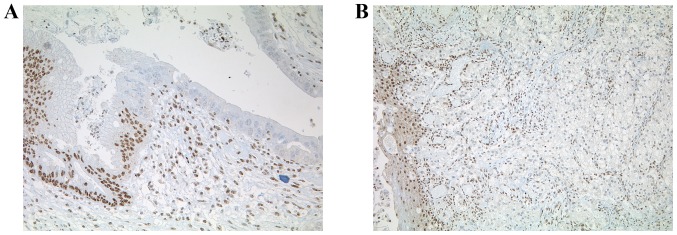
BAP1 IHC on TMA slides, which included all of the 332 esophageal adenocarcinomas, was performed. Fibroblasts or inflammatory cells served as an internal positive control. Tumors were considered to be BAP1 negative only if all the neoplastic cells exhibited a complete nuclear loss of expression, while the internal control was positive. Three out of the 332 adenocarcinomas demonstrated nuclear loss of BAP1, amongst which was one case with a concomitant Barrett dysplasia that also exhibited loss of BAP1 in the dysplasia (0.9%) (Fig. 1). In these cases, IHC was repeated on whole sections with an identical negative staining pattern. A total of 329 out of 332 adenocarcinomas revealed an intense and uniform nuclear staining pattern (Fig. 2).
Figure 1.
Staining for BAP1 on the TMAs of 332 esophageal adenocarcinomas. (A) Nuclear staining for BAP1 (original magnification, ×50). (B) Loss of nuclear staining for BAP1 with positive internal control of fibroblasts (original magnification, ×50). TMA, tissue microarray; BAP1, BRCA1-associated protein.
Figure 2.
Whole sections of BAP1 immunohistochemistry. (A) Loss of BAP1 nuclear staining for high-grade Barrett dysplasia (original magnification, ×200). (B) Esophageal adenocarcinoma with loss of nuclear staining for BAP1 (original magnification, ×100). BAP1, BRCA1-associated protein.
Discussion
Inactivating mutations of BAP1 are associated with various malignancies, including squamous cell carcinomas of the esophagus (16). In the majority of cancer types, BAP1 mutations are associated with advanced tumor stages and poor prognosis (24). Malignant mesothelioma harboring BAP1 mutations are associated with a markedly improved prognosis compared with wild-type BAP1 mesotheliomas. BAP1 mutations in malignant mesothelioma appear to be an early event in carcinogenesis, which may impair the carcinogenic damage of smoking, thereby exerting a strong genetic-environmental effect (11,24).
To the best of our knowledge, the present study provides the first description of BAP1 function in adenocarcinomas of the esophagus. In contrast with squamous cell carcinomas of the esophagus, where 44% of cases showed a reduced expression of BAP1 (16), it was possible to demonstrate that BAP1 loss is a rare event, since three cases with a loss of BAP1 expression were identified out of a total of 332 esophageal adenocarcinomas. Furthermore, it was possible to show that BAP1 loss is potentially an early event in esophageal adenocarcinoma, since the loss of BAP1 in one adenocarcinoma and its precursor lesion of Barrett high-grade dysplasia was detected. On the other hand, the loss of a functional alteration of BAP1 in intraepithelial preinvasive neoplasia may lend support to an argument for the importance of BAP1 loss in this particular tumor specimen, supporting an environmental influence on carcinogenesis. The findings of the present retrospective analysis underline the results of sequencing analyses [e.g., The Cancer Genome Atlas (TCGA), provisional for adenocarcinoma of the esophagus], which revealed deletion vs. mutation of BAP1 in 1.6 vs. 2.1% of cases (see http://www.cbioportal.org).
In conclusion, the present study has demonstrated that mutations of BAP1 are a rare and early event in esophageal adenocarcinomas.
References
- 1.Fassan M, Cagol M, Pennelli G, Rizzetto C, Giacomelli L, Battaglia G, Zaninotto G, Ancona E, Ruol A, Rugge M. Programmed cell death 4 protein in esophageal cancer. Oncol Rep. 2010;24:135–139. doi: 10.3892/or_00000838. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JC. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ Health Perspect. 1993;100:9–20. doi: 10.1289/ehp.931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y, Fu D, Shen XZ. The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 2010;1806:1–6. doi: 10.1016/j.bbcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, Creaney J, Lake RA, Zakowski MF, Reva B, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, Liu J, O'Rourke K, Dixit VM, Wilson AC. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koopmans AE, Verdijk RM, Brouwer RW, van den Bosch TP, van den Berg MM, Vaarwater J, Kockx CE, Paridaens D, Naus NC, Nellist M, et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. 2014;27:1321–1330. doi: 10.1038/modpathol.2014.43. [DOI] [PubMed] [Google Scholar]
- 8.Arzt L, Quehenberger F, Halbwedl I, Mairinger T, Popper HH. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res. 2014;20:145–151. doi: 10.1007/s12253-013-9677-2. [DOI] [PubMed] [Google Scholar]
- 9.Farzin M, Toon CW, Clarkson A, Sioson L, Watson N, Andrici J, Gill AJ. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47:302–307. doi: 10.1097/PAT.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 10.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zauderer MG, Bott M, McMillan R, Sima CS, Rusch V, Krug LM, Ladanyi M. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol. 2013;8:1430–1433. doi: 10.1097/JTO.0b013e31829e7ef9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng0912-1072b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur P, Peña-Llopis S, Christie A, Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie XJ, Brugarolas J. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alakus H, Yost SE, Woo B, French R, Lin GY, Jepsen K, Frazer KA, Lowy AM, Harismendy O. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J Transl Med. 2015;13:122. doi: 10.1186/s12967-015-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori T, Sumii M, Fujishima F, Ueno K, Emi M, Nagasaki M, Ishioka C, Chiba N. Somatic alteration and depleted nuclear expression of BAP1 in human esophageal squamous cell carcinoma. Cancer Sci. 2015;106:1118–1129. doi: 10.1111/cas.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebe S, Driml J, Nasu M, Pastorino S, Zangiabadi A, Henderson D, Carbone M. BAP1 hereditary cancer predisposition syndrome: A case report and review of literature. Biomark Res. 2015;3:14. doi: 10.1186/s40364-015-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d'Enghien C, Richaudeau B, Renaudin X, Sellers J, Nicolas A, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92:974–980. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilarski R, Cebulla CM, Massengill JB, Rai K, Rich T, Strong L, McGillivray B, Asrat MJ, Davidorf FH, Abdel-Rahman MH. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer. 2014;53:177–182. doi: 10.1002/gcc.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon R, Mirlacher M, Sauter G. Tissue microarrays. Methods Mol Med. 2005;114:257–268. doi: 10.1385/1-59259-923-0:257. [DOI] [PubMed] [Google Scholar]
- 23.Helbig D, Ihle MA, Putz K, Pütz K, Tantcheva-Poor I, Mauch C, Büttner R, Quaas A. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2016;7:21763–21774. doi: 10.18632/oncotarget.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luchini C, Veronese N, Yachida S, Cheng L, Nottegar A, Stubbs B, Solmi M, Capelli P, Pea A, Barbareschi M, et al. Different prognostic roles of tumor suppressor gene BAP1 in cancer: A systematic review with meta-analysis. Genes Chromosomes Cancer. 2016;55:741–749. doi: 10.1002/gcc.22381. [DOI] [PubMed] [Google Scholar]