Abstract
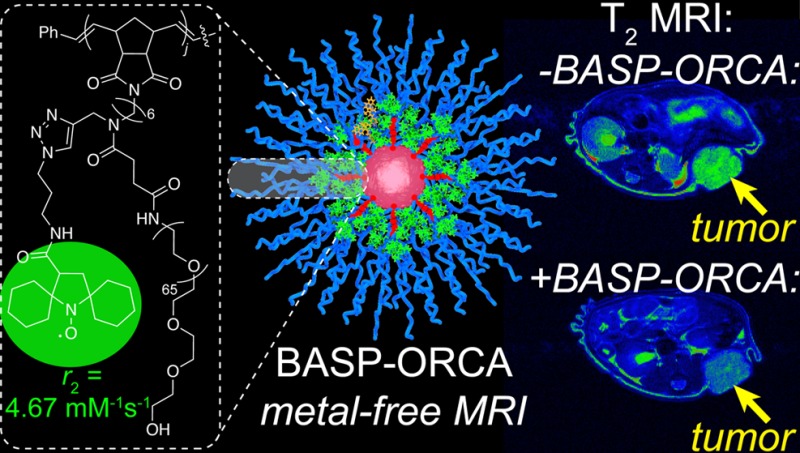
Metal-free magnetic resonance imaging (MRI) agents could overcome the established toxicity associated with metal-based agents in some patient populations and enable new modes of functional MRI in vivo. Herein, we report nitroxide-functionalized brush-arm star polymer organic radical contrast agents (BASP-ORCAs) that overcome the low contrast and poor in vivo stability associated with nitroxide-based MRI contrast agents. As a consequence of their unique nanoarchitectures, BASP-ORCAs possess per-nitroxide transverse relaxivities up to ∼44-fold greater than common nitroxides, exceptional stability in highly reducing environments, and low toxicity. These features combine to provide for accumulation of a sufficient concentration of BASP-ORCA in murine subcutaneous tumors up to 20 h following systemic administration such that MRI contrast on par with metal-based agents is observed. BASP-ORCAs are, to our knowledge, the first nitroxide MRI contrast agents capable of tumor imaging over long time periods using clinical high-field 1H MRI techniques.
Short abstract
Nitroxide-based brush-arm star-polymer organic radical contrast agents—rush-arm star polymer organic radical contrast agents—display high transverse relaxivity and stability against reduction, which enables metal-free in vivo magnetic resonance imaging of tumors in mice 20 h following systemic administration.
Introduction
Among the many imaging modalities for medical diagnostics, magnetic resonance imaging (MRI) is one of the most useful thanks to its ability to noninvasively generate three-dimensional detailed anatomical images with high spatial resolution while not requiring an ionizing source and remaining insensitive to depth.1−4 Current clinical MRI methods depict the spatial distribution and chemical environment of water protons (1H) within a region of interest (ROI); to enhance the differences between native water 1H and ROIs, contrast agents are often employed. These contrast agents are divided into two primary classes: T1 contrast agents (e.g., paramagnetic metals such as gadolinium or manganese) that afford positive-contrast images primarily by locally reducing the water 1H longitudinal relaxation time (spin–lattice, T1), and T2 contrast agents (e.g., superparamagnetic iron oxide nanoparticles) that afford negative-contrast images by locally reducing the water 1H transverse relaxation time (spin–spin, T2).5,6 The corresponding water 1H relaxivities (r1 and r2, respectively) of a contrast agent characterize the extent to which the agent decreases the T1 and T2 times of water 1H. Contrast agents with greater r1 and r2 values provide increased image contrast compared to those with lower values at the same concentration.6,7
Most MRI contrast agents with large r1 and/or r2 values contain metals that possess several unpaired electrons. For example, small molecule8−13 and nanoparticle-based14−21 contrast agents featuring Gd, Mn, Fe-oxide, and other metals have been reported to function as either T1 or T2 contrast agents or both. Furthermore, metal-based contrast agents that display advanced functions such as multimodal imaging,8−10,12,13,17,20,21 enhanced target-specific accumulation,14,18,19 and/or sensing8,11−14 have been developed. Despite their unquestionable utility, metal-based contrast agents, especially nanoparticle ones that tend to accumulate in biological tissues, may present toxicity concerns in some patient populations. For example, Gd-based agents, perhaps the most widely used T1 contrast agents in the clinic, are associated with potentially lethal nephrogenic systemic fibrosis, and they have recently been linked to a rising prevalence of toxic Gd ions in the environment.5,22−28 In addition, several T2 contrast agents based on Fe-nanoparticles have been stopped from further development or withdrawn from the market due to safety concerns.29−32 Moreover, according to the FDA, Fe-based products including ferumoxytol (Feraheme), the only FDA-approved superparamagnetic iron oxide nanoparticle currently available on the market, carry a risk of potentially life-threatening allergic reactions.33−35 Thus, there is extensive interest in the development of “metal-free” MRI contrast agents that make use of entirely organic-based components. Such agents could enable MRI in at-risk patient populations, and they could potentially open new avenues for functional/responsive MRI based on in vivo organic transformations. Furthermore, organic nanoparticle contrast agents could provide safe alternatives in MR imaging applications that may require long-term tissue accumulation, such as tumor imaging.
Four main classes of metal-free MRI contrast agents have been the most widely studied: paramagnetic nitroxide-based organic radical contrast agents (ORCAs), hyperpolarized 13C agents, 19F MRI contrast agents, and chemical exchange saturation transfer (CEST) contrast agents. While 19F MRI and CEST agents have undergone many advances in recent years,36−43 these approaches often suffer from low sensitivity, and in some cases, require a high contrast agent concentration (10–50 mM), long imaging times, and/or potentially harmful high-intensity radio frequency fields. Hyperpolarized 13C agents, on the other hand, can theoretically afford up to 105 sensitivity improvements; nevertheless, issues including short hyperpolarization lifetimes that lead to limited imaging times, complexity in terms of the chemistry and instrumentation required for generation of the hyperpolarized agent, and a rather small substrate scope remain major challenges.44−46 Furthermore, 19F MRI, CEST, and hyperpolarized 13C agents rely on imaging mechanisms that are not currently common in the clinic.44−51 In contrast, nitroxide ORCAs rely on standard water relaxation mechanisms to achieve MRI contrast; they could in principle be immediately translated to clinical applications. However, several key challenges limit the clinical feasibility of nitroxide ORCAs. First, nitroxide radicals only possess one unpaired electron. As a result, compared to metal-based contrast agents such as Gd3+ (seven unpaired electrons) or Mn2+ (five unpaired electrons), nitroxide ORCAs inherently suffer from much lower water 1H relaxivity. One strategy to achieve higher molecular relaxivity is to use a poly(nitroxide) where the relatively low per nitroxide relaxivity is multiplied by the number of nitroxides bound to a polymer scaffold. The second major limitation of nitroxide ORCAs is that they are typically reduced rapidly in vivo (half-lives on the order of minutes) to diamagnetic hydroxylamines, thus rendering them ineffective as contrast agents shortly after injection.52−55 Initial efforts to utilize nitroxides as MRI contrast agents exposed these shortcomings,56,57 and though their rapid bioreduction has been cleverly exploited to enable redox-mapping in vitro and in vivo,58−62 an in vivo-stable nitroxide ORCA that allows for longitudinal studies over clinically meaningful time scales following systemic administration has yet to be developed.
Macromolecular nitroxide ORCAs with long-term in vivo stability could be particularly useful for tumor imaging. Nanoparticles of suitable size (∼10–200 nm) are known to passively accumulate in tumors, especially in murine models, via the enhanced permeation and retention effect, but hours to tens of hours are often needed to reach maximal accumulation.63−69 To our knowledge, there are no nitroxide-based molecules or materials with demonstrated capability to provide in vivo MRI contrast after such long times. This problem is exacerbated in murine models where imaging is often used for preclinical studies of disease development: murine tissues contain higher levels of metabolic antioxidants, which lead to faster nitroxide reduction rates.70,71 Thus, the development of stable nitroxide-based macromolecular ORCAs with high relaxivities could open a new arena of MRI applications, whereby the accumulation of contrast agents in diseased tissues could be monitored by MRI without off-site toxicity concerns.55,72,73 Moreover, the synthetic versatility of polymeric materials could facilitate future image-guided drug delivery strategies.
Herein, we report the design, synthesis, and biological evaluation of a new class of nitroxide macromolecules—brush-arm star polymer ORCAs (BASP-ORCAs)—with unique structures that are designed to overcome the aforementioned challenges associated with tumor MRI with nitroxide-based contrast agents. BASP-ORCAs contain a high concentration of reduction-resistant nitroxide groups bound in an interlayer between a poly(ethylene glycol) (PEG) shell and a polyacetal core. Due to their shielded and dense nitroxide layer, yet hydrophilic PEGylated nanostructures, BASP-ORCAs simultaneously possess the highest known water 1H transverse relaxivities and stabilities for nitroxide ORCAs. In addition, the modularity of BASP synthesis was exploited to install near-infrared fluorophores into BASP-ORCAs and thereby achieve near-infrared fluorescence (NIRF) imaging in concert with MRI. Leveraging this combination of features, BASP-ORCAs were successfully employed for longitudinal MR and NIRF imaging of tumors with MRI contrast enhancement on par with metal-based contrast agents observed up to 1 day following systemic administration, which has, to our knowledge, never been achieved with a paramagnetic organic agent. Notably, though previous studies on nitroxide MRI contrast agents focused on T1-weighted imaging, BASP-ORCAs operate most effectively as T2 contrast agents, which is advantageous given that high-field instruments are being increasingly adopted in the clinic, and r2 often remains similar or increases with magnetic field strength.74 Thus, BASP-ORCAs not only overcome the challenges that have plagued all previous nitroxide-based MRI contrast agents, and thereby facilitate the first longitudinal imaging of tumors with a nitroxide ORCA, but they are also naturally amenable to current and future clinical high-field MRI instruments.
Results and Discussion
BASP-ORCA Design and Synthesis
One of the most common ways to increase the relaxivity of MRI contrast agents (including nitroxides) involves attaching them to a rigid macromolecular scaffold.6,47,52,53,75−78 For example, Rajca and co-workers appended a spirocyclohexyl nitroxide derivative (“chex”)79 to the surface of dendrimers to produce chex-dendrimer ORCAs where the per-chexr1 was 0.42 mM–1 s–1 compared to r1 = 0.14 mM–1 s–1 for the model nitroxide 3-carboxy-2,2,5,5-tetramethyl-l-pyrrolidinyloxy (3-CP).52,53 In a later study, we appended chex to the core of PEGylated branched-bottlebrush polymers.80 The resulting “chex-bottlebrush” had a per-chexr1 of 0.32 mM–1 s–1, which was approximately 50% greater than the chex-macromonomer used to synthesize these polymers (chex-MM, Figure 1a). In this system, r2 also increased from 0.30 mM–1 s–1 for chex-MM to 0.82 mM–1 s–1 for the chex-bottlebrush polymer, thus demonstrating that increasing the macromolecular size and chex density leads to increases in both r1 and r2, with a greater increase in r2.80 In an effort to further increase these relaxivity values, we sought to incorporate chex into our BASP macromolecules wherein the nitroxides would be bound at a rigid core–shell interface.80−83 On the basis of this novel structure compared to previous systems, we hypothesized that BASPs could provide enhanced relaxivity and nitroxide stability potentially making tumor imaging in vivo possible. Moreover, the control and robustness of BASP synthesis would enable the scalable production of BASP-ORCAs with optimal sizes for tumor accumulation, which is difficult with previous macromolecular systems such as dendrimers and bottlebrush polymers.84−86
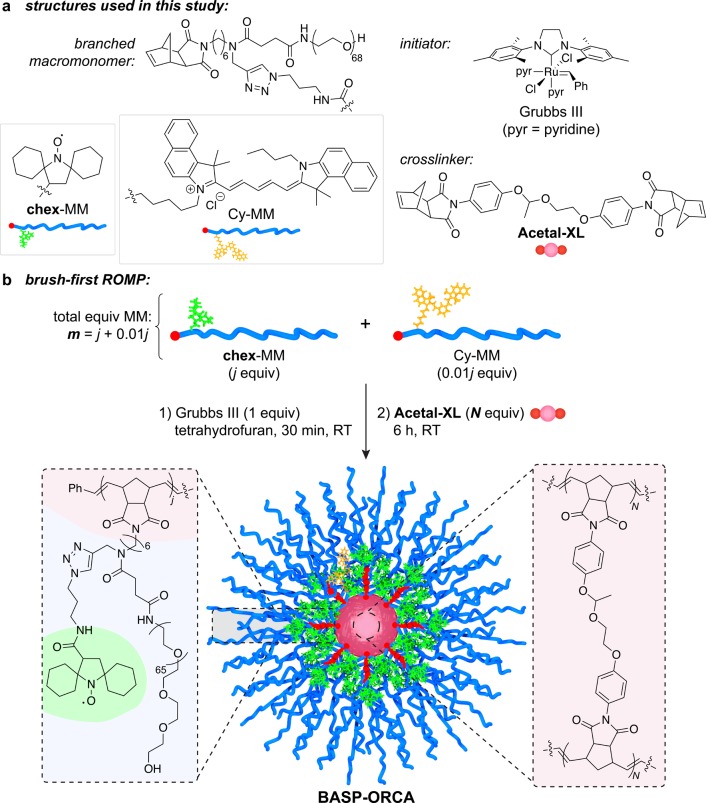
Figure 1.
(a) Chemical structures of BASP components studied in this work. (b) General brush-first ROMP procedure. Branched MMs chex-MM and Cy-MM are combined in the ratio j : 0.01j. This combination of MMs is exposed to 1.0 equiv of Grubbs III initiator to produce a living bottlebrush with an average degree of polymerization (DP) = j + 0.01j = m. N equiv of acetal-XL is then added (in aliquots of 5 equiv of acetal-XL every 5 min) to provide the final BASP-ORCA. The properties of the BASP-ORCAs are defined by their m and N values (Table 1).
BASP-ORCAs were synthesized by brush-first ring-opening metathesis polymerization (ROMP) as depicted in Figure 1.84,86−88 Norbornene-based branched macromonomers (MMs, Figure 1a) featuring 3 kDa PEG and either chex (chex-MM) or Cy5.5 dye (Cy-MM, Figure 1a) were copolymerized by exposure to Grubbs third-generation bis-pyridine initiator89 (Grubbs III, Figure 1a; reaction stoichiometry: j equiv. chex-MM to 0.01j Cy-MM to 1.0 Grubbs III) for 30 min (Figure 1b). The resulting living bottlebrush polymers with an average degree of polymerization (DP) of ∼j + 0.01j = m were then cross-linked via portionwise addition of N equiv of bis-norbornene acetal cross-linker acetal-XL(84) (Figure 1a) to the reaction mixture to generate the desired BASP-ORCA (Figure 1b). With this method, the BASP-ORCA size is determined by the MM to Grubbs III to acetal-XL ratios (i.e., m and N values). Much less Cy-MM (0.01j) relative to chex-MM (j) was used to bridge the difference in concentration requirements between MRI (mM to μM) and NIRF (nM to pM).1,6
To identify optimal conditions for the synthesis of BASP-ORCAs with narrow size distributions and average diameters of ∼25–40 nm, as well as high water solubility and relaxivity, we screened m and N values from 5−10 and 15–30, respectively (Table 1). Gel permeation chromatography (GPC) revealed nearly quantitative MM-to-bottlebrush conversion as well as ≥85% bottlebrush-to-BASP conversion for all m and N values (Figure S1). The BASP-ORCA diameters as determined by dynamic light scattering (DLS) and transmission electron microscopy (TEM) ranged from ∼28 to ∼49 nm (Table 1). In general, for the same bottlebrush arm length (m), the BASP-ORCA size increased with the amount of acetal-XL added (N). In addition, the BASP-ORCA aqueous solubility (Table 1) increased with m. A representative TEM image for the m = 7.07 and N = 20 BASP-ORCA (referred to as BASP-ORCA1 throughout the remainder of this work) is provided in Figure 2a. The aqueous solubility of BASP-ORCA1 was the highest amongst the BASP-ORCAs prepared, and its hydrodynamic diameter (Dh) of 31 ± 4 nm is suitable for extended in vivo circulation and tumor accumulation.66−68
Table 1. Characterization Data for BASP-ORCAs and Control Compounds.
| composition |
diameter |
relaxivity |
|||||
|---|---|---|---|---|---|---|---|
| name | m | N | Dh/nm | DTEM/nm | r1/mM–1 s–1 | r2/mM–1 s–1 | notes |
| 3-CPa | 0.15 | 0.17 | |||||
| chex-MMb | 0.21 | 0.30 | |||||
| chex-dendrimera | 0.44 | 0.86 | |||||
| chex-bottlebrushb | 55.55 | 17b | n.d. | 0.32 | 0.82 | ||
| BASP-ORCA | 5.05 | 20 | 31 ± 2 | n.d. | 0.27 | 6.92 | poor solubility (<10 mg/mL) |
| BASP-ORCA | 5.05 | 30 | 49 ± 6 | n.d. | 0.53 | 7.11 | poor solubility (<10 mg/mL) |
| BASP-ORCA1 | 7.07 | 20 | 31 ± 4 | 37 ± 7 | 0.41 | 4.67 | good solubility (>50 mg/mL) |
| BASP-ORCA | 7.07 | 30 | 36 ± 3 | n.d. | 0.35 | 7.40 | poor solubility (<10 mg/mL) |
| BASP-ORCA | 9.99 | 15 | 28 ± 3 | 38 ± 10 | 0.33 | 2.90 | low relaxivity |
| BASP-ORCA | 9.99 | 30 | 33 ± 4 | 39 ± 10 | 0.37 | 4.52 | low relaxivity |
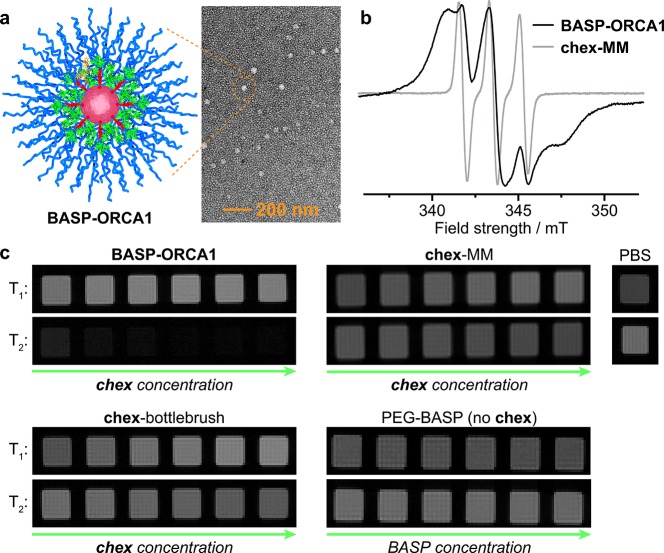
Figure 2.
(a) Transmission electron microscopy image of BASP-ORCA1 (DTEM = 37 ± 7 nm) after being negatively stained with uranyl acetate; the reported diameter (DTEM) represents the mean and standard deviation of >150 individual particle measurements. (b) Electron paramagnetic resonance (EPR) spectra for BASP-ORCA1 and chex-MM. (c) T1 and T2-weighted MRI phantoms for BASP-ORCA1, chex-MM, PBS buffer, chex-bottlebrush, and a PEG-BASP lacking chex. The concentration of chex-containing samples (BASP-ORCA1, chex-MM, and chex-bottlebrush) ranges from 1 mM to 4 mM chex. The concentration of PEG-BASP lacking chex ranges from 6 mg/mL to 21 mg/mL, which is equivalent to the mass per volume concentration range of BASP-ORCA1.
Characterization of BASP-ORCA Magnetic Properties
Electron paramagnetic resonance spectroscopy (EPR) was used to confirm the presence of chex in BASP-ORCAs, as well as to study the chex environment in BASP-ORCA1. The spin concentrations were ≥85% for all BASP-ORCAs. The height-normalized EPR spectra for BASP-ORCA1 and chex-MM80 are shown in Figure 2b. The spectrum for BASP-ORCA1 is significantly broader than chex-MM, which is consistent with the larger and more rigid BASP nanostructure where chex is bound at the dense interface between the acetal cross-linker core and the PEG shell (Figures 1b and 2b). The BASP-ORCA1 spectrum was simulated using the procedure developed by Budil, Freed, and co-workers90 (see Supporting Information section A for details), which allows for characterization of the chex mobility in terms of the correlation time for rotational diffusion (τ). The spectrum was best fitted by superimposing two computed components (Figure S3): 22% corresponded to a relatively fast-moving nitroxide with τ = 0.2 ns, while 78% corresponded to a slow-moving nitroxide with τ = 10.0 ns. The faster-moving component likely corresponds to nitroxides that are furthest from the BASP-ORCA1 acetal core (Figure 1b), while the slow-moving component corresponds to nitroxides that are close to and/or entangled within the acetal core. Notably, the τ of 10.0 ns measured for the slow component in BASP-ORCA1 is quite large, which suggests that a majority of the chex groups are in a rigid environment. For comparison, in our previously reported chex-dendrimer ORCAs,52,53 TEMPO-labeled bottlebrush polymers,81,82 and BASPs,83 the largest τ measured was ∼1 ns.
Next, we evaluated the longitudinal (r1) and transverse (r2) relaxivities of these BASP-ORCAs using a Bruker 7 T MRI scanner. The per-chexr1 values as a function of m and N (Table 1) ranged from 0.27 to 0.53 mM–1 s–1; they were not significantly increased compared to Rajca’s chex-dendrimer and our chex-bottlebrush polymers. However, the per-chexr2 values ranged from 2.90 to 7.40 mM–1 s–1, which is ∼3.5- to ∼9.0-fold greater than the per-chexr2 in our chex-bottlebrush polymers and ∼17- to ∼44-fold greater than 3-CP (Table 1).80BASP-ORCA1 displayed a per-chexr2 value of 4.67 mM–1 s–1. Though this value was not the highest we measured, we selected BASP-ORCA1 for translation to biological studies because it offered the best balance of high relaxivity, solubility (greater than 50 mg/mL, Table 1), and size. Given the number-average molar mass of BASP-ORCA1 as determined by gel permeation chromatography and static light scattering (Mn = 4.75 × 105 g/mol, Đ = 1.32), we estimate that each BASP-ORCA1 particle contains an average of 92 chex groups. Thus, the estimated average molecular r1 and r2 values for BASP-ORCA1 are 37.6 mM–1 s–1 and 428.8 mM–1 s–1, respectively, which are greater than those for the commonly used FDA-approved Gd-based contrast agent Magnevist (r1 = 3.1 mM–1 s–1 and r2 = 5.4 mM–1 s–1 at 7 T) and iron-based nanoparticles such as Feraheme (r1 = 3.1 mM–1 s–1 and r2 = 68 mM–1 s–1 at 7 T).91−94
MR phantom images of phosphate-buffered saline (PBS) solutions of BASP-ORCA1, chex-MM, and our previously reported chex-bottlebrush polymer at various chex concentrations (from 1 mM−4 mM chex) as well as a PEG-BASP that lacks chex (at equivalent mass fractions to BASP-ORCA1) are provided in (Figure 2c), along with images for “blank” PBS buffer. The T1-weighted images for BASP-ORCA1, and chex-bottlebrush polymer are not obviously different, while the T2-weighted images clearly show a large reduction in signal for BASP-ORCA1. The PEG-BASP with no chex shows no difference in contrast as a function of concentration, which confirms that chex is required to observe changes in image contrast.
The data presented above demonstrate that the high nitroxide density of BASP-ORCA1, which is a consequence of its unique cross-linked multilayer nanostructure, affords an increased magnetization capability that leads to r2 enhancement. This finding is consistent with reports where nitroxides are utilized as magnetic catalysts for outer-sphere relaxation processes.95−97 Most importantly, the exceptionally high r2 of BASP-ORCA1 overcomes one of the major limitations of nitroxide-based contrast agents: inherently low contrast.
Ascorbate Quenching Kinetics of BASP-ORCAs
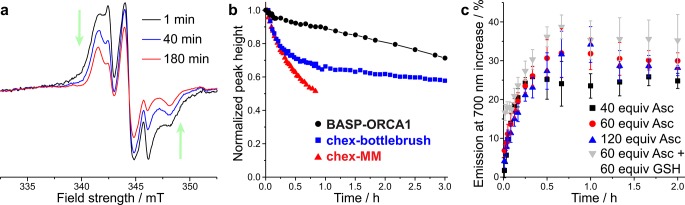
As discussed above, nitroxide-based ORCAs typically suffer from rapid reduction to diamagnetic hydroxylamines under biologically relevant conditions. Among the many potential biological reducing agents, ascorbate (Asc) is known to play a major role in in vivo nitroxide reduction,54,98,99 and Asc-induced reduction can be amplified by glutathione (GSH).80,99 We hypothesized that the rigid chex environment in our BASP-ORCAs could help to lower the rate of chex reduction. To test this hypothesis, we collected EPR spectra for BASP-ORCA1 at various times following exposure to 20 equiv of Asc and 20 equiv of GSH per nitroxide (both reagents were present in 10 mM concentrations). EPR spectra collected 1, 40, and 180 min after exposure to these conditions are provided in Figure 3a. The changes in peak height as a function of time are indicative of nitroxide reduction. The normalized peak height of the EPR spectra are plotted versus time in Figure 3b. Reduction kinetics data for our previous chex-bottlebrush polymers and chex-MM are provided for comparison.80 In contrast to the chex-bottlebrush and chex-MM samples, which both display an initial rapid chex reduction phase in the first hour, the reduction of chex in BASP-ORCA1 was significantly retarded with nearly 85% remaining after 1 h, and 70% remaining after 3 h (compared to 65% and 57%, respectively, for the chex-bottlebrush). On the basis of the integrated peak heights as a function of time, the second-order rate constants for BASP-ORCA1 reduction in the initial (first 10 min) and late (>1 h) stages of the reduction process were calculated: kearly = 0.0376 M–1 s–1 and klate ≈ 0.00672 M–1 s–1) (Table S1).52,53,80 Simulations revealed that the EPR spectra collected during the reduction process consisted of a “fast” and a “slow” component (Figure S3). Interestingly, τ for the “fast” component remained constant at 0.2 ns, while τ for the “slow” component became increasingly larger with time (11.0 ns at 40 min and 13.2 ns at 180 min). Therefore, even after 3 h there persists an extremely reduction resistant and slow moving nitroxide population. The presence of these very stable nitroxides within BASP-ORCA1 may enable T2-weighted MRI over longer time scales than have been possible with previous nitroxide contrast agents (vide infra).
Figure 3.
(a) EPR spectra for BASP-ORCA1 1, 40, and 180 min following exposure to 20 equiv of sodium ascorbate (Asc) per nitroxide. (b) Ascorbate reduction kinetics for BASP-ORCA1, chex-bottlebrush, and chex-MM. (c) Cy5.5 emission at 700 nm in response to Asc and glutathione (GSH); the reported values represent the mean and standard error of the mean (SEM) (n = 3).
Fluorescence Properties of BASP-ORCAs
As noted above, Cy5.5 was also incorporated into these BASP-ORCAs (see Figure S4 for BASP-ORCA1 absorption and emission spectra confirming the presence of Cy5.5) in order to simultaneously use NIRF as an imaging modality for comparison to MRI. Nitroxides are well-known to quench fluorescence via catalysis of nonemissive photophysical processes such as intersystem crossing. This quenching requires close interaction between the nitroxide and the fluorophore; the systems with the greatest quenching typically feature the nitroxide directly linked to the fluorophore via π bonds (i.e., electronic conjugation).100−102 Given the fact that chex and Cy5.5 are incorporated into BASP-ORCAs via two different macromonomers and that the mobility of chex is limited in these constructs, we reasoned that Cy5.5 quenching would be minimal; therefore, we could potentially use Cy5.5 emission as a fairly constant descriptor of particle concentration regardless of the extent of chex reduction.
To test this hypothesis, we exposed BASP-ORCA1 to a large excess of Asc (40–120 equiv. to chex) in water, and monitored the resulting Cy5.5 emission. In agreement with our expectation, only a 25 ± 2% to 30 ± 2% increase in fluorescence emission was observed (Figure 3c). Moreover, addition of GSH (60 equiv) as a coreductant along with 60 equiv of Asc gave only a 35 ± 7% increase in fluorescence. Taken together, these data suggest that Cy5.5 fluorescence is minimally quenched by chex in BASP-ORCA1. For comparison, exposure of our previously reported chex-bottlebrush polymer containing Cy5.5 to excess Asc or Asc+GSH led to 119 ± 5% and 250 ± 5% increases in fluorescence, respectively.80 Notably, the time required to achieve a fluorescence plateau varied significantly between BASP-ORCA1 (approximately 40 min) and our chex-bottlebrush polymer (a few minutes). Collectively, these data suggest that the BASP nanostructure provides greater steric shielding and isolation of chex and Cy5.5 compared to the chex-bottlebrush polymer.
In Vitro Cytotoxicity and in Vivo Gross Toxicity, Pharmacokinetics (PK), and Biodistribution (BD) of BASP-ORCA1 in Non-Tumor-Bearing Mice
Next, we investigated the performance of BASP-ORCA1 in biological assays. As discussed above, one potential advantage of ORCAs is their low toxicity. To assess the toxicity of BASP-ORCA1, we first conducted in vitro human umbilical vein endothelial cell (HUVEC) and HeLa cell viability assays. In these assays, the cells were incubated with varied concentrations of BASP-ORCA1 for 72 h. Cell viability was determined by the CellTiter-Glo assay (Supplemental Figure S5). The half-maximal inhibitory concentrations of BASP-ORCA1, i.e., the concentrations that led to 50% cell death, were 1.5 mg/mL (280 μM chex) and 4.5 mg/mL (830 μM chex) in HUVEC and HeLa cells, respectively. These results confirm that BASP-ORCA1 induces negligible in vitro cytotoxicity at practical concentrations.85,86 Next, the in vivo gross toxicity of BASP-ORCA1 was assessed. Healthy BALB/c mice were administered increasing doses (from 5 to 30 mg or 0.2 to 1.5 g/kg, respectively) of BASP-ORCA1 via tail vein injection. The animal body masses and behaviors were monitored over the course of 30 days. Loss of ≥10% body mass is generally considered to be a sign of unacceptable toxicity.103,104 As shown in Figure S6, even the highest dose of BASP-ORCA1 (administered to n = 4 animals) induced no significant decrease in body mass, which suggests that these particles are well-tolerated up to their solubility-limiting dose.
The pharmacokinetics (PK) and biodistribution (BD) of BASP-ORCA1 were monitored in healthy, nontumor bearing BALB/c mice (n = 3) using NIRF imaging (IVIS, Cy5.5 λex/λem = 640/700 nm). For PK analysis, blood samples were collected via cardiac puncture at various time points from 1 to 48 h. Percent injected dose was plotted as a function of time (Figure S7a). As is common for spherical PEGylated nanostructures, BASP-ORCA1 exhibited a two-phase clearance behavior, with an early distribution phase of ∼6 h, followed by a steady elimination phase.67,86 Fitting the data presented in Figure S7a with a standard two-compartment model yielded a blood compartment half-life for BASP-ORCA1 of 10 h.105 This long half-life is attributed to the nanoscale size of BASP-ORCA1, which limits renal clearance, and its PEGylated corona.66,69 Consistent with these results and a plethora of studies on PEGylated nanoparticles,65−69 BD analysis revealed that a majority of BASP-ORCA1 accumulated in the liver, with increasing accumulation over 72 h (Figure S7b). Less accumulation in the kidney and negligible accumulation in other tissues was observed. Fluorescence in extracted lung tissue is attributed to a high concentration of BASP-ORCA1 in the blood. Notably, fluorescence images of fecal samples (Figure S7c) suggest that BASP-ORCA1 is ultimately cleared from the body via excretion.
BASP-ORCA1 BD in Tumor-Bearing Mice
Given the long circulation of BASP-ORCA1, we hypothesized that this particle would passively accumulate in subcutaneous tumors following systemic injection. To test this hypothesis, we first established a tumor model via subcutaneous injection of a mixture of 2.0 × 106 lung carcinoma cells (A549, ATCC), Matrigel, and PBS buffer into a hind flank of NCR-NU mice (n = 4). When the average tumor volume was ∼1 cm, BASP-ORCA1 was administered at a dose of 0.23 mmol chex/kg (1.2 g BASP-ORCA1/kg) via tail vein injection. NIRF images collected 20 h after administration indicated substantial tumor accumulation of BASP-ORCA1, which is consistent with other reports for PEGylated nanoparticles of similar size including our related drug-conjugated BASPs (Figure 4a).65,66,69,86Ex vivo BD data were consistent with our studies on nontumor bearing BALB/c mice (i.e., liver accumulation and persistence in blood) with the addition of significant tumor accumulation (Figure 4b and Figure S8).
Figure 4.

(a) In vivo NIRF images
of NCR nude mouse before
and 20 h after injection of BASP-ORCA1 (see Supporting Information for details). (b) Ex vivo NIRF images of selected organs (see Supporting Information for details). Units of
radiant efficiency:  .
.
MRI and NIRF Imaging with BASP-ORCA1 in Tumor-Bearing Mice
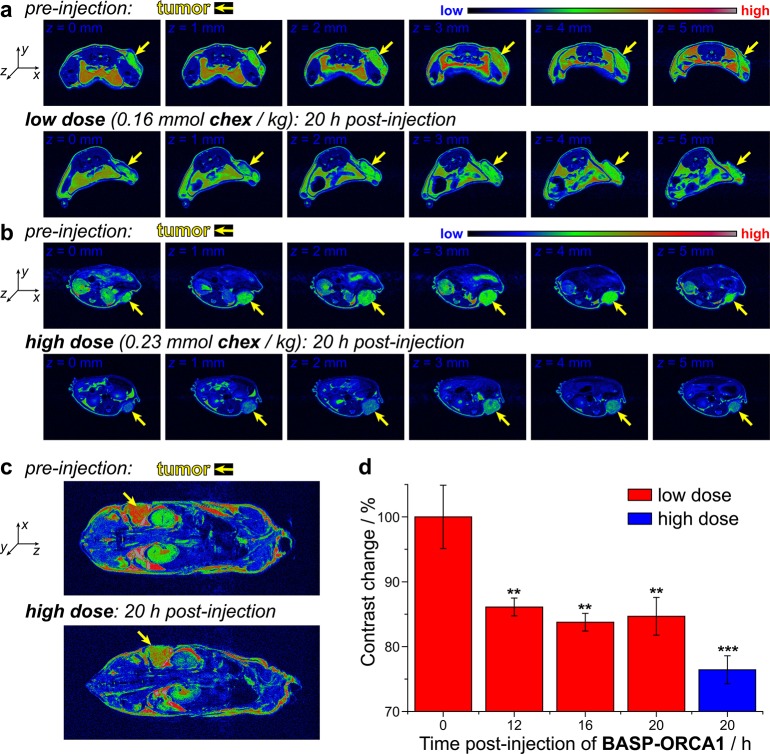
The low toxicity, long circulation half-life, and tumor accumulation of BASP-ORCA1, along with its exceptional stability and relaxivity, suggested that this particle could be suitable for MRI of tumors following systemic injection and accumulation; a feat that, to our knowledge, has not yet been achieved with ORCAs. Two groups of A459 tumor-bearing NCR-NU mice were administered different doses of BASP-ORCA1 via tail-vein injection: the “low dose” group (n = 3) received 0.16 mmol chex/kg (0.8 g BASP-ORCA1/kg), while the “high dose” group (n = 4) received 0.23 mmol chex/kg (1.2 g BASP-ORCA1/kg). The mice were anaesthetized and MR images were collected at various time points: 12, 16, and 20 h postinjection for the low dose group and 20 h postinjection for the high dose group. The images from each time point were compared to images collected before BASP-ORCA1 injection. Figures 5a shows T2-weighted false-colored images for a selected mouse from the low dose group imaged before BASP-ORCA1 injection (top row of images) and 20 h (bottom row of images) after BASP-ORCA1 injection. From left-to-right the images correspond to progressive slices of the same animal in the z-axis with the tumor indicated with a yellow arrow in each image. Figure 5b shows an analogous set of images for a selected mouse from the high dose group. Contrast differences between the preinjection and postinjection images can be observed at both dose levels, with greater contrast observed in the high dose animal (Figure 5b). Whole animal images similarly revealed a clear difference in tumor contrast (Figure 5c, yellow arrows).
Figure 5.
(a) T2-weighted MR images of tumor bearing NCR nude mouse before (top row) and 20 h after (bottom row) injection of 0.16 mmol chex/kg (“low dose”) of BASP-ORCA1. Each series of images corresponds to progressive slices in the z-axis through the tumor of the same mouse. (b) T2-weighted MR images of tumor bearing NCR nude mouse before (top row) and 20 h after (bottom row) injection of 0.23 mmol chex/kg (“high dose”) of BASP-ORCA1. Each series of images corresponds to progressive slices in the z-axis through the tumor of the same mouse. (c) T2-weighted coronal MR images before (top) and 20 h after (bottom) injection of 0.23 mmol chex/kg (“high dose”) of BASP-ORCA1. (d) Percent MRI contrast change at various times following BASP-ORCA1 injection compared to preinjection. Statistical comparisons (n = 3 for low dose group; n = 4 for high dose group; reported values represent mean ± SEM) to preinjection images were made with a student t test: **P ≤ 0.05, ***P ≤ 0.001.
The percent negative contrast enhancement (i.e., the amount of signal reduction) before and after BASP-ORCA1 administration was quantified by image analysis (Figure 5d). Signal reductions ranging from 14 ± 2% to 16 ± 2% (P ≤ 0.05) were observed for the 12 to 20 h time points in the low dose group (Figure 5d, red bars). In the high dose group, a 24 ± 2% (P ≤ 0.001) signal reduction was observed 20 h after BASP-ORCA1 administration (Figure 5d, blue bar). The BASP-ORCA1 dose–response effect suggests that the observed contrast differences between pre- and postinjection are due to accumulation of BASP-ORCA1 in the tumors. Keeping in mind that MRI phantoms revealed no observable contrast enhancement for PEG-BASPs that lack chex (Figure 2c), these MRI data imply that 20 h following injection there is a sufficient concentration of chex radicals present on the BASP-ORCA1 in the tumor to impart contrast. To confirm the presence of chex radicals in the tumors, the same mice that were imaged by MRI were sacrificed 21 h after BASP-ORCA1 administration and their tissue homogenates and blood were analyzed by EPR spectroscopy (Figure 6a). From these spectra, the radical concentration per gram of protein in each tissue sample, the latter obtained via a bicinchoninic acid assay (BCA), was evaluated and normalized by the radical concentration per gram of protein in muscle tissue (Figure 6b). In agreement with our MRI data, the concentration of free radicals in the tumor was quite high after BASP-ORCA1 injection; the measured value of 0.25 ± 0.04 μmol chex/g chex/g of protein corresponds to 4.5% of the injected dose of chex radicals. Moreover, consistent with our in vivo NIRF imaging results (vide supra), relatively high radical concentrations were observed in the liver and kidney, which suggests that the clearance of BASP-ORCA1 proceeded mostly through these organs. Notably, the murine liver contains a high concentration of Asc (millimolar range); our observation of radicals in the liver is further evidence of the extremely stable nature of the chex units in BASP-ORCA1 (Note: in our previous chex-bottlebrush polymers, there was very little chex radical in the liver following 30 min and none observed after 24 h). A high chex concentration was also observed in the heart, which is in accord with a long blood compartment half-life and is consistent with our PK data obtained by NIRF imaging. Finally, NIRF imaging of these homogenates provided fluorescence radiant efficiencies that were in good agreement with our spin concentrations (Figure 6b), which suggests that the chex radicals and Cy5.5 dyes are still colocalized within the BASP-ORCA1 construct after biodistribution. Unlike our previous chex-bottlebrush polymers, which displayed dramatic increases in fluorescence as chex was reduced, the signal uniformity offered by BASP-ORCA1 provides for straightforward multimodal confirmation of BD.
Figure 6.
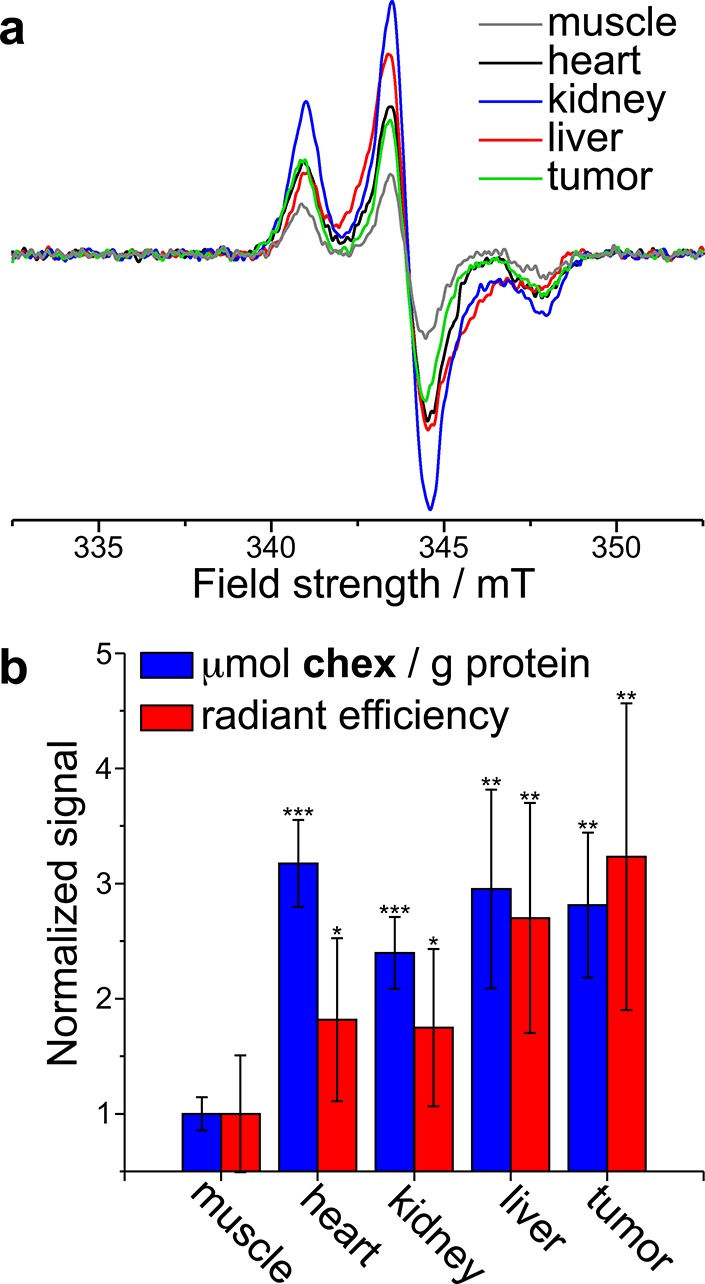
(a) EPR spectra obtained for homogenized tissue samples collected for the same mice imaged in Figure 5 22 h following BASP-ORCA1 injection. (b) Blue: Muscle-normalized concentration of chex per gram of protein as obtained from EPR double integration of tissue homogenates. Red: Muscle-normalized concentration of Cy5.5 in tissue homogenates as obtained from NIRF imaging. Statistical comparisons (n = 4; reported values represent mean ± SEM) to muscle signal were made with a Student’s t test: *not significant, **P ≤ 0.05, ***P ≤ 0.001.
To the best of our knowledge, BASP-ORCA1 is the first nitroxide MRI contrast agent capable of providing significant contrast 20 h after injection, which is a testament to its unique structural features that combine optimal size for tumor accumulation with a high nitroxide density and stability. To set these results in context, we compared our data to recent literature examples of MRI-contrast agents that rely on metals to achieve tumor imaging following systemic administration. For example, Kataoka and co-workers recently reported on a new class of Gd-based nanoparticles (T1 contrast agents) for MRI of tumors. In their study, a ∼40% contrast enhancement (at 0.05 mmol Gd/kg iv dose) was observed 4 h following injection into mice bearing subcutaneous C26 tumors. Notably, the commercially available small molecule contrast agent Gd-DTPA exhibited negligible contrast enhancement (at 0.23 mmol Gd/kg iv dose) after 4 h.16 This example highlights the importance of a nanoparticle system for extended circulation and tumor imaging. The same group reported Fe-based nanoparticles (T2 contrast agents) for tumor imaging in a similar murine model (subcutaneous C26 tumors). Here, an approximately 25% contrast difference was observed 24 h following intravenous administration of 0.45 mg Fe/kg. Notably, less than 10% contrast enhancement was observed using commercially available Resovist (at 0.45 mg Fe/kg intravenous dose).15 It should be noted that the instrument parameters used to obtain T2-weighted images in this work were similar to those used above in our studies; thus, our results for BASP-ORCA1 are on par with recently reported nanoparticle MRI contrast agents that rely on metals to achieve contrast.
Conclusion
We have developed a nitroxide-based macromolecular MRI contrast agent —BASP-ORCA1— that enables simultaneous MRI and NIRF imaging in vivo over time scales suitable for tumor imaging following systemic injection. BASP-ORCA1 addresses the two major challenges that have historically limited nitroxide-based organic radical contrast agents for MRI: low relaxivity and poor stability. These functions were made possible by the brush-arm star polymer (BASP) nanostructure, which places a dense layer of chex nitroxides at the interface between a rigid poly(acetal) core and a hydrophilic PEG shell. Altogether, BASP-ORCA1 displayed unprecedented per-nitroxide and per-molecule transverse relaxivities for organic radical contrast agents, exceptional stability, high water solubility, low in vitro and in vivo toxicity, and a long blood compartment half-life. These features combined to facilitate the imaging of subcutaneous tumors in mice 20 h after tail-vein injection, providing contrast enhancements on par with commercial and literature examples of metal-based contrast agents. This work suggests that organic radicals can be viable alternatives to metal-based MRI contrast agents, and sets the stage for the development of theranostic systems that combine organic radical contrast agents with therapeutic payloads to achieve simultaneous tumor imaging and drug delivery without concerns over long-term tissue accumulation of metals.
Acknowledgments
We thank the NIH-NIBIB (1R21EB018529-01A1 for J.A.J. and A.R.; R01 EB-019950-01A1 for A.R.) and the National Science Foundation (Graduate Research Fellowship for H.V.-T.N.) for support of this research. A.J. thanks the NIH (U01-NS090451). P.H. was supported by a Wellcome Trust-MIT Postdoctoral Fellowship. This work was supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. We thank the Koch Institute Swanson Biotechnology Center for technical support, specifically Dr. S. Malstrom and Ms. W. Huang. We thank Dr. A. Detappe and Dr. P. Ghoroghchian for very fruitful discussions and consultations.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.7b00253.
Synthesis and characterization data for BASPs, as well as supplementary figures, methods and materials, experimental procedures, in vitro, in vivo, and ex vivo supplementary data (PDF)
The authors declare no competing financial interest.
Dedication
¶ We dedicate this work to our co-author, Michael D. Boska, who passed away on May 13, 2017 in a one-man hang glider accident. Hang gliding was Mike's hobby and his dream come true for nearly 40 years. He spent his last moments doing what he loved. This loss is tremendous on multiple levels. Mike was an incredible asset to our research, the community, and the University of Nebraska Medical Center. His contributions will positively impact the medical field for years to come.
Supplementary Material
References
- Cheon J.; Lee J.-H. Synergistically Intergrated Nanoparticles as Multimodal Probes for Nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]
- Na H. B.; Song I. C.; Hyeon T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. 10.1002/adma.200802366. [DOI] [Google Scholar]
- Lee D.-E.; Koo H.; Sun I.-C.; Ryu J. H.; Kim K.; Kwon I.-C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. 10.1039/C2CS15261D. [DOI] [PubMed] [Google Scholar]
- Villaraza A. J. L.; Bumb A.; Brechbiel M. W. Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G.-L.; Kramberger I.; Davis J. J. Environmentally Responsive MRI Contrast Agents. Chem. Commun. 2013, 49, 9704–9721. 10.1039/c3cc44268c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastarone D. J.; Harrison V. S. R.; Eckermann A. L.; Parigi G.; Luchinat C.; Meade T. J. A Modular System for the Synthesis of Multiplexed Magnetic Resonance Probes. J. Am. Chem. Soc. 2011, 133, 5329–5337. 10.1021/ja1099616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravan P.; Ellison J. J.; McMurry T. J.; Lauffer R. B. Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- Tu C.; Nagao R.; Louie A. Y. Multimodal Magnetic-resonance/Optical-imaging Contrast Agent Sensitive to NADH. Angew. Chem., Int. Ed. 2009, 48, 6547–6551. 10.1002/anie.200900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison V. S. R.; Carney C. E.; MacRenaris K. W.; Meade T. J. A Multimeric MR-optical Contrast Agent for Multimodal Imaging. Chem. Commun. 2014, 50, 11469–11471. 10.1039/C4CC05651E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison V. S. R.; Carney C. E.; MacRenaris K. W.; Waters E. A.; Meade T. J. Multimeric Near IR-MR Contrast Agent for Multimodal in vivo Imaging. J. Am. Chem. Soc. 2015, 137, 9108–9116. 10.1021/jacs.5b04509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRenaris K. W.; Ma Z.; Krueger R. L.; Carney C. E.; Meade T. J. Cell-permeable Esterase-activated Ca(II)-Sensitive MRI Contrast Agent. Bioconjugate Chem. 2016, 27, 465–473. 10.1021/acs.bioconjchem.5b00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y.; Tomat E.; Hwang K.; Atanasijevic T.; Nam W.; Jasanoff A. P.; Lippard S. Manganese Displacement from Zinpyr-1 Allows Zinc Detection by Fluorescence Microscopy and Magnetic Resonance Imaging. Chem. Commun. 2010, 46, 4139–4141. 10.1039/c0cc00179a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Jing X.; Liu T.; Han G.; Li H.; Duan C. Dual-functional Gadolinium-based Copper(II) Probe for Selective Magnetic Resonance Imaging and Fluorescence Sensing. Inorg. Chem. 2012, 51, 2325–2331. 10.1021/ic202322f. [DOI] [PubMed] [Google Scholar]
- Mi P.; Kokuryo D.; Cabral H.; Wu H.; Terada Y.; Saga T.; Aoki I.; Nishiyama N.; Kataoka K. A pH-activatable Nanoparticle with Signal-aplification Capabilities for non-invasive Imaging of Tumor Malignancy. Nat. Nanotechnol. 2016, 11, 724–730. 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- Kokuryo D.; Anraku Y.; Kishimura A.; Tanaka S.; Kano M. R.; Kershaw J.; Nishiyama N.; Saga T.; Aoki I.; Kataoka K. SPIO-PICsome: Development of a Highly Sensitive and Stealth-capable MRI Nano-agent for Tumor Detection using SPIO-loaded Unilamellar Polyion Complex Vesicles (PICsomes). J. Controlled Release 2013, 169, 220–227. 10.1016/j.jconrel.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Mi P.; Kokuryo D.; Cabral H.; Kumagai M.; Nomoto T.; Aoki I.; Terada Y.; Kishimura A.; Nishiyama N.; Kataoka K. Hydrothermally Synthesized PEGylated Calcium Phosphate Nanoparticles Incorporating Gd-DTPA for Contrast Enhanced MRI Diagnosis of Solid Tumors. J. Controlled Release 2014, 174, 63–71. 10.1016/j.jconrel.2013.10.038. [DOI] [PubMed] [Google Scholar]
- Chou S.-W.; Shau Y.-H.; Wu P.-C.; Yang Y.-S.; Shieh D.-B.; Chen C.-C. in vitro and in vivo Studies of FePt Nanoparticles for Dual Modal CT/MRI Molecular Imaging. J. Am. Chem. Soc. 2010, 132, 13270–13278. 10.1021/ja1035013. [DOI] [PubMed] [Google Scholar]
- Holbrook R. J.; Rammohan N.; Rotz M. W.; MacRenaris K. W.; Preslar A. T.; Meade T. J. Gd(III)-dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. 10.1021/acs.nanolett.6b00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls F. J.; Rotz M. W.; Ghuman H.; MacRenaris K. W.; Meade T. J.; Modo M. DNA-gadolinium-gold Nanoparticles for in vivo T1MR Imaging of Transplanted Human Neural Stem Cells. Biomaterials 2016, 77, 291–306. 10.1016/j.biomaterials.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-S.; Lee J.-H.; Shin T.-H.; Song H.-T.; Kim E. Y.; Cheon J. Self-confirming “AND” Logic Nanoparticles for Fault-free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. 10.1021/ja104503g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medarova Z.; Pham W.; Farrar C.; Petkova V.; Moore A. in vivo Imaging of siRNA Delivery and Silencing in Tumors. Nat. Med. 2007, 13, 372–377. 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- Shellock F. G.; Kanal E. Safety of Magnetic Resonance Imaging Contrast Agents. J. Magn. Reson. Imaging. 1999, 10, 477–484. . [DOI] [PubMed] [Google Scholar]
- Swaminathan S.; Horn T. D.; Pellowski D.; Abul-Ezz S.; Bornhorst J. A.; et al. Nephrogenic Systemic Fibrosis, Gadolinium, and Iron Mobilization. N. Engl. J. Med. 2007, 357, 720–722. 10.1056/NEJMc070248. [DOI] [PubMed] [Google Scholar]
- Shin T.-H.; Choi Y.; Kim S.; Cheon J. Recent Advances in Magnetic Nanoparticle-based Multi-modal Imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. 10.1039/C4CS00345D. [DOI] [PubMed] [Google Scholar]
- Verwilst P.; Park S.; Yoon B.; Kim J. S. Recent Advances in Gd-chelate Based Bimodal Optical/MRI Contrast Agents. Chem. Soc. Rev. 2015, 44, 1791–1806. 10.1039/C4CS00336E. [DOI] [PubMed] [Google Scholar]
- Mendichovszky I. A.; Marks S. D.; Simcock C. M.; Olsen O. E. Gadolinium and Nephrogenic Systemic Fibrosis: Time to Tighten Practice. Pediatr. Radiol. 2008, 38, 489–496. 10.1007/s00247-007-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatje V.; Bruland K. W.; Flegal A. R. Increases in Anthropogenic Gadolinium Anomalies and Rare Earth Element Concentration in San Francisco Bay over a 20 Year Record. Environ. Sci. Technol. 2016, 50, 4159–4168. 10.1021/acs.est.5b04322. [DOI] [PubMed] [Google Scholar]
- Nardone B.; Saddleton E.; Laumann A. E.; Edwards B. J.; Raisch D. W.; McKoy J. M.; Belknap S. M.; Bull C.; Haryani A.; Cowper S. E.; Abu-Alfa A. K.; Miller F. H.; Godinez-Puig V.; Dharnidharka V. R.; West D. P. Pediatric Nephrogenic Systemic Fibrosis is Rarely Reported: a RADAR Report. Pediatr. Radiol. 2014, 44, 173–180. 10.1007/s00247-013-2795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We thank one of the reviewers for pointing out to us safety issues associated with T2 contrast agents based on iron-nanoparticles.
- Wang Y-X. J. Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application. Quant. Imaging. Med. Surg. 2011, 1, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-X. J. Current Status of Superparamagnetic Iron Oxide Contrast Agents for Liver Magnetic Resonance Imaging. World J. Gastroenterol. 2015, 21, 13400–13402. 10.3748/wjg.v21.i47.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.; Liu H.; Bhakoo K. K.; Lu L.; Chen Z. A Metabonomic Analysis of Organ Specific Response to USPIO Administration. Biomaterials 2011, 32, 6558–6569. 10.1016/j.biomaterials.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Vasanawala S. S.; Nguyen K.-L.; Hope M. D.; Bridges M. D.; Hope T. A.; Reeder S. B.; Bashir M. R. Safety and Technique of Ferumoxytol Administration for MRI. Magn. Reson. Med. 2016, 75, 2107–2111. 10.1002/mrm.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor A. S.; Jokerst J. V.; Ghanouni P.; Campbell J. L.; Mittra E.; Gambhir S. S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. 10.2967/jnumed.116.181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraheme (Ferumoxytol): Drug Safety Communication – Warnings Strengthened and Prescribing Instruction Changed; U. S. Food & Drug Administration (FDA), 2015, https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm440479.htm.
- Lim Y. T.; Noh Y.-W.; Cho J.-H.; Han J. H.; Choi B. S.; Kwon J.; Hong K. S.; Gokarna A.; Cho Y.-H.; Chung B. H. Multiplexed Imaging of Therapeutic Cells with Multispectrally Encoded Magnetofluorescent Nanocomposite Emulsions. J. Am. Chem. Soc. 2009, 131, 17145–17154. 10.1021/ja904472z. [DOI] [PubMed] [Google Scholar]
- Rolfe B. E.; Blakey I.; Squires O.; Peng H.; Boase N. R. B.; Alexander C.; Parsons P. G.; Boyle G. M.; Whittaker A. K.; Thurecht K. J. Multimodal Polymer Nanoparticles with Combined 19F Magnetic Resonance and Optical Detection for Tunable, Targeted, Multimodal Imaging in vivo. J. Am. Chem. Soc. 2014, 136, 2413–2419. 10.1021/ja410351h. [DOI] [PubMed] [Google Scholar]
- Patrick M. J.; Janjic J. M.; Teng H.; O’Hear M. R.; Brown C. W.; Stokum J. A.; Schmidt B. F.; Ahrens E. T.; Waggoner A. S. Intracellular pH Measurements Using Perfluorocarbon Nanoemulsions. J. Am. Chem. Soc. 2013, 135, 18445–18457. 10.1021/ja407573m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shir A.; Yadav N. N.; Gilad A. A.; van Zijl P. C. M.; McMahon M. T.; Bulte J. W. M. Single 19F Probe for Simultaneous Detection of Multiple Metal Ions Using miCEST MRI. J. Am. Chem. Soc. 2015, 137, 78–81. 10.1021/ja511313k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. L.; Li Y.; Mao X.; Chen H.; Staedtke V.; Bai R.; Ma W.; Lin R.; Li Y.; Liu G.; Cui H. One-component Supramolecular Filament Hydrogels as Theranostic Label-free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11, 797–805. 10.1021/acsnano.6b07196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrauto G.; Di Gregorio E.; Baroni S.; Aime S. Frequency-encoded MRI-CEST Agents Based on Paramagnetic Liposomes/RBC Aggregates. Nano Lett. 2014, 14, 6857–6862. 10.1021/nl5026612. [DOI] [PubMed] [Google Scholar]
- Ratnakar S. J.; Soesbe T. C.; Lumata L. L.; Do Q. N.; Viswanathan S.; Lin C.-Y.; Sherry A. D.; Kovacs Z. Modulation of CEST Images in vivo by T1 Relaxation: a New Approach in the Design of Responsive PARACEST Agents. J. Am. Chem. Soc. 2013, 135, 14904–14907. 10.1021/ja406738y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrauto G.; Delli Castelli D.; Di Gregorio E.; Langereis S.; Burdinski D.; Grull H.; Terreno E.; Aime S. Lanthanide-loaded Erythrocytes as Highly Sensitive Chemical Exchange Saturation Transfer MRI Contrast Agents. J. Am. Chem. Soc. 2014, 136, 638–641. 10.1021/ja411793u. [DOI] [PubMed] [Google Scholar]
- Terreno E.; Castelli D. D.; Viale A.; Aime S. Challenges for Molecular Magnetic Resonance Imaging. Chem. Rev. 2010, 110, 3019–3042. 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- Glunde K.; Artemov D.; Penet M.-F.; Jacobs M. A.; Bhujwalla Z. M. Magnetic Resonance Spectroscopy in Metabolic and Molecular Imaging and Diagnostic of Cancer. Chem. Rev. 2010, 110, 3043–3059. 10.1021/cr9004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R.; Gambhir S. S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- Harvey P.; Kuprov I.; Parker D. Lanthanide Complexes as Paramagnetic Probes for 19F Magnetic Resonance. Eur. J. Inorg. Chem. 2012, 2012, 2015–2022. 10.1002/ejic.201100894. [DOI] [Google Scholar]
- Boase N. R. B.; Blakey I.; Thurecht K. J. Molecular Imaging with Polymers. Polym. Chem. 2012, 3, 1384–1389. 10.1039/c2py20132a. [DOI] [Google Scholar]
- Tirotta I.; Dichiarante V.; Pigliacelli C.; Cavallo G.; Terraneo G.; Bombelli F. B.; Metrangolo P.; Resnati G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev. 2015, 115, 1106–1129. 10.1021/cr500286d. [DOI] [PubMed] [Google Scholar]
- Aime S.; Castelli D. D.; Crich S. G.; Gianolio E.; Terreno E. Pushing the Sensitivity Envelope of Lanthanide-based Magnetic Resonance Imaging (MRI) Contrast Agents for Molecular Imaging Applications. Acc. Chem. Res. 2009, 42, 822–831. 10.1021/ar800192p. [DOI] [PubMed] [Google Scholar]
- Liu G.; Song X.; Chan K. W. Y.; McMahon M. T. Nuts and Bolts of Chemical Exchange Saturation Transfer MRI. NMR Biomed. 2013, 26, 810–828. 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajca A.; Wang Y.; Boska M.; Paletta J. T.; Olankitwanit A.; Swanson M. A.; Mitchell D. G.; Eaton S. S.; Eaton G. R.; Rajca S. Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. 10.1021/ja3079829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajca A.; Wang Y.; Boska M.; Paletta J. T.; Olankitwanit A.; Swanson M. A.; Mitchell D. G.; Eaton S. S.; Eaton G. R.; Rajca S. Correction to Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2014, 136, 3318–3318. 10.1021/ja413028d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo F.; Soule B. P.; Matsumoto K.-I.; Matusmoto S.; Cook J. A.; Hyodo E.; Sowers A. L.; Krishna M. C.; Mitchell J. B. Assessment of Tissue Redox Status Using Metabolic Responsive Contrast Agents and Magnetic Resonance Imaging. J. Pharm. Pharmacol. 2008, 60, 1049–1060. 10.1211/jpp.60.8.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo F.; Chuang K.-H.; Goloshevsky A. G.; Sulima A.; Griffiths G. L.; Mitchell J. B.; Koretsky A. P.; Krishna M. C. Brain Redox Imaging Using Blood-brain Barrier-permeable Nitroxide MRI Contrast Agent. J. Cereb. Blood Flow Metab. 2008, 28, 1165–1174. 10.1038/jcbfm.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch R. C. Work in Progress: Methods of Contrast Enhancement for NMR Imaging and Potential Applications. A Subject Review. Radiology 1983, 147, 781–788. 10.1148/radiology.147.3.6342034. [DOI] [PubMed] [Google Scholar]
- Brasch R. C.; London D. A.; Wesbey G. E.; Tozer T. N.; Nitecki D. E.; Williams R. D.; Doemeny J.; Tuck L. D.; Lallemand D. P. Work in Progress: Nuclear Magnetic Resonance Study of a Paramagnetic Nitroxide Contrast Agent for Enhancement of Renal Structures in Experimental Animals. Radiology 1983, 147, 773–779. 10.1148/radiology.147.3.6844613. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.-I.; Hyodo F.; Matsumoto A.; Koretsky A. P.; Sowers A. L.; Mitchell J. B.; Krishna M. C. High-resolution Mapping of Tumor Redox Status by Magnetic Resonance Imaging Using Nitroxides as Redox-sensitive Contrast Agents. Clin. Cancer Res. 2006, 12, 2455–2462. 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- Hyodo F.; Matsumoto K.-I.; Matsumoto A.; Mitchell J. B.; Krishna M. C. Probing the Intracellular Redox Status of Tumors with Magnetic Resonance Imaging and Redox-sensitive Contrast Agents. Cancer Res. 2006, 66, 9921–9928. 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- Zhelev Z.; Bakalova R.; Aoki I.; Lazarova D.; Saga T. Imaging of Superoxide Generation in the Dopaminergic Area of the Brain in Parkinson’s Disease, Using Mito-TEMPO. ACS Chem. Neurosci. 2013, 4, 1439–1445. 10.1021/cn400159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. M.; Sowers A. L.; Degraff W.; Bernardo M.; Thetford A.; Krishna M. C.; Mitchell J. B. A Novel Nitroxide is an Effective Brain Redox Imaging Contrast Agent and in vivo Radioprotector. Free Radical Biol. Med. 2011, 51, 780–790. 10.1016/j.freeradbiomed.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev Z.; Bakalova R.; Aoki I.; Matsumoto K.-I.; Gadjeva V.; Anzai K.; Kanno I. Nitroxide Radicals for Labelling of Conventional Therapeutics and Noninvasive Magnetic Resonance Imaging of Their Permeability for Blood-brain Barrier: Relationship between Structure, Blood Clearance, and MRI Signal Dynamic in the Brain. Mol. Pharmaceutics 2009, 6, 504–512. 10.1021/mp800175k. [DOI] [PubMed] [Google Scholar]
- Doane T. L.; Burda C. The Unique Role of Nanoparticles in Nanomedicine: Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. 10.1039/c2cs15260f. [DOI] [PubMed] [Google Scholar]
- Jokerst J. V.; Gambhir S. S. Molecular Imaging with Theranostic Nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. 10.1021/ar200106e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joralemon M. J.; McRae S.; Emrick T. PEGylated Polymers for Medicine: from Conjugation to Self-assembled Systems. Chem. Commun. 2010, 46, 1377–1393. 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- Torchilin V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Delivery Rev. 2011, 63, 131–135. 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Anraku Y.; Kishimura A.; Kobayashi A.; Oba M.; Kataoka K. Size-controlled Long-circulating PICsome as a Ruler to Measure Critical Cut-off Disposition Size into Normal and Tumor Tissues. Chem. Commun. 2011, 47, 6054–6056. 10.1039/c1cc11465d. [DOI] [PubMed] [Google Scholar]
- Cabral H.; Matsumoto Y.; Mizuno K.; Chen Q.; Murakami M.; Kimura M.; Terada Y.; Kano M. R.; Miyazono K.; Uesaka M.; Nishiyama N.; Kataoka K. Accumulation of Sub-100 nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotechnol. 2011, 6, 815–823. 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- Jokerst J. V.; Lobovkina T.; Zare R. N.; Gambhir S. S. Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine 2011, 6, 715–728. 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. A. Metabolism of brain tissue slices and suspensions from various mammals. J. Neurophysiol. 1948, 11, 473–484. [DOI] [PubMed] [Google Scholar]
- Tolmasoff J. M.; Ono T.; Cutler R. G. Superoxide dismutase: correlation with lifespan and specific metabolic rate in primate species. Proc. Natl. Acad. Sci. U. S. A. 1980, 77, 2777–2781. 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev Z.; Bakalova R.; Aoki I.; Matsumoto K.-I.; Gadjeva V.; Anzai K.; Kanno I. Nitroxyl Radicals as Low Toxic Spin-labels for Non-invasive Magnetic Resonance Imaging of Blood-brain Barrier Permeability for Conventional Therapeutics. Chem. Commun. 2009, 53–55. 10.1039/B816878D. [DOI] [PubMed] [Google Scholar]
- Samuni Y.; Gamson J.; Samuni A.; Yamada K.; Russo A.; Krishna M. C.; Mitchell J. B. Factors Influencing Nitroxide Reduction and Cytotoxicity in Vitro. Antioxid. Redox Signaling 2004, 6, 587–595. 10.1089/152308604773934341. [DOI] [PubMed] [Google Scholar]
- Merbach A. S.; Helm L.; Toth E.. The Chemistry of Contrast Agent in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons: Chichester, 2013. [Google Scholar]
- Caravan P. Protein-targeted Gadolinium-based Magnetic Resonance Imaging (MRI) Contrast Agents: Design and Mechanism of Action. Acc. Chem. Res. 2009, 42, 851–862. 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- Winalski C. S.; Shortkroff S.; Mulkern R. V.; Schneider E.; Rosen G. M. Magnetic Resonance Relaxivity of Dendrimer-linked Nitroxides. Magn. Reson. Med. 2002, 48, 965–972. 10.1002/mrm.10312. [DOI] [PubMed] [Google Scholar]
- Winalski C. S.; Shortkroff S.; Schneider E.; Yoshioka H.; Mulkern R. V.; Rosen G. M. Targeted Dendrimer-based Contrast Agents for Articular Cartilage Assessment by MR Imaging. Osteoarthr. Cartil. 2008, 16, 815–822. 10.1016/j.joca.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Francese G.; Dunand F. A.; Loosli C.; Merbach A. E.; Decurtins S. Functionalization of PAMAM Dendrimers with Nitronyl Nitroxide Radicals as Models for the Outer-sphere Relaxation in Dentritic Potential MRI Contrast Agents. Magn. Reson. Chem. 2003, 41, 81–83. 10.1002/mrc.1151. [DOI] [Google Scholar]
- Paletta J. T.; Pink M.; Foley B.; Rajca S.; Rajca A. Synthesis and Reduction Kinetics of Sterically Shielded Pyrrolidine Nitroxides. Org. Lett. 2012, 14, 5322–5325. 10.1021/ol302506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M. A.; McCombs J. R.; Wang Y.; Paletta J. T.; Morton S. W.; Dreaden E. C.; Boska M. D.; Ottaviani M. F.; Hammond P. T.; Rajca A.; Johnson J. A. Redox Responsive Branched Bottebrush Polymers for In Vivo MRI and Fluorescence Imaging. Nat. Commun. 2014, 5, 5460. 10.1038/ncomms6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Li Y.; Burts A. O.; Ottaviani M. F.; Tirrell D. A.; Johnson J. A.; Turro N. J.; Grubbs R. H. EPR Study of Spin Labeled Brush Polymers in Organic Solvents. J. Am. Chem. Soc. 2011, 133, 19953–19959. 10.1021/ja2085349. [DOI] [PubMed] [Google Scholar]
- Burts A. O.; Li Y.; Zhukhovitskiy A. V.; Patel P. R.; Grubbs R. H.; Ottaviani M. F.; Turro N. J.; Johnson J. A. Using EPR to Compare PEG-branch-nitroxide “Bivalent-brush Polymers” and Traditional PEG Bottle-brush Polymers: Branching Makes a Difference. Macromolecules 2012, 45, 8310–8318. 10.1021/ma301874d. [DOI] [Google Scholar]
- Liu J.; Burts A. O.; Li Y.; Zhukhovitskiy A. V.; Ottaviani M. F.; Turro N. J.; Johnson J. A. Brush-first” Method for the Parallel Synthesis of Photocleavable, Nitroxide-labeled poly(ethylene glycol) Star Polymers. J. Am. Chem. Soc. 2012, 134, 16337–16344. 10.1021/ja3067176. [DOI] [PubMed] [Google Scholar]
- Gao A. X.; Liao L.; Johnson J. A. Synthesis of Acid-labile PEG and PEG-doxorubicin-conjugate Nanoparticles via Brush-first ROMP. ACS Macro Lett. 2014, 3, 854–857. 10.1021/mz5004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L.; Liu J.; Dreaden E. C.; Morton S. W.; Shopsowitz K. E.; Hammond P. T.; Johnson J. A. A Convergent Synthetic Platform for Single-nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. 10.1021/ja502011g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J. C.; Bruno P. M.; Nguyen H. V.-T.; Liao L.; Liu J.; Hemann M. T.; Johnson J. A. Using an RNAi Signature Assay to Guide the Design of Three-drug Conjugated Nanoparticles with Validated Mechanisms, in vivo Efficacy, and Low Toxicity. J. Am. Chem. Soc. 2016, 138, 12494–12501. 10.1021/jacs.6b06321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Gao A. X.; Johnson J. A. Particles Without a Box: Brush-first Synthesis of Photodegradable PEG Star Polymers under Ambient Conditions. J. Visualized Exp. 2013, 80, e50874. 10.3791/50874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burts A. O.; Liao L.; Lu Y. Y.; Tirrell D. A.; Johnson J. A. Brush-first and Click: Efficient Synthesis of Nanoparticles that Degrade and Release Doxorubicin in Response to Light. Photochem. Photobiol. 2014, 90, 380–385. 10.1111/php.12182. [DOI] [PubMed] [Google Scholar]
- Love J. A.; Morgan J. P.; Trnka T. M.; Grubbs R. H. A Practical and Highly Active Ruthenium-based Catalyst that Effects the Cross Metathesis of Acrylonitrile. Angew. Chem., Int. Ed. 2002, 41, 4035–4037. . [DOI] [PubMed] [Google Scholar]
- Budil D. E.; Lee S.; Saxena S.; Freed J. H. Nonlinear-least-square Analysis of Slow-motion EPR Spectra in One and Two Dimensions Using a Modified Levenberg-Marquardt-algorithm. J. Magn. Reson., Ser. A 1996, 120, 155–189. 10.1006/jmra.1996.0113. [DOI] [Google Scholar]
- Na H. B.; Lee J. H.; An K.; Park Y. I.; Park M.; Lee I. S.; Nam D.-H.; Kim S. T.; Kim S.-H.; Kim S.-W.; et al. Development of a T1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew. Chem., Int. Ed. 2007, 46, 5397–5401. 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- Detappe A.; Kunjachan S.; Sancey L.; Motto-Ros V.; Biancur D.; Drane P.; Guieze R.; Makrigiorgos G. M.; Tillement O.; Langer R.; et al. Advanced multimodal nanoparticles delay tumor progression with clinical radiation therapy. J. Controlled Release 2016, 238, 103–113. 10.1016/j.jconrel.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Sancey L.; Kotb S.; Truillet C.; Appaix F.; Marais A.; Thomas E.; van der Sanden B.; Klein J. P.; Laurent B.; Cottier M.; et al. Long-Term in Vivo Clearance of Gadolinium-Based AGuIX Nanoparticles and Their Biocompatibility after Systemic Injection. ACS Nano 2015, 9, 2477–2488. 10.1021/acsnano.5b00552. [DOI] [PubMed] [Google Scholar]
- Wei H.; Bruns O. T.; Kaul M. G.; Hansen E. C.; Barch M.; Wisniowska A.; Chen O.; Chen Y.; Li N.; Okada S.; Cordero J. M.; Heine M.; Farrar C. T.; Montana D. M.; Adam G.; Ittrich H.; Jasanoff A.; Nielsen P.; Bawendi M. G. Exceedingly Small Iron Oxide Nanoparticles as Positive MRI Contrast Agents. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 2325–2330. 10.1073/pnas.1620145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Lei X.; Jockusch S.; Chen J. Y.-C.; Frunzi M.; Johnson J. A.; Lawler R. G.; Murata Y.; Murata M.; Komatsu K.; Turro N. J. A Magnetic Switch for Spin-catalyzed Interconversion of Nuclear Spin Isomers. J. Am. Chem. Soc. 2010, 132, 4042–4043. 10.1021/ja910282p. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lei X.; Lawler R. G.; Murata Y.; Komatsu K.; Turro N. J. Distance-dependent Paramagnet-enhanced Nuclear Spin Relaxation of H2@C60 Derivatives Covalently Linked to a Nitroxide Radical. J. Phys. Chem. Lett. 2010, 1, 2135–2138. 10.1021/jz100645w. [DOI] [Google Scholar]
- Sartori E.; Ruzzi M.; Lawler R. G.; Turro N. J. Nitroxide Paramagnet-induced Para-ortho Conversion and Nuclear Spin Relaxation of H2 in Organic Solvents. J. Am. Chem. Soc. 2008, 130, 12752–12756. 10.1021/ja8037195. [DOI] [PubMed] [Google Scholar]
- Keana J. F. W.; Pou S.; Rosen G. M. Nitroxides as Potential Contrast Enhancing Agent for MRI Application: Influence of Structure on the Rate of Reduction by Rat Hepatocytes, Whole Liver Homogenate, Subcellular Fractions, and Ascorbate. Magn. Reson. Med. 1987, 5, 525–536. 10.1002/mrm.1910050603. [DOI] [PubMed] [Google Scholar]
- Bobko A. A.; Kirilyuk I. A.; Grigor’ev I. A.; Zweier J. L.; Khramtsov V. V. Reversible Reduction of Nitroxides to Hydroxylamines: Role for Ascorbate and Glutathione. Free Radical Biol. Med. 2007, 42, 404–412. 10.1016/j.freeradbiomed.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinco J. P.; Fairfull-Smith K. E.; Morrow B. J.; Bottle S. E. Profluorescent Nitroxides as Sensitive Probes of Oxidative Change and Free Radical Reactions. Aust. J. Chem. 2011, 64, 373–389. 10.1071/CH10442. [DOI] [Google Scholar]
- Yang Y.; Zhao Q.; Feng W.; Li F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. 10.1021/cr2004103. [DOI] [PubMed] [Google Scholar]
- Ahn H.-Y.; Fairfull-Smith K. E.; Morrow B. J.; Lussini V.; Kim B.; Bondar M. V.; Bottle S. E.; Belfield K. D. Two-photon Fluorescence Microscopy Imaging of Cellular Oxidative Stress Using Profluorescent Nitroxides. J. Am. Chem. Soc. 2012, 134, 4721–4730. 10.1021/ja210315x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P.; Aboagye E. O.; Balkwill F.; Balmain A.; Bruder G.; Chaplin D. J.; Double J. A.; Everitt J.; Farningham D. A. H.; Glennie M. J.; Kelland L. R.; Robinson V.; Stratford I. J.; Tozer G. M.; Watson S.; Wedge S. R.; Eccles S. A. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K.; Sewell F.; Allais L.; Delongeas J.-L.; Donald E.; Festag M.; Kervyn S.; Ockert D.; Nogues V.; Palmer H.; Popovic M.; Roosen W.; Schoenmakers A.; Somers K.; Stark C.; Stei P.; Robinson S. A Global Pharmaceutical Company Initiative: an Evidence-based Approach to Define the Upper Limit of Body Weight Loss in Short Term Toxicity Studies. Regul. Toxicol. Pharmacol. 2013, 67, 27–38. 10.1016/j.yrtph.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Rowland M.; Benet L. Z.; Graham G. G. Clearance Concepts in Pharmacokinetics. J. Pharmacokinet. Biopharm. 1973, 1, 123–136. 10.1007/BF01059626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.