Abstract
The formation of atmospheric cloud droplets due to secondary organic aerosol (SOA) particles is important for quantifying the Earth’s radiative balance under future, possibly warmer, climates, yet is only poorly understood. While cloud activation may be parametrized using the surface tension depression that coincides with surfactant partitioning to the gas–droplet interface, the extent to which cloud activation is influenced by both the chemical structure and reactivity of the individual molecules comprising this surfactant pool is largely unknown. We report herein considerable differences in the surface tension depression of aqueous pendant droplets that contain synthetically prepared ozonolysis products derived from α-pinene and β-caryophyllene, the most abundant of the monoterpenes and sesquiterpenes, respectively, that are emitted over the planet’s vast forest ecosystems. Oxidation products derived from β-caryophyllene were found to exhibit significantly higher surface activity than those prepared from α-pinene, with the critical supersaturation required for cloud droplet activation reduced by 50% for β-caryophyllene aldehyde at 1 mM. These considerable reductions in the critical supersaturation were found to coincide with free energies of adsorption that exceed ∼25 kJ/mol, or just one hydrogen bond equivalent, depending on the ammonium sulfate and oxidation product concentration in the solution. Additional experiments showed that aldehyde-containing oxidation products exist in equilibrium with hydrated forms in aqueous solution, which may modulate their bulk solubility and surface activity. Equilibration time scales on the order of 10–5 to 10–4 s calculated for micrometer-sized aerosol particles indicate instantaneous surface tension depression in the activation processes leading to cloud formation in the atmosphere. Our findings highlight the underlying importance of molecular structure and reactivity when considering cloud condensation activity in the presence of SOA particles.
Short abstract
Surface tension depression caused by atmospheric ozonolysis products derived from α-pinene and β-caryophyllene highlight the importance of molecular structure and reactivity when considering cloud activation potential of secondary organic aerosol.
Introduction
Despite the abundance of biogenic terpene-derived secondary organic aerosol (SOA) particles in the lower troposphere1 and their potential cooling effects for vast forested regions of the globe,2−6 the mechanisms that lead to their growth and that drive climate-relevant cloud interactions of SOA material4,7−10 remain elusive. A current area of intense research regarding the properties of SOA particles focuses on the role of surface-active organic compounds. Indeed, we have proposed that because the SOA gas–particle interface is first to encounter gas–phase species, surface-localized molecules may significantly influence SOA particle growth as well as their propensity to serve as cloud condensation nuclei (CCN).11 While many studies report the presence of numerous different organic compounds within SOA particles,4,12,13 only a subset of these compounds are expected to be sufficiently surface active to reside at the aqueous particle surface. This subset, which may be composed of surface-active species having amphiphilic or surfactant-like behavior14−16 and may thus be termed an “organic surfactant pool”, has been hypothesized to influence surface processes that ultimately lead to cloud activation by lowering the surface tension of cloud-forming droplets containing aerosol nuclei.
Overall changes in the surface tension caused by surface-active molecules may alter the critical supersaturation required for cloud droplet activation, thereby influencing the propensity of SOA particles to nucleate cloud droplets.14,17−21 A recent publication by Ruehl and co-workers suggests that the bulk–surface partitioning of surface-active organic compounds can result in a concentrated organic film near the gas–particle interface. This organic film can significantly reduce the surface tension and result in a larger droplet diameter before activation.22 While current models have taken into account the contribution of organic species to cloud activity through the bulk solubility effect,23 surface tension depression effects have largely been neglected, in part because of a lack of reliable direct dynamic surface tension measurements of relevant individual surface-active organic compounds.11,14,22,24
Within this context, we decided to investigate the ozonolysis oxidation products derived from α-pinene and β-caryophyllene, which we hypothesized might exhibit higher surface activity than previously investigated isoprene derivatives11 due to their more surfactant-like structures. α-Pinene is the most abundant monoterpene (i.e., C10) (∼50 Tg C y–1)25,26 in the troposphere and is primarily emitted over the boreal forest ecosystems of the northern hemisphere—the largest terrestrial biome on Earth.27,28 Sesquiterpenes (i.e., C15), of which β-caryophyllene is the most abundant29−45 (ca. 5–7 Tg y–1),46,47 are believed to be present in lower quantities in the atmosphere (∼15 Tg C y–1 for sesquiterpene emissions).34,47,26,48 Yet, sesquiterpenes exhibit higher reactivity toward ozone when compared to monoterpenes,31,34,44,49−51 which may lead to an underestimation of emissions detected for these precursors and therefore their resulting SOA material. Sesquiterpenes also have been shown to afford higher comparative aerosol yields than isoprene and abundant monoterpenes, and consequently may contribute significantly to SOA particle formation.29,42,52,53
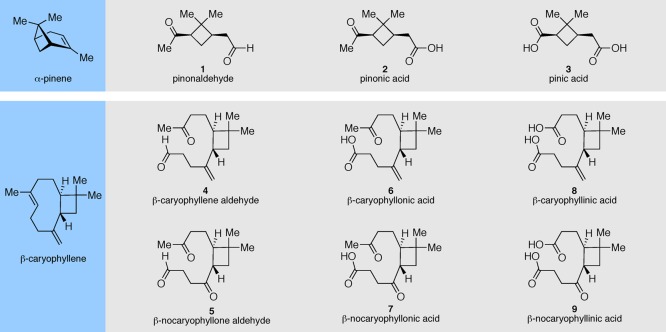
Motivated by field and laboratory studies reporting various proposed oxidation products derived from α-pinene and β-caryophyllene,12,44,50,54,55 and given the potentially significant role that these organic species might play in various atmospheric processes, including SOA formation,10,13,56−58 we study here a series of structurally related monomeric ozonolysis products derived from β-caryophyllene and α-pinene (Figure 1). The specific compounds synthesized have been proposed as constituents relevant to SOA material in field and laboratory aerosol studies29−45,59−61 and may therefore serve as homogeneous standards to further corroborate those studies. Moreover, we present dynamic surface tension measurements of this series of compounds and provide estimations of their individual supersaturation ratios, which allow for prediction of CCN activity.
Figure 1.
Atmospheric oxidation products derived from α-pinene and β-caryophyllene synthesized and measured in this study.
Results and Discussion
Synthesis of α-Pinene and β-Caryophyllene Oxidation Products
Experimental procedures for the preparation of all synthesized compounds studied are described in the Supporting Information. β-Caryophyllene aldehyde (4) and β-nocaryophyllone aldehyde (5) were synthesized in an similar fashion to procedures reported by Parshintsev and co-workers.33 Additionally, β-caryophyllonic acid (6) and β-nocaryophyllonic acid (7) were prepared analogously to procedures reported by van Eijck and co-workers.36 Attempts to synthesize β-caryophyllinic acid (8) according to procedures published by the same group were unsuccessful, however. We developed an alternative approach for preparing compounds 8 and 9 that is described in the Supporting Information. cis-Pinonic acid (98%) was purchased from Sigma-Aldrich and used as received. With the exception of β-caryophyllinic acid (8) and β-nocaryophyllinic acid (9), all synthesized compounds required iterative purification using silica gel chromatagraphy until determined to be ≥95% pure by the 1H NMR spectroscopy prior to performing surface tension measurements. Compound-specific purification procedures are included in the Supporting Information. Pinonaldehyde (1), pinonic acid (2), and pinic acid (3) are present as a low viscosity oil, powdery solid, and highly viscous oil, respectively. All synthetic β-caryophyllene oxidation products were isolated as highly viscous oils, with the exception of β-nocaryophyllinic acid, which was isolated as a powdery solid (Figure S1).
In aqueous environments, aldehydes may readily react with water and thus exist in equilibrium with the hydrate or geminal diol form (Figure 2). In order to monitor the potential formation of the hydrate in aqueous solutions of pinonaldehyde (1), β-caryophyllene aldehyde (4), and β-nocaryophyllone aldehyde (5), solutions (2 mg mL–1) of each compound in D2O were prepared and monitored by 1H and 13C NMR spectroscopy for 72 h. The presence of the hydrate in D2O was observed for all three aldehyde oxidation products by 1H and 13C NMR spectroscopic data collected after only 10 min (Figure 2A; hydrates 10, 11, and 12 were formed from aldehydes 1, 4, and 5, respectively). Hydrate formation resulted in a mixture containing approximately 55% hydrate and 45% aldehyde for both pinonaldehyde (1) and β-nocaryophyllone aldehyde (5) in D2O (Figure 2B). The ratio of aldehyde-to-hydrate did not change any further over the course of days, revealing that an aldehyde–hydrate equilibrium is rapidly reached before 10 min for these compounds. In addition, the ratio of aldehyde-to-hydrate did not change with the addition of ammonium sulfate (100 mM in D2O) over 72 h. Note that the solubility of β-caryophyllene aldehyde (4) in D2O was insufficient for accurate quantification of an aldehyde–hydrate ratio, and therefore hydrate formation from this compound was only qualitatively observed.
Figure 2.
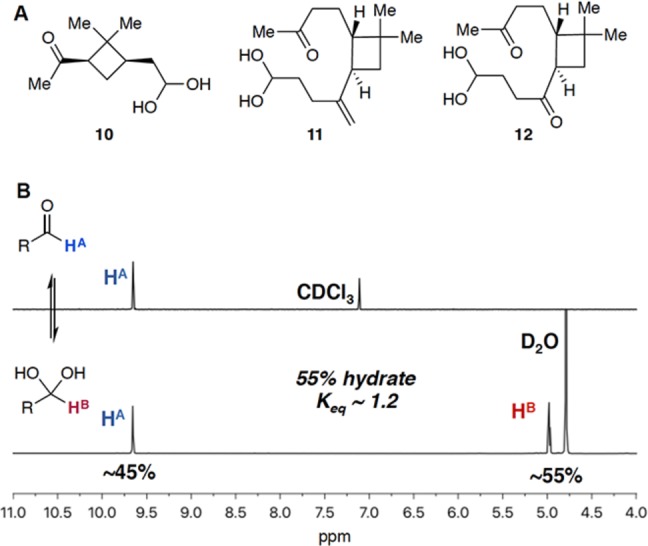
(A) In aqueous solution, aldehydes 1, 4, and 5 exist in equilibrium with their hydrate forms 10, 11, and 12, respectively. (B) 1H NMR spectroscopic data taken in D2O reveals an aldehyde–hydrate mixture containing approximately 55% of the hydrate form for both pinonaldehyde (1, 10) and β-nocaryophyllone aldehyde (5, 12). The shown spectra are for pinonaldehyde (1, 10). Note that the sparing solubility of β-caryophyllene aldehyde (4) in D2O resulted in poor signal-to-noise in the NMR spectra, and hydrate formation (i.e., 11) from this compound was only qualitatively observed.
Dynamic Interfacial Tension Measurements
The surface tension of suspended droplets was measured using pendant drop tensiometry (PDT) over the course of 10 min for solutions of varying concentrations (0–30 mM) in either deionized H2O or 1.0 M ammonium sulfate solution. Similar equilibration times have been reported for dynamic surface tension studies of PM10 size fraction of aerosol particles collected in an urban setting.24 Concentrations of α-pinene-derived compounds in water solutions ranged from 0 to 30 mM for pinonic acid (2) and pinic acid (3) and from 0 to 10 mM for pinonaldehyde (1) due to insolubility above 10 mM. All β-caryophyllene oxidation products (i.e., compounds 4–9) were insoluble above 1 mM, and therefore 0–1 mM concentrations were measured for these compounds. Solutions in 1.0 M ammonium sulfate were prepared using the same solute concentrations as those measured in water. Concentrations that could not be measured due to compound insolubility in 1.0 M ammonium sulfate are noted where applicable. With the exception of pinonic acid,16,21,62 we report unprecedented dynamic surface tension experiments for the presented series of compounds.
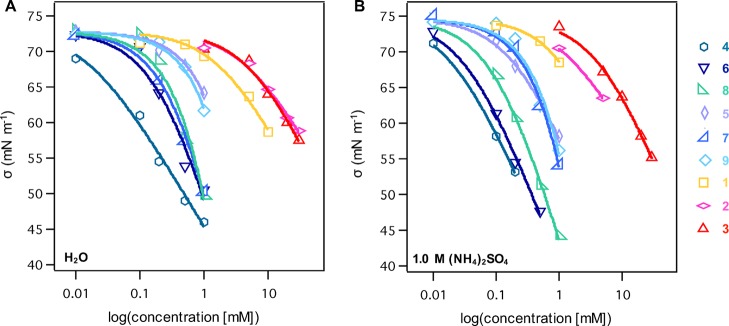
In Figure 3, the interfacial tension (σ) is plotted as a function of solute concentration on a logarithmic scale for suspended drops of solutions containing the synthesized four-membered ring oxidation products derived from α-pinene and β-caryophyllene in deionized water and 1.0 M ammonium sulfate, and the data are fitted to the Szyszkowski–Langmuir equation.14,63−65 Below we present the observations of droplets formed using water and ammonium sulfate for the α-pinene oxidation products, and then discuss our findings for the β-caryophyllene oxidation products.
Figure 3.
Comparison of interfacial tension (σ) as a function of solute concentration and Szyszkowski–Langmuir fitted curves for α-pinene- and β-caryophyllene-derived oxidation products in water (A) and 1.0 M ammonium sulfate (B): pinonaldehyde (1), pinonic acid (2), pinic acid (3), β-caryophyllene aldehyde (4), β-nocaryophyllone aldehyde (5), β-caryophyllonic acid (6), β-nocaryophyllonic acid (7), β-caryophyllinic acid (8), and β-nocaryophyllinic acid (9). All error values range from 0.06 to 0.69 mN m–1.
The surface tension measurements for the α-pinene-derived oxidation products in water reveal that pinonaldehyde (1) is the most surface active of the three compounds measured, reaching a 20% decrease relative to the interfacial tension of water at the highest measured concentration (10 mM) after 10 min (Figure S3). The surface tension depression caused by pinonaldehyde (1) at 10 mM in water is comparable to that exhibited by pinonic acid (2) and pinic acid (3) present at three times that concentration in water (Figure 3A). The substantially higher surface tension depression of pinonaldehyde (1) relative to pinonic acid (2) and pinic acid (3) highlights a considerable dependence of surface activity on structural functionality, as pinonic acid (2) and pinic acid (3) bear highly polar carboxylic acid moieties while pinonaldehyde (1) exists as an aldehyde–hydrate mixture with 10 in the aqueous droplets measured (Figure 2).
As reported previously, the surface tension of the droplets is raised by approximately 3% upon addition of 1.0 M ammonium sulfate in water.11 The results obtained for the α-pinene-derived oxidation products in 1.0 M ammonium sulfate (Figure 3B) show that pinonaldehyde (1) and pinonic acid (2) exhibit enhanced surface activity likely caused by a “salting out” effect in the presence of ammonium sulfate, which increases the solute concentration at the droplet surface due to decreased solubility with the addition of inorganic salt.11 The influence of “salting out” effects on surface activity of organic species may have a significant influence on the overall surface tension of atmospheric aerosols, as a substantial portion of the aerosol bulk contains inorganic salts such as (NH4)2SO4.66−68 For example, 1 mM solutions of pinonaldehyde (1) and pinonic acid (2) in 1.0 M ammonium sulfate each resulted in respective surface tension decreases of 8% and 6% compared to 5% and 2%, respectively, at the same concentration in water after 10 min, while a slight decrease in the surface activity of pinic acid (3) was observed at 1 mM in 1.0 M ammonium sulfate compared to in water (Figure S3). Slight enhancement of the surface tension lowering effects was only observed at the higher concentrations measured for pinic acid (3) in the presence of 1.0 M ammonium sulfate.
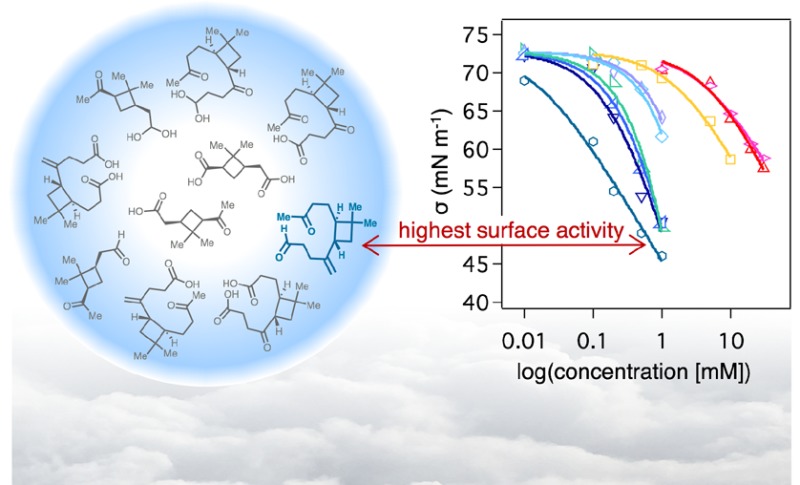
Turning to the β-caryophyllene oxidation products, we reemphasize that surface tension was measured between 0.01 mM and 1 mM due to their low solubility in water at concentrations above 1 mM (Figure 3A). Of the six β-caryophyllene oxidation products, β-caryophyllene aldehyde (4) was found to exhibit the highest surface activity in water solutions at all concentrations measured, resulting in an overall 37% decrease at 1 mM compared to the interfacial tension of water (Figure S4), the greatest surface tension depression we have observed for all oxidation products we have studied to date.11 Unexpectedly, β-nocaryophyllone aldehyde (5) exhibited the least surface activity of all six derivatives. This result for nocaryophyllone aldehyde (5) is potentially attributed to an interdependent decrease in surface activity and increase in solubility, which may be modulated by the existence of a hydrate–aldehyde mixture containing 55% of the hydrate (i.e., 12) in water rather than pure aldehyde content (Figure 2). The hydrate form of β-nocaryophyllone aldehyde is likely to increase its propensity to hydrogen bond with solvent water molecules within the bulk of the droplet, causing lower surface activity than anticipated. In contrast, β-caryophyllene aldehyde (4) is substantially more hydrophobic than β-nocaryophyllone aldehyde (5), due to the presence of an intact C=C double bond adjacent to the four-membered ring rather than a ketone, and therefore the hydrate form of β-caryophyllene aldehyde (i.e., 11) is unlikely to enhance its bulk aqueous solubility to the same extent. Of the β-caryophyllene oxidation products containing carboxylic acid functional groups, the most polar of the four compounds, β-nocaryophyllinic acid (9), is the least surface active, likely due to its higher solubility in water.
In the presence of 1.0 M ammonium sulfate, all six β-caryophyllene oxidation products exhibited “salting out” effects (Figures 3B and S5), which were most pronounced for the oxidation products that retain the exocyclic C=C double bond of β-caryophyllene compared to those bearing a ketone at that same position. For example, the two monoacid β-caryophyllene oxidation products, β-caryophyllonic acid (6) and β-nocaryophyllonic acid (7), exhibited comparable surface activity at 1 mM in water; however, upon addition of 1.0 M ammonium sulfate, “salting out” effects were much more pronounced for β-caryophyllonic acid (6) than for β-nocaryophyllonic acid (7).
Comparing the surface activity of all compounds studied at 1 mM concentration, Figure 4 highlights that the β-caryophyllene oxidation products are significantly more surface active than the measured α-pinene-derived oxidation products. The stark difference emphasizes that the surface depression behavior exhibited by the monoterpene (C10) and sesquiterpene (C15) ozone-mediated oxidation products studied here depends on carbon chain length and degree of oxidation. Yet, even the two least surface active compounds studied here, pinonic acid (2) and pinic acid (3), still exhibit larger surface tension depression than α-IEPOX, the most surface active of the IEPOX and tetraol oxidation products derived from isoprene.11
Figure 4.
Dynamic surface tension measurements comparing 1 mM solutions in water (A) and 1.0 M ammonium sulfate (B) for all compounds in this study: pinonaldehyde (1), pinonic acid (2), pinic acid (3), β-caryophyllene aldehyde (4), β-nocaryophyllone aldehyde (5), β-caryophyllonic acid (6), β-nocaryophyllonic acid (7), β-caryophyllinic acid (8), and β-nocaryophyllinic acid (9). β-Caryophyllene aldehyde (4) and β-caryophyllonic acid (6) were insoluble at 1 mM in 1.0 M ammonium sulfate and therefore are not shown.
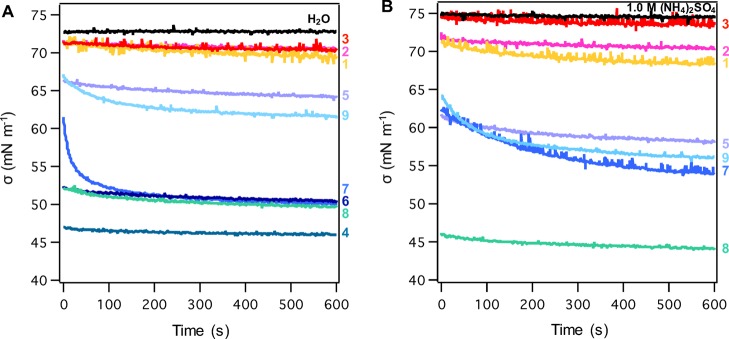
Kinetics of Interfacial Tension and Equilibration Time Scales
The values of dynamic surface tension σ(t) are time dependent. Curves of σ(t) presented in Figure 3 can be typically divided into three kinetic regions: (I) rapid fall region where σ rapidly decreases from the value of pure water (72.8 mN m–1) to σ0 within the initial time step of the measurement; (II) meso-equilibrium region, in which σ(t) slowly decreases with a characteristic time scale of tm; and (III) equilibrium region where the minimum value σm is reached. This time-dependent curve can be described by the following equation:24,69
| 1 |
where σ0, σm, tm, and n are fitting parameters. The optimized values of these parameters are listed in Table S1 for the obtained σ(t) data set.
For most of the compounds studied, a significant depression in surface tension was achieved in the rapid fall region, as indicated by the lower values of σ0 than that of pure water (Table S1). This surface tension depression can be treated as instantaneous in any aerosol and cloud process in the atmosphere. Further decrease of σ(t) occurred in the meso-equilibrium region, with a characteristic time tm of equilibration that ranged from 10 to 1000 s (Table S1). At tm, σ(tm) = (σ0+ σm)/2, meaning that equilibration was not yet reached. The final equilibration time was about twice of tm (teq = 2tm).24 Note that these time scales were measured for aqueous pendant droplets with diameters of 1.9–2.1 mm in the PDT laboratory experiments. These droplets were therefore much larger than typically sized aerosol and cloud droplets in the atmosphere. To estimate the surface tension effect for cloud activation, we calculated the equilibration time scale for a 1 μm droplet using the following equation:
| 2 |
where Ddrop represents the average diameter of droplets used in the laboratory experiments (Table S1). The droplet diameter of 1 μm is a typical size of activated aerosol particles. As reference, a particle with a 200 nm dry diameter has an activation diameter of 1.07 μm, assuming a hygroscopic parameter κ of 0.1 and a surface tension equal to that of pure water. The calculated equilibration time scales for 1 μm droplets were on the order of 10–5 to 10–4 s (Table S1). These results indicate that the surface tension depression observed in the laboratory experiments can occur instantaneously in the cloud activation processes both in online instruments and in the atmosphere, given that the surfactant concentrations are similar to those measured in the laboratory.
Nozière and co-workers suggested that the equilibration time scale can steeply increase with a decreasing surfactant concentration.24 Ambient particles having a low concentration of strong surfactant might exhibit delayed equilibrium. In the present study, we do not intend to extrapolate the results to a lower concentration because this extrapolation may have a large uncertainty.
Calculation of Cloud Activation Potential
Cloud droplet formation via water vapor condensation onto SOA particles is known to be heavily influenced by concentration-dependent surface tension depression effects caused by surface active species, which can reduce the critical supersaturation at the moment of cloud droplet activation.14 As described in our previous study11 and by the McNeill laboratory,14,66 the critical supersaturation ratio (sc*/sc) for cloud activation can be determined using Köhler theory14,70 from the equilibrium surface tension of a given solute concentration in water (σ) and the surface tension of water (σw) (72.8 mN m–1),71 and is expressed as follows:
| 3 |
Equation 3 assumes that the effect of surface-active organic species on equilibrium CCN activity is based purely on surface tension if the bulk solute concentration remains constant.66 We note, however, that the critical supersaturation ratio calculated by eq 3 neglects the effect of surfactant partitioning on the Raoult effect,72,73 which may potentially increase the critical supersaturation ratio for small particles by reducing the bulk solute concentration and therefore partially counteract the effect of surface tension depression. However, as noted by Sareen and co-workers previously, under heterogeneous SOA formation conditions the bulk solute composition of the particle is expected to be dominated by salt, and this solute content is expected to remain constant if gas-phase species are continuously taken up at the aerosol surface as they partition to the particle phase.66 Therefore, we make the assumption that the effect of organic species on equilibrium CCN activity is purely surface tension based in the present study. From the equilibrium surface tension (calculated as the average of the final 20 values of 3–5 10 min acquisitions) (Tables S2 and S3), sc*/sc was calculated at all concentrations measured for each compound in both water and 1.0 M ammonium sulfate (Tables 1 and 2). The equilibrium results were reported herein because such equilibrium can be reached instantaneously in a typically sized particle at activation point (see above). The concentrations of 0.01 to 30 mM are relevant to the total surfactant concentrations in ambient conditions.74 As an estimate, a particle composed of 20% to 100% mass fraction of secondary organic material has a total organic concentration of 1–10 M at dry condition. The hygroscopic growth of a particle from dry to cloud activation corresponds to a volume dilution factor of 20 to 1000. The activated particle thus has a total organic concentration of 1 to 500 mM, and the concentration of an individual compound can be much lower. Even so, the studied individual compounds can serve as representatives of a broad spectrum of monomeric carboxylic acids and aldehydes produced from the ozonolysis of α-pinene and β-caryophyllene precursors, and the total concentrations can be sufficiently high, thus having a significant effect on cloud activation. Similar calculations indicate that a typically sized ammonium sulfate particle (30–500 nm dry diameter) has an ammonium sulfate concentration of 1 to 100 mM. The effect of adding 1.0 M ammonium sulfate observed in the present study can thus serve as an upper limit estimate for the presence of salts in organic particles.
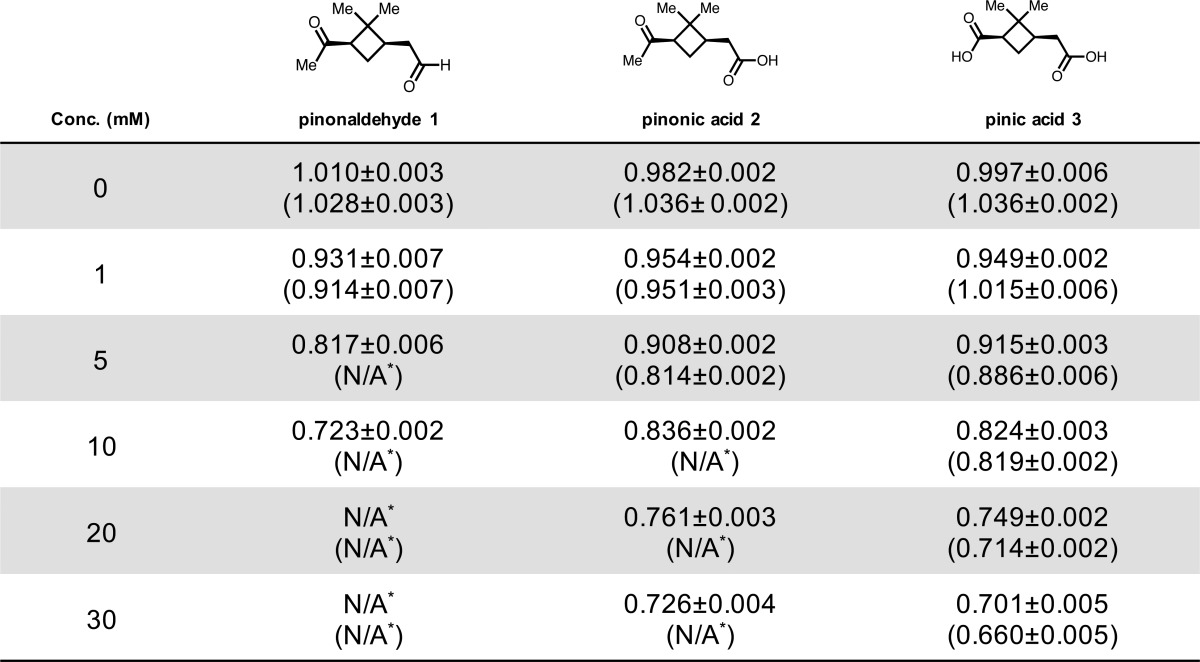
Table 1. Supersaturation Ratios (sc*/sc) for α-Pinene-Derived Oxidation Products at 0–30 mM in Water and 1.0 M Ammonium Sulfate (Values in Parentheses)a.
Asterisk (*) notes that value was not obtained due to insolubility at the indicated concentration. Note: Pinonaldehyde (1) exists in equilibrium with its hydrate form as described in the text.
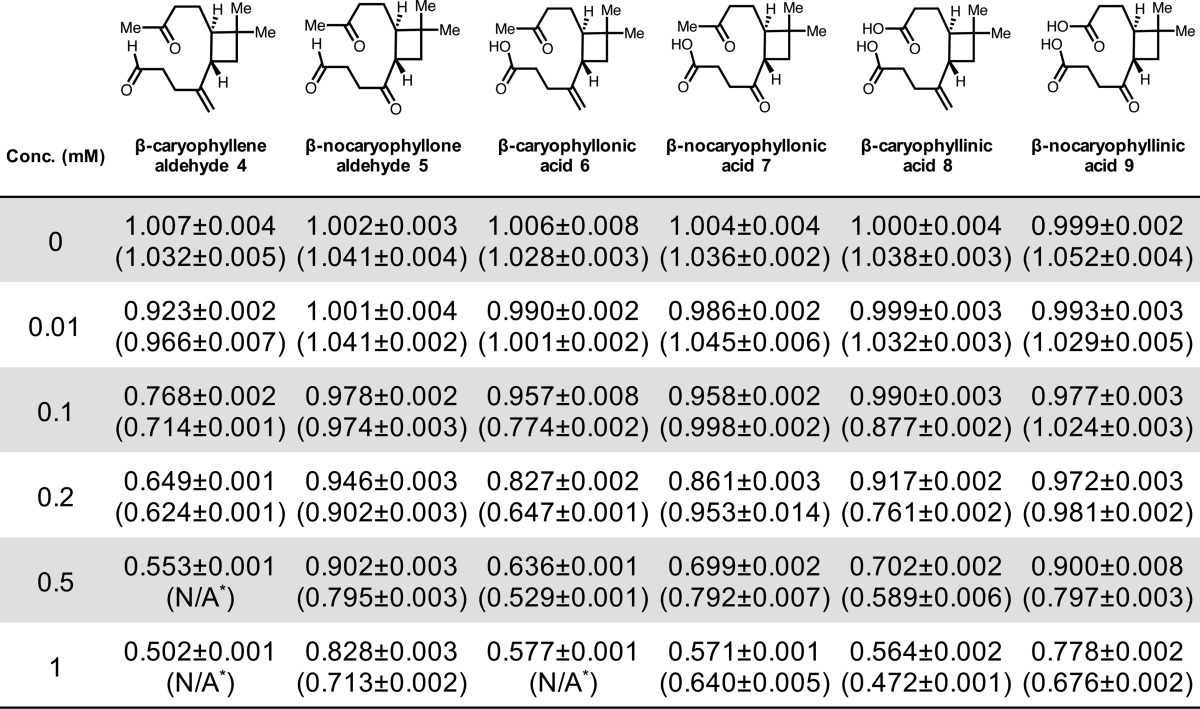
Table 2. Supersaturation Ratios (sc*/sc) for β-Caryophyllene-Derived Oxidation Products at 0–1 mM in Water and 1.0 M Ammonium Sulfate (Values in Parentheses)a.
Asterisk (*) notes that value was not obtained due to insolubility at the indicated concentration. Note: Aldehyde containing compounds 4 and 5 exist in equilibrium with hydrate forms as described in the text.
We previously reported an sc*/sc of 0.90 (10% decrease) for 10 mM trans-β-IEPOX in water.11 As shown in Table 1, a comparable sc/sc value of 0.93 was calculated for pinonaldehyde (1) in water at 1 mM. Additionally, Table 2 shows a similar sc*/sc value of 0.92 for β-caryophyllene aldehyde (4) at a concentration of 0.01 mM in water, which is 1000 times lower than the concentration producing a comparable supersaturation ratio (10 mM) for trans-β-IEPOX in water.11 Of all the oxidation products we have studied to date, β-caryophyllene aldehyde (4) was shown to decrease sc/sc by the largest extent (50% in water), highlighting the highly amphiphilic and surfactant-like nature of this compound as well as the other monomeric sesquiterpene oxidation products studied relative to monomeric oxidation products derived from α-pinene and isoprene.
Atmospheric Implications
According to the 2013 Intergovernmental Panel on Climate Change Report,6 atmospheric aerosol–cloud interactions remain among the least understood of processes within the climate system. Investigation of sesquiterpenes in the context of SOA–cloud interactions has been particularly rare, in part due to experimental shortcomings related to measuring SOA cloud activation properties coupled with the characteristic complexity and sparse level of chemical understanding regarding sesquiterpenes and their oxidation products compared to other SOA precursors. Comparison of CCN activity of sesquiterpene- and monoterpene-derived SOA samples using cloud condensation nuclei counters (CCNC) has demonstrated that CCN activity of SOA from β-caryophyllene and other sesquiterpenes is lower than that of monoterpene SOA as quantified by CCNC detection.71,75,76 However, CCN activity of β-caryophyllene SOA, for example, was reported to be greater when measured using a static diffusion (SD) CCNC than when measured with a continuous flow (CF) CCNC, highlighting a possibility for measurement variability by CCNC detection.52,75 Additionally, β-caryophyllene contributions to secondary organic CCN were revisited by Asa-Awuku and co-workers in 2012,77 who measured higher CCN activity and hygroscopicity than reported previously and concluded that β-caryophyllene-derived SOA formed in the presence of ozone may be a potentially important source of biogenic CCN.75−77 CCN activity experiments performed using cloud condensation nucleus counters (CCNC) and related hygroscopicity tandem differential mobility analyzers (HTDMA) indirectly quantify CCN activity by measuring population or growth of nucleated droplets rather than Raoult’s term and surface tension, which are key parameters related to cloud activation outlined by Köhler theory.14,24,70 Additionally, HTDMA and CCNC instruments typically collect measurements in a time regime on the order of seconds to minutes. In their 2014 publication, Nozière and co-workers suggested that bulk-to-surface partitioning of low-concentration surface-active organic compounds may reach equilibrium beyond time scales detectable by these instruments.24 The experimental results and calculations in the present study, however, suggest that such a delayed equilibrium might be less important for abundant oxidation products at conditions relevant to cloud activation. Nevertheless, Nozière and co-workers highlighted the importance of using bulk surface tension studies of SOA samples as an alternative approach to CCNC and HTDMA experiments to gain a more comprehensive understanding of the role of surface active organics in SOA particle CCN activity.24 Our findings improve upon the growing insight obtained from surface tension studies related to atmospheric aerosol by providing molecule-specific insight into the aqueous surface tension depression induced by pure standards of oxidation products relevant to terpene-derived SOA material.
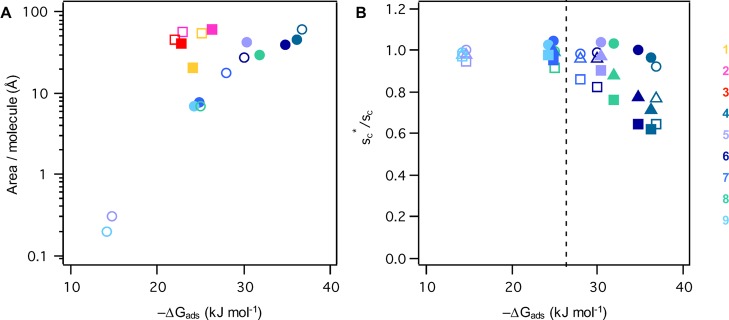
Oxidation of α-pinene and β-caryophyllene in the atmosphere generates a plethora of products of different molecular weight and containing diverse sets of functional groups that impact their surface activities. In order to deconvolute the complexity associated with analyzing this mixture, we focused on several abundant monomeric acid- and aldehyde-containing ozonolysis products. As shown in Figure 5, molecular properties such as the surface area per molecule adsorbed at the air/water interface of the pendant drop correlate seemingly well with the free energy of adsorption (both parameters were obtained from the Szyszkowski–Langmuir equation as outlined in the Supporting Information), at least for the β-caryophyllene oxidation product series examined here. Moreover, our analysis indicates that, for the β-caryophyllene oxidation products, free energies of adsorption larger than ∼25 kJ/mol (just one hydrogen bond equivalent) coincide with considerable reductions in supersaturation ratios, depending on ammonium sulfate and oxidation product concentration in solution.
Figure 5.
(A) Area per molecule adsorbed at the air/water interface of pendant drops as a function of the free energy of adsorption obtained from the Szyszkowski–Langmuir equation. Results are for solutions in water (empty symbols) and 1.0 M ammonium sulfate (filled symbols) for the α-pinene (squares) and the β-caryophyllene (circles) series: Pinonaldehyde (1), pinonic acid (2), pinic acid (3), β-caryophyllene aldehyde (4), β-nocaryophyllone aldehyde (5), β-caryophyllonic acid (6), β-nocaryophyllonic acid (7), β-caryophyllinic acid (8), and β-nocaryophyllinic acid (9). (B) Supersaturation ratio as a function of free energy of adsorption of the β-caryophyllene series studied here. Results are for solutions in water (empty symbols) and 1.0 M ammonium sulfate (filled symbols) for concentrations of 0.01 (circles), 0.1 (triangles), and 0.2 (squares) mmol/L. β-Caryophyllene aldehyde (4) and β-caryophyllonic acid (6) were insoluble at 1 mM in 1.0 M ammonium sulfate and therefore are not shown.
We expect that molecular-level measurements such as the ones presented here will be useful for developing structure–function relationships across atmospheric organic species and serve as benchmarks for future studies that could involve recently developed complementary surface tension measurements in more atmospherically transferable droplet-size regimes78,79 as well as other surface-specific climate-relevant aerosol techniques.80−82
Ultimately, given the inherent complexity of studying the surface tension of SOA-related systems in the context of cloud droplet condensation, we take the specific approach of cataloging the relative surface tension depression exhibited by individual α-pinene and β-caryophyllene oxidation products in order to gain chemical insight into the correlation between surface activity and molecular structure and reactivity. Among the series of compounds studied, β-caryophyllene aldehyde (4) shows significant cloud activation potentials, indicating that it might play a substantial role in the atmosphere. Our investigations also reveal that aqueous solutions of terpene oxidation products containing aldehyde functional groups exist in equilibrium with hydrated forms, which may modulate their bulk solubility and surface activity. Overall, our findings highlight substantial differences in surface tension to consider for this given series of monomeric terpene-derived ozonolysis products, and the data obtained in this study should prove valuable for chemical comparison to laboratory and field CCN studies.
Conclusions
In summary, we present dynamic surface tension measurements of synthetically prepared homogeneous oxidation products derived from α-pinene and β-caryophyllene and provide calculated equilibration time scales and supersaturation ratios of relevance to predicting CCN activity. Equilibration time scales calculated for a 1 μm aerosol particle provide an estimate of the surface tension depression effect on cloud activation for typically sized atmospheric particles. The results of these experiments also demonstrate that the oxidation products derived from β-caryophyllene exhibit significantly greater surface tension depression behavior, and, consequently, cloud activation potentials, than those prepared from α-pinene. β-Caryophyllene aldehyde (4) was found to be the most surface active of all compounds we have studied to date, with a calculated supersaturation ratio of 50% at 1 mM concentration, and 8% at 10 μM concentration. We also revealed that hydrate formation from oxidation products containing aldehydes was rapid, leading to approximately equal mixtures of the aldehyde and hydrate forms within aqueous solutions of the compounds investigated. Our findings provide specific molecular-level understanding of the relative surface tension effects exhibited by α-pinene and β-caryophyllene oxidation products toward investigating their role as surface-active organic compounds in SOA–cloud interactions.
Acknowledgments
A.G.B. and M.A.U. gratefully acknowledge NSF Graduate Research fellowships. M.A.U. and P.L. also acknowledge National Aeronautics and Space Administration Earth and Space (NASA ESS) fellowships. M.A.U. additionally acknowledges support from a P.E.O. Scholar Award. F.M.G. gratefully acknowledges support from the Alexander von Humboldt Foundation. We thank Prof. Allen Goldstein and Dr. Lindsay Yee (UC Berkeley) for helpful discussions. This work was supported by the National Science Foundation (NSF) under Grant No. CHE-1607640.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00112.
General methods, experimental procedures, 1H and 13C NMR data and spectra, and dynamic surface tension details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ng N. L.; Canagaratna M. R.; Zhang Q.; Jimenez J. L.; Tian J.; Ulbrich I. M.; Kroll J. H.; Docherty K. S.; Chhabra P. S.; Bahreini R.; Murphy S. M.; Seinfeld J. H.; Hildebrandt L.; Donahue N. M.; DeCarlo P. F.; Lanz V. A.; Prévôt A. S. H.; Dinar E.; Rudich Y.; Worsnop D. R. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2010, 10 (10), 4625–4641. 10.5194/acp-10-4625-2010. [DOI] [Google Scholar]
- Baltensperger U.; Kalberer M.; Dommen J.; Paulsen D.; Alfarra M. R.; Coe H.; Fisseha R.; Gascho A.; Gysel M.; Nyeki S.; Sax M.; Steinbacher M.; Prevot A. S. H.; Sjogren S.; Weingartner E.; Zenobi R. Secondary organic aerosols from anthropogenic and biogenic precursors. Faraday Discuss. 2005, 130, 265–278. 10.1039/b417367h. [DOI] [PubMed] [Google Scholar]
- Ebben C. J.; Shrestha M.; Martinez I. S.; Corrigan A. L.; Frossard A. A.; Song W. W.; Worton D. R.; Petäjä T.; Williams J.; Russell L. M.; Kulmala M.; Goldstein A. H.; Artaxo P.; Martin S. T.; Thomson R. J.; Geiger F. M. Organic Constituents on the Surfaces of Aerosol Particles from Southern Finland, Amazonia, and California Studied by Vibrational Sum Frequency Generation. J. Phys. Chem. A 2012, 116 (32), 8271–8290. 10.1021/jp302631z. [DOI] [PubMed] [Google Scholar]
- Hallquist M.; Wenger J. C.; Baltensperger U.; Rudich Y.; Simpson D.; Claeys M.; Dommen J.; Donahue N. M.; George C.; Goldstein A. H.; Hamilton J. F.; Herrmann H.; Hoffmann T.; Iinuma Y.; Jang M.; Jenkin M. E.; Jimenez J. L.; Kiendler-Scharr A.; Maenhaut W.; McFiggans G.; Mentel T. F.; Monod A.; Prévôt A. S. H.; Seinfeld J. H.; Surratt J. D.; Szmigielski R.; Wildt J. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009, 9 (14), 5155–5236. 10.5194/acp-9-5155-2009. [DOI] [Google Scholar]
- Williams J.; Crowley J.; Fischer H.; Harder H.; Martinez M.; Petäjä T.; Rinne J.; Bäck J.; Boy M.; Dal Maso M.; Hakala J.; Kajos M.; Keronen P.; Rantala P.; Aalto J.; Aaltonen H.; Paatero J.; Vesala T.; Hakola H.; Levula J.; Pohja T.; Herrmann F.; Auld J.; Mesarchaki E.; Song W.; Yassaa N.; Nölscher A.; Johnson A. M.; Custer T.; Sinha V.; Thieser J.; Pouvesle N.; Taraborrelli D.; Tang M. J.; Bozem H.; Hosaynali-Beygi Z.; Axinte R.; Oswald R.; Novelli A.; Kubistin D.; Hens K.; Javed U.; Trawny K.; Breitenberger C.; Hidalgo P. J.; Ebben C. J.; Geiger F. M.; Corrigan A. L.; Russell L. M.; Ouwersloot H. G.; Vilà-Guerau de Arellano J.; Ganzeveld L.; Vogel A.; Beck M.; Bayerle A.; Kampf C. J.; Bertelmann M.; Köllner F.; Hoffmann T.; Valverde J.; González D.; Riekkola M. L.; Kulmala M.; Lelieveld J. The summertime Boreal forest field measurement intensive (HUMPPA-COPEC-2010): an overview of meteorological and chemical influences. Atmos. Chem. Phys. 2011, 11 (20), 10599–10618. 10.5194/acp-11-10599-2011. [DOI] [Google Scholar]
- Beck M.; Hoffmann T. A detailed MSn study for the molecular identification of a dimer formed from oxidation of pinene. Atmos. Environ. 2016, 130, 120–126. 10.1016/j.atmosenv.2015.09.012. [DOI] [Google Scholar]
- Goldstein A. H.; Galbally I. E. Known and Unexplored Organic Constituents in the Earth’s Atmosphere. Environ. Sci. Technol. 2007, 41 (5), 1514–1521. 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
- Galbally I. E.; Lawson S. J.; Weeks I. A.; Bentley S. T.; Gillett R. W.; Meyer M.; Goldstein A. H. Volatile organic compounds in marine air at Cape Grim, Australia. Environmental Chemistry 2007, 4 (3), 178–182. 10.1071/EN07024. [DOI] [Google Scholar]
- Riipinen I.; Pierce J. R.; Yli-Juuti T.; Nieminen T.; Häkkinen S.; Ehn M.; Junninen H.; Lehtipalo K.; Petäjä T.; Slowik J.; Chang R.; Shantz N. C.; Abbatt J.; Leaitch W. R.; Kerminen V. M.; Worsnop D. R.; Pandis S. N.; Donahue N. M.; Kulmala M. Organic condensation: a vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations. Atmos. Chem. Phys. 2011, 11 (8), 3865–3878. 10.5194/acp-11-3865-2011. [DOI] [Google Scholar]
- Carlton A. G.; Wiedinmyer C.; Kroll J. H. A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9 (14), 4987–5005. 10.5194/acp-9-4987-2009. [DOI] [Google Scholar]
- Upshur M. A.; Strick B. F.; McNeill V. F.; Thomson R. J.; Geiger F. M. Climate-relevant physical properties of molecular constituents for isoprene-derived secondary organic aerosol material. Atmos. Chem. Phys. 2014, 14 (19), 10731–10740. 10.5194/acp-14-10731-2014. [DOI] [Google Scholar]
- Ehn M.; Thornton J. A.; Kleist E.; Sipila M.; Junninen H.; Pullinen I.; Springer M.; Rubach F.; Tillmann R.; Lee B.; Lopez-Hilfiker F.; Andres S.; Acir I.-H.; Rissanen M.; Jokinen T.; Schobesberger S.; Kangasluoma J.; Kontkanen J.; Nieminen T.; Kurten T.; Nielsen L. B.; Jorgensen S.; Kjaergaard H. G.; Canagaratna M.; Maso M. D.; Berndt T.; Petaja T.; Wahner A.; Kerminen V.-M.; Kulmala M.; Worsnop D. R.; Wildt J.; Mentel T. F. A large source of low-volatility secondary organic aerosol. Nature 2014, 506 (7489), 476–479. 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- Kroll J. H.; Seinfeld J. H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42 (16), 3593–3624. 10.1016/j.atmosenv.2008.01.003. [DOI] [Google Scholar]
- McNeill V. F.; Sareen N.; Schwier A. N.. Surface-Active Organics in Atmospheric Aerosols. In Atmospheric and Aerosol Chemistry; McNeill F. V., Ariya A. P., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; pp 201–259. [DOI] [PubMed] [Google Scholar]
- Woo J. L.; Kim D. D.; Schwier A. N.; Li R.; McNeill V. F. Aqueous aerosol SOA formation: impact on aerosol physical properties. Faraday Discuss. 2013, 165, 357. 10.1039/c3fd00032j. [DOI] [PubMed] [Google Scholar]
- Tuckermann R.; Cammenga H. K. The surface tension of aqueous solutions of some atmospheric water-soluble organic compounds. Atmos. Environ. 2004, 38 (36), 6135–6138. 10.1016/j.atmosenv.2004.08.005. [DOI] [Google Scholar]
- Prenni A. J.; Petters M. D.; Kreidenweis S. M.; DeMott P. J.; Ziemann P. J. Cloud droplet activation of secondary organic aerosol. J. Geophys. Res. 2007, 112, D10223. 10.1029/2006JD007963. [DOI] [Google Scholar]
- Wex H.; Petters M. D.; Carrico C. M.; Hallbauer E.; Massling A.; McMeeking G. R.; Poulain L.; Wu Z.; Kreidenweis S. M.; Stratmann F. Towards closing the gap between hygroscopic growth and activation for secondary organic aerosol: Part 1 – Evidence from measurements. Atmos. Chem. Phys. 2009, 9 (12), 3987–3997. 10.5194/acp-9-3987-2009. [DOI] [Google Scholar]
- Good N.; Topping D. O.; Duplissy J.; Gysel M.; Meyer N. K.; Metzger A.; Turner S. F.; Baltensperger U.; Ristovski Z.; Weingartner E.; Coe H.; McFiggans G. Widening the gap between measurement and modelling of secondary organic aerosol properties?. Atmos. Chem. Phys. 2010, 10 (6), 2577–2593. 10.5194/acp-10-2577-2010. [DOI] [Google Scholar]
- Renbaum-Wolff L.; Song M.; Marcolli C.; Zhang Y.; Liu P. F.; Grayson J. W.; Geiger F. M.; Martin S. T.; Bertram A. K. Observations and implications of liquid–liquid phase separation at high relative humidities in secondary organic material produced by α-pinene ozonolysis without inorganic salts. Atmos. Chem. Phys. 2016, 16 (12), 7969–7979. 10.5194/acp-16-7969-2016. [DOI] [Google Scholar]
- Shulman M. L.; Jacobson M. C.; Carlson R. J.; Synovec R. E.; Young T. E. Dissolution behavior and surface tension effects of organic compounds in nucleating cloud droplets. Geophys. Res. Lett. 1996, 23 (3), 277–280. 10.1029/95GL03810. [DOI] [Google Scholar]
- Ruehl C. R.; Davies J. F.; Wilson K. R. An interfacial mechanism for cloud droplet formation on organic aerosols. Science 2016, 351 (6280), 1447–1450. 10.1126/science.aad4889. [DOI] [PubMed] [Google Scholar]
- Petters M. D.; Kreidenweis S. M. A single parameter representation of hygroscopic growth and cloud condensation nucleus activity. Atmos. Chem. Phys. 2007, 7 (8), 1961–1971. 10.5194/acp-7-1961-2007. [DOI] [Google Scholar]
- Nozière B.; Baduel C.; Jaffrezo J.-L. The dynamic surface tension of atmospheric aerosol surfactants reveals new aspects of cloud activation. Nat. Commun. 2014, 5, 3335. 10.1038/ncomms4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak R. K.; Presto A. A.; Lane T. E.; Stanier C. O.; Donahue N. M.; Pandis S. N. Ozonolysis of α-pinene: parameterization of secondary organic aerosol mass fraction. Atmos. Chem. Phys. 2007, 7 (14), 3811–3821. 10.5194/acp-7-3811-2007. [DOI] [Google Scholar]
- Guenther A.; Hewitt C. N.; Erickson D.; Fall R.; Geron C.; Graedel T.; Harley P.; Klinger L.; Lerdau M.; McKay W. A.; Pierce T.; Scholes B.; Steinbrecher R.; Tallamraju R.; Taylor J.; Zimmerman P. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100 (D5), 8873–8892. 10.1029/94JD02950. [DOI] [Google Scholar]
- Hakola H.; Tarvainen V.; Laurila T.; Hiltunen V.; Hellén H.; Keronen P. Seasonal variation of VOC concentrations above a boreal coniferous forest. Atmos. Environ. 2003, 37 (12), 1623–1634. 10.1016/S1352-2310(03)00014-1. [DOI] [Google Scholar]
- Zhang X.; McVay R. C.; Huang D. D.; Dalleska N. F.; Aumont B.; Flagan R. C.; Seinfeld J. H. Formation and evolution of molecular products in α-pinene secondary organic aerosol. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (46), 14168–14173. 10.1073/pnas.1517742112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulyanontvittaya T.; Duhl T.; Wiedinmyer C.; Helmig D.; Matsunaga S.; Potosnak M.; Milford J.; Guenther A. Monoterpene and Sesquiterpene Emission Estimates for the United States. Environ. Sci. Technol. 2008, 42 (5), 1623–1629. 10.1021/es702274e. [DOI] [PubMed] [Google Scholar]
- Helmig D.; Ortega J.; Duhl T.; Tanner D.; Guenther A.; Harley P.; Wiedinmyer C.; Milford J.; Sakulyanontvittaya T. Sesquiterpene Emissions from Pine Trees – Identifications, Emission Rates and Flux Estimates for the Contiguous United States. Environ. Sci. Technol. 2007, 41 (5), 1545–1553. 10.1021/es0618907. [DOI] [PubMed] [Google Scholar]
- Winterhalter R.; Herrmann F.; Kanawati B.; Nguyen T. L.; Peeters J.; Vereecken L.; Moortgat G. K. The gas-phase ozonolysis of β-caryophyllene (C15H24). Part I: an experimental study. Phys. Chem. Chem. Phys. 2009, 11 (21), 4152–4172. 10.1039/b817824k. [DOI] [PubMed] [Google Scholar]
- Jaoui M.; Lewandowski M.; Kleindienst T. E.; Offenberg J. H.; Edney E. O. β-caryophyllinic acid: An atmospheric tracer for β-caryophyllene secondary organic aerosol. Geophys. Res. Lett. 2007, 34 (5), L05816. 10.1029/2006GL028827. [DOI] [Google Scholar]
- Parshintsev J.; Nurmi J.; Kilpeläinen I.; Hartonen K.; Kulmala M.; Riekkola M.-L. Preparation of β-caryophyllene oxidation products and their determination in ambient aerosol samples. Anal. Bioanal. Chem. 2008, 390 (3), 913–919. 10.1007/s00216-007-1755-4. [DOI] [PubMed] [Google Scholar]
- Jaoui M.; Leungsakul S.; Kamens R. M. Gas and Particle Products Distribution from the Reaction of β-Caryophyllene with Ozone. J. Atmos. Chem. 2003, 45 (3), 261–287. 10.1023/A:1024263430285. [DOI] [Google Scholar]
- Hartonen K.; Parshintsev J.; Vilja V.-P.; Tiala H.; Knuuti S.; Lai C. K.; Riekkola M.-L. Gas chromatographic vapor pressure determination of atmospherically relevant oxidation products of β-caryophyllene and α-pinene. Atmos. Environ. 2013, 81, 330–338. 10.1016/j.atmosenv.2013.09.023. [DOI] [Google Scholar]
- van Eijck A.; Opatz T.; Taraborrelli D.; Sander R.; Hoffmann T. New tracer compounds for secondary organic aerosol formation from β-caryophyllene oxidation. Atmos. Environ. 2013, 80, 122–130. 10.1016/j.atmosenv.2013.07.060. [DOI] [Google Scholar]
- Fu P.; Kawamura K.; Chen J.; Barrie L. A. Isoprene, Monoterpene, and Sesquiterpene Oxidation Products in the High Arctic Aerosols during Late Winter to Early Summer. Environ. Sci. Technol. 2009, 43 (11), 4022–4028. 10.1021/es803669a. [DOI] [PubMed] [Google Scholar]
- Lewandowski M.; Jaoui M.; Offenberg J. H.; Kleindienst T. E.; Edney E. O.; Sheesley R. J.; Schauer J. J. Primary and Secondary Contributions to Ambient PM in the Midwestern United States. Environ. Sci. Technol. 2008, 42 (9), 3303–3309. 10.1021/es0720412. [DOI] [PubMed] [Google Scholar]
- Kleindienst T. E.; Jaoui M.; Lewandowski M.; Offenberg J. H.; Lewis C. W.; Bhave P. V.; Edney E. O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41 (37), 8288–8300. 10.1016/j.atmosenv.2007.06.045. [DOI] [Google Scholar]
- Bouvier-Brown N. C.; Goldstein A. H.; Gilman J. B.; Kuster W. C.; de Gouw J. A. In-situ ambient quantification of monoterpenes, sesquiterpenes, and related oxygenated compounds during BEARPEX 2007: implications for gas- and particle-phase chemistry. Atmos. Chem. Phys. 2009, 9 (15), 5505–5518. 10.5194/acp-9-5505-2009. [DOI] [Google Scholar]
- Lee A.; Goldstein A. H.; Kroll J. H.; Ng N. L.; Varutbangkul V.; Flagan R. C.; Seinfeld J. H. Gas-phase products and secondary aerosol yields from the photooxidation of 16 different terpenes. J. Geophys. Res. 2006, 111, D17305. 10.1029/2006JD007050. [DOI] [Google Scholar]
- Lee A.; Goldstein A. H.; Keywood M. D.; Gao S.; Varutbangkul V.; Bahreini R.; Ng N. L.; Flagan R. C.; Seinfeld J. H. Gas-phase products and secondary aerosol yields from the ozonolysis of ten different terpenes. J. Geophys. Res. 2006, 111, D07302. 10.1029/2005JD006437. [DOI] [Google Scholar]
- Li Y. J.; Chen Q.; Guzman M. I.; Chan C. K.; Martin S. T. Second-generation products contribute substantially to the particle-phase organic material produced by β-caryophyllene ozonolysis. Atmos. Chem. Phys. 2011, 11 (1), 121–132. 10.5194/acp-11-121-2011. [DOI] [Google Scholar]
- Bonn B.; Moortgat G. K. Sesquiterpene ozonolysis: Origin of atmospheric new particle formation from biogenic hydrocarbons. Geophys. Res. Lett. 2003, 30 (11), 1585. 10.1029/2003GL017000. [DOI] [Google Scholar]
- Chan M. N.; Surratt J. D.; Chan A. W. H.; Schilling K.; Offenberg J. H.; Lewandowski M.; Edney E. O.; Kleindienst T. E.; Jaoui M.; Edgerton E. S.; Tanner R. L.; Shaw S. L.; Zheng M.; Knipping E. M.; Seinfeld J. H. Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene. Atmos. Chem. Phys. 2011, 11 (4), 1735–1751. 10.5194/acp-11-1735-2011. [DOI] [Google Scholar]
- Henrot A. J.; Stanelle T.; Schröder S.; Siegenthaler C.; Taraborrelli D.; Schultz M. G. Implementation of the biogenic emission model MEGAN(v2.1) into the ECHAM6-HAMMOZ chemistry climate model. Basic results and sensitivity tests. Geosci. Model Dev. 2016, 2016, 1–43. 10.5194/gmd-2016-248. [DOI] [Google Scholar]
- Guenther A. B.; Jiang X.; Heald C. L.; Sakulyanontvittaya T.; Duhl T.; Emmons L. K.; Wang X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5 (6), 1471–1492. 10.5194/gmd-5-1471-2012. [DOI] [Google Scholar]
- Henze D. K.; Seinfeld J. H.; Ng N. L.; Kroll J. H.; Fu T. M.; Jacob D. J.; Heald C. L. Global modeling of secondary organic aerosol formation from aromatic hydrocarbons: high- vs. low-yield pathways. Atmos. Chem. Phys. 2008, 8 (9), 2405–2420. 10.5194/acp-8-2405-2008. [DOI] [Google Scholar]
- Nguyen T. L.; Winterhalter R.; Moortgat G.; Kanawati B.; Peeters J.; Vereecken L. The gas-phase ozonolysis of β-caryophyllene (C15H24). Part II: A theoretical study. Phys. Chem. Chem. Phys. 2009, 11 (21), 4173–4183. 10.1039/b817913a. [DOI] [PubMed] [Google Scholar]
- Shu Y.; Atkinson R. Atmospheric lifetimes and fates of a series of sesquiterpenes. J. Geophys. Res. 1995, 100 (D4), 7275–7281. 10.1029/95JD00368. [DOI] [Google Scholar]
- Griffin R. J.; Cocker D. R.; Flagan R. C.; Seinfeld J. H. Organic aerosol formation from the oxidation of biogenic hydrocarbons. Journal of Geophysical Research: Atmospheres 1999, 104 (D3), 3555–3567. 10.1029/1998JD100049. [DOI] [Google Scholar]
- Frosch M.; Bilde M.; Nenes A.; Praplan A. P.; Jurányi Z.; Dommen J.; Gysel M.; Weingartner E.; Baltensperger U. CCN activity and volatility of β-caryophyllene secondary organic aerosol. Atmos. Chem. Phys. 2013, 13 (4), 2283–2297. 10.5194/acp-13-2283-2013. [DOI] [Google Scholar]
- Griffin R. J.; Cocker D. R.; Seinfeld J. H.; Dabdub D. Estimate of global atmospheric organic aerosol from oxidation of biogenic hydrocarbons. Geophys. Res. Lett. 1999, 26 (17), 2721–2724. 10.1029/1999GL900476. [DOI] [Google Scholar]
- Atkinson R.; Arey J. Atmospheric Chemistry of Biogenic Organic Compounds. Acc. Chem. Res. 1998, 31 (9), 574–583. 10.1021/ar970143z. [DOI] [Google Scholar]
- Goldstein A. H.; McKay M.; Kurpius M. R.; Schade G. W.; Lee A.; Holzinger R.; Rasmussen R. A. Forest thinning experiment confirms ozone deposition to forest canopy is dominated by reaction with biogenic VOCs. Geophys. Res. Lett. 2004, 31, L22106. 10.1029/2004GL021259. [DOI] [Google Scholar]
- Claeys M.; Iinuma Y.; Szmigielski R.; Surratt J. D.; Blockhuys F.; Van Alsenoy C.; Böge O.; Sierau B.; Gómez-González Y.; Vermeylen R.; Van der Veken P.; Shahgholi M.; Chan A. W. H.; Herrmann H.; Seinfeld J. H.; Maenhaut W. Terpenylic Acid and Related Compounds from the Oxidation of α-Pinene: Implications for New Particle Formation and Growth above Forests. Environ. Sci. Technol. 2009, 43 (18), 6976–6982. 10.1021/es9007596. [DOI] [PubMed] [Google Scholar]
- Lin Y.-H.; Zhang Z.; Docherty K. S.; Zhang H.; Budisulistiorini S. H.; Rubitschun C. L.; Shaw S. L.; Knipping E. M.; Edgerton E. S.; Kleindienst T. E.; Gold A.; Surratt J. D. Isoprene Epoxydiols as Precursors to Secondary Organic Aerosol Formation: Acid-Catalyzed Reactive Uptake Studies with Authentic Compounds. Environ. Sci. Technol. 2012, 46 (1), 250–258. 10.1021/es202554c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings W. P.; Koehler C. A.; Bailey E. L.; De Haan D. O. Secondary Organic Aerosol Formation by Glyoxal Hydration and Oligomer Formation: Humidity Effects and Equilibrium Shifts during Analysis. Environ. Sci. Technol. 2005, 39 (22), 8728–8735. 10.1021/es050446l. [DOI] [PubMed] [Google Scholar]
- Vestenius M.; Hellén H.; Levula J.; Kuronen P.; Helminen K. J.; Nieminen T.; Kulmala M.; Hakola H. Acidic reaction products of monoterpenes and sesquiterpenes in atmospheric fine particles in a boreal forest. Atmos. Chem. Phys. 2014, 14 (15), 7883–7893. 10.5194/acp-14-7883-2014. [DOI] [Google Scholar]
- Wyche K. P.; Monks P. S.; Smallbone K. L.; Hamilton J. F.; Alfarra M. R.; Rickard A. R.; McFiggans G. B.; Jenkin M. E.; Bloss W. J.; Ryan A. C.; Hewitt C. N.; MacKenzie A. R. Mapping gas-phase organic reactivity and concomitant secondary organic aerosol formation: chemometric dimension reduction techniques for the deconvolution of complex atmospheric data sets. Atmos. Chem. Phys. 2015, 15 (14), 8077–8100. 10.5194/acp-15-8077-2015. [DOI] [Google Scholar]
- Nozière B.; Kalberer M.; Claeys M.; Allan J.; D’Anna B.; Decesari S.; Finessi E.; Glasius M.; Grgić I.; Hamilton J. F.; Hoffmann T.; Iinuma Y.; Jaoui M.; Kahnt A.; Kampf C. J.; Kourtchev I.; Maenhaut W.; Marsden N.; Saarikoski S.; Schnelle-Kreis J.; Surratt J. D.; Szidat S.; Szmigielski R.; Wisthaler A. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015, 115 (10), 3919–3983. 10.1021/cr5003485. [DOI] [PubMed] [Google Scholar]
- Li X.; Hede T.; Tu Y.; Leck C.; Ågren H. Surface-Active cis-Pinonic Acid in Atmospheric Droplets: A Molecular Dynamics Study. J. Phys. Chem. Lett. 2010, 1 (4), 769–773. 10.1021/jz9004784. [DOI] [Google Scholar]
- von Szyszkowski B. Experimentelle Studien über kapillare Eigenschaften der wasserigen Losungen von Fettsauren. (Experimental studies of the capillary properties of aqueous solutions of fatty acids). Z. Phys. Chem. 1908, 64, 385–414. 10.1515/zpch-1908-0125. [DOI] [Google Scholar]
- Meissner H. P.; Michaels A. S. Surface Tensions of Pure Liquids and Liquid Mixtures. Ind. Eng. Chem. 1949, 41 (12), 2782–2787. 10.1021/ie50480a028. [DOI] [Google Scholar]
- Henning S.; Rosenørn T.; D’Anna B.; Gola A. A.; Svenningsson B.; Bilde M. Cloud droplet activation and surface tension of mixtures of slightly soluble organics and inorganic salt. Atmos. Chem. Phys. 2005, 5 (2), 575–582. 10.5194/acp-5-575-2005. [DOI] [Google Scholar]
- Sareen N.; Schwier A. N.; Shapiro E. L.; Mitroo D.; McNeill V. F. Secondary organic material formed by methylglyoxal in aqueous aerosol mimics. Atmos. Chem. Phys. 2010, 10 (3), 997–1016. 10.5194/acp-10-997-2010. [DOI] [Google Scholar]
- Li Z.; Williams A. L.; Rood M. J. Influence of Soluble Surfactant Properties on the Activation of Aerosol Particles Containing Inorganic Solute. J. Atmos. Sci. 1998, 55 (10), 1859–1866. . [DOI] [Google Scholar]
- Cruz C. N.; Pandis S. N. The effect of organic coatings on the cloud condensation nuclei activation of inorganic atmospheric aerosol. Journal of Geophysical Research: Atmospheres 1998, 103 (D11), 13111–13123. 10.1029/98JD00979. [DOI] [Google Scholar]
- Xi Yuan H.; Rosen M. J. Dynamic surface tension of aqueous surfactant solutions: I. Basic paremeters. J. Colloid Interface Sci. 1988, 124 (2), 652–659. 10.1016/0021-9797(88)90203-2. [DOI] [Google Scholar]
- Köhler H. The nucleus in and the growth of hygroscopic droplets. Trans. Faraday Soc. 1936, 32, 1152–1161. 10.1039/TF9363201152. [DOI] [Google Scholar]
- Engelhart G. J.; Asa-Awuku A.; Nenes A.; Pandis S. N. CCN activity and droplet growth kinetics of fresh and aged monoterpene secondary organic aerosol. Atmos. Chem. Phys. 2008, 8 (14), 3937–3949. 10.5194/acp-8-3937-2008. [DOI] [Google Scholar]
- Sorjamaa R.; Svenningsson B.; Raatikainen T.; Henning S.; Bilde M.; Laaksonen A. The role of surfactants in Köhler theory reconsidered. Atmos. Chem. Phys. 2004, 4 (8), 2107–2117. 10.5194/acp-4-2107-2004. [DOI] [Google Scholar]
- Prisle N. L.; Asmi A.; Topping D.; Partanen A. I.; Romakkaniemi S.; Dal Maso M.; Kulmala M.; Laaksonen A.; Lehtinen K. E. J.; McFiggans G.; Kokkola H. Surfactant effects in global simulations of cloud droplet activation. Geophys. Res. Lett. 2012, 10.1029/2011GL050467. [DOI] [Google Scholar]
- Gérard V.; Nozière B.; Baduel C.; Fine L.; Frossard A. A.; Cohen R. C. Anionic, Cationic, and Nonionic Surfactants in Atmospheric Aerosols from the Baltic Coast at Askö, Sweden: Implications for Cloud Droplet Activation. Environ. Sci. Technol. 2016, 50 (6), 2974–2982. 10.1021/acs.est.5b05809. [DOI] [PubMed] [Google Scholar]
- Asa-Awuku A.; Engelhart G. J.; Lee B. H.; Pandis S. N.; Nenes A. Relating CCN activity, volatility, and droplet growth kinetics of β-caryophyllene secondary organic aerosol. Atmos. Chem. Phys. 2009, 9 (3), 795–812. 10.5194/acp-9-795-2009. [DOI] [Google Scholar]
- Huff Hartz K. E.; Rosenørn T.; Ferchak S. R.; Raymond T. M.; Bilde M.; Donahue N. M.; Pandis S. N. Cloud condensation nuclei activation of monoterpene and sesquiterpene secondary organic aerosol. J. Geophys. Res. Atmos. 2005, 110, D14208. 10.1029/2004JD005754. [DOI] [Google Scholar]
- Tang X.; Cocker D. R. III; Asa-Awuku A. Are sesquiterpenes a good source of secondary organic cloud condensation nuclei (CCN)? Revisiting β-caryophyllene CCN. Atmos. Chem. Phys. 2012, 12 (18), 8377–8388. 10.5194/acp-12-8377-2012. [DOI] [Google Scholar]
- Morris H. S.; Grassian V. H.; Tivanski A. V. Humidity-dependent surface tension measurements of individual inorganic and organic submicrometre liquid particles. Chemical Science 2015, 6 (5), 3242–3247. 10.1039/C4SC03716B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdek B. R.; Power R. M.; Simpson S. H.; Reid J. P.; Royall C. P. Precise, contactless measurements of the surface tension of picolitre aerosol droplets. Chemical Science 2016, 7 (1), 274–285. 10.1039/C5SC03184B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebben C. J.; Strick B. F.; Upshur M. A.; Chase H. M.; Achtyl J. L.; Thomson R. J.; Geiger F. M. Towards the identification of molecular constituents associated with the surfaces of isoprene-derived secondary organic aerosol (SOA) particles. Atmos. Chem. Phys. 2014, 14 (5), 2303–2314. 10.5194/acp-14-2303-2014. [DOI] [Google Scholar]
- Ho J.; Psciuk B. T.; Chase H. M.; Rudshteyn B.; Upshur M. A.; Fu L.; Thomson R. J.; Wang H.-F.; Geiger F. M.; Batista V. S. Sum Frequency Generation Spectroscopy and Molecular Dynamics Simulations Reveal a Rotationally Fluid Adsorption State of α-Pinene on Silica. J. Phys. Chem. C 2016, 120, 12578–12589. 10.1021/acs.jpcc.6b03158. [DOI] [Google Scholar]
- Chase H. M.; Ho J.; Upshur M. A.; Thomson R. J.; Batista V. S.; Geiger F. M. Unanticipated Stickiness of α-Pinene. J. Phys. Chem. A 2017, 121, 3239–3246. 10.1021/acs.jpca.6b12653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.