SUMMARY
The kidney contains the functional units, the nephrons, surrounded by the renal interstitium. Previously, we discovered that, once Six2-expressing nephron progenitor cells and Foxd1-expressing renal interstitial progenitor cells form at the onset of kidney development, descendant cells from these populations contribute exclusively to the main body of nephrons and renal interstitial tissues, respectively, indicating a lineage boundary between the nephron and renal interstitial compartments. Currently, it is unclear how lineages are regulated during kidney organogenesis. We demonstrate that nephron progenitor cells lacking Pax2 fail to differentiate into nephron cells, but can switch fates into renal interstitium-like cell types. These data suggest that Pax2 function maintains nephron progenitor cells by repressing a renal interstitial cell program. Thus, the lineage boundary between the nephron and renal interstitial compartments is maintained by the Pax2 activity in nephron progenitor cells during kidney organogenesis.
Keywords: kidney, nephron, progenitor, cell fate, PAX2
eTOC BLURB
The lineage boundary exists between the nephron and interstitial compartments in the kidney. It is currently unclear how lineage boundaries are formed during kidney development. Naiman et al. show that repression of interstitial fates in nephron progenitor cells by Pax2 establishes the nephron-interstitium boundary throughout kidney organogenesis.
INTRODUCTION
The kidney is a vital organ removing metabolic waste products from the blood and maintaining water/ion balance for the body. The functional unit of the kidney, the nephron, is surrounded by the renal interstitial tissues (Little et al., 2007). The human and mouse kidney contain about one million and 13,000 nephrons, respectively (Bertram et al., 2014; Cebrian et al., 2004). Reduced nephron endowment is associated with the future development of hypertension and potentially chronic kidney diseases (CKD), leading to end stage renal disease (ESRD) (Luyckx and Brenner, 2015), a significant, growing economic health burden in the USA. In mammals, nephrons are generated only during kidney development: no de novo nephron-forming ability resides within the adult kidney (Hartman et al., 2007; Romagnani et al., 2013). An understanding of the regulatory mechanisms governing nephron formation is critical not only to clarify how the functional kidney forms a full complement of nephrons, but also to develop therapeutic strategies to increase nephron endowment where premature birth, malnutrition or other pathological conditions have reduced nephron number (Hendry et al., 2013; Taguchi et al., 2014; Takasato et al., 2015).
During mammalian embryogenesis, the urogenital system including the kidney arises from the intermediate mesoderm of the developing embryo (Saxen, 1987; Stewart and Bouchard, 2014). Formation of the kidney is initiated by reciprocal interactions of two adjacent tissues, the ureteric bud and metanephric mesenchyme, from 10.5 days post coitus (dpc) of mouse development (Costantini and Kopan, 2010; Little and McMahon, 2012). As the ureteric bud grows into the metanephric mesenchyme, the Six2-expressing (Six2+) condensed cap mesenchyme becomes surrounded by the Foxd1+ cortical stroma (the renal capsule and nephrogenic interstitium) in the mesenchyme (Hatini et al., 1996; Levinson and Mendelsohn, 2003). While the ureteric tip grows and branches repetitively to form the collecting duct system of the kidney (Costantini, 2012), cap mesenchyme cells remain condensed around each branch tip. A subset of cap mesenchyme cells commits to a nephron differentiation program through the establishment of a pretubular aggregate (Stark et al., 1994). The pretubular aggregate subsequently undergoes epithelial formation to a renal vesicle, which further differentiates into the comma- and S-shaped bodies, and eventually the main body of the nephron (the nephron epithelium): the visceral (podocyte) and parietal epithelium of the glomerulus, proximal tubule, loop of Henle and distal tubule (Boyle et al., 2007; Kobayashi et al., 2008).
The mature nephron is surrounded by the renal cortical and medullary interstitium (Alcorn et al., 1999; Lemley and Kriz, 1991). Podocytes of the glomerulus surround the glomerular capillary system, including the mesangium and endothelium (Boyle et al., 2014; Lin et al., 2014; Quaggin and Kreidberg, 2008). Outside of the glomerulus, the vasculature is supported by perivascular cells (pericytes) (Herzlinger and Hurtado, 2014; Sequeira Lopez and Gomez, 2011). The renal capsule, the nephrogenic, renal cortical and medullary interstitium, the mesangium and the peri-vasculature compose the renal interstitium (Little et al., 2007).
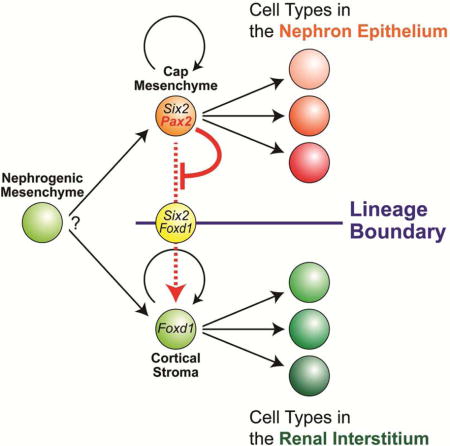
Our fate map analysis previously revealed that the Six2+ cap mesenchyme is a multipotent self-renewing progenitor population for the nephron epithelium (Kobayashi et al., 2008). Furthermore, we identified that the Foxd1+ cortical stroma is another multipotent self-renewing progenitor population for the renal interstitium (Kobayashi et al., 2014). The Six2+ cap mesenchyme and Foxd1+ cortical stroma exclusively contribute to nephron epithelial and renal interstitial tissues, respectively, although there are minor cell fate changes of contribution of Foxd1+ cells to the nephron lineage (Brunskill et al., 2014; Kobayashi et al., 2014). Mature nephron and renal interstitial compartments are also maintained during kidney fibrosis and nephron regeneration in adults with little (LeBleu et al., 2013; Pippin et al., 2013) or no evidence (Humphreys et al., 2008; Humphreys et al., 2010) of cell type switching between the lineage-restricted cellular compartments. Thus, once nephron and renal interstitial progenitor cells are specified at the onset of kidney development, their descendants maintain distinct fates segregated by a lineage boundary in normal, diseased and regenerating kidneys. However, it is currently unclear how such lineage boundaries are regulated during kidney development.
The paired-domain transcriptional regulator Pax2 is expressed in multiple urogenital tissues, including the nephric (Wolffian) duct, cap mesenchyme, and differentiating nephron and collecting duct system of the developing kidney (Dressler et al., 1990). Global inactivation of Pax2 in the mouse results in agenesis of the kidney, ureter and male reproductive tract due to degenerating nephric ducts prior to the initiation of (metanephric) kidney development (Ranghini and Dressler, 2015; Torres et al., 1995), while combined removal of Pax2 and Pax8 leads to a complete absence of nephric duct development, also resulting in kidney agenesis (Bouchard et al., 2002). Although Pax2 has been widely recognized as a key regulatory factor in kidney development (Brophy et al., 2001; Rothenpieler and Dressler, 1993), because of the kidney agenesis in Pax2-null mouse mutants, Pax2 function in distinct tissues within the developing kidney has not been defined in vivo.
Our data herein suggest that Pax2 function in the cap mesenchyme is required to maintain nephron progenitor cells mainly by repressing transdifferentiation into renal interstitium-like cell fates. Thus, Pax2 activity in nephron progenitor cells maintains the lineage boundary between the nephron and renal interstitial compartments during mammalian kidney organogenesis.
RESULTS
PAX2 is co-expressed with SIX2 in the cap mesenchyme, but not with FOXD1 in the renal cortical stroma during kidney organogenesis
PAX2 is expressed in the mesenchyme surrounding the PAX2-expressing (PAX2+) ureteric epithelium and differentiated derivatives of SIX2+ progenitors in the developing kidney (Dressler and Douglass, 1992). First, we closely examined PAX2 expression in progenitor populations during early stages of kidney organogenesis. Around the posterior nephric duct at 10.5 dpc, PAX2 expression was detected in SIX2+ metanephric mesenchyme cells and SIX2− cells in the nephric duct and ureteric bud. At this stage, FOXD1 expression was largely absent except for a few mesenchymal cells starting to express FOXD1 at very low levels outside of the SIX2+ metanephric mesenchyme (Fig 1A–D). One day later at 11.5 dpc, when the ureteric bud grows and branches once to form two ureteric tips, PAX2 expression was observed in SIX2+ cap mesenchyme cells and SIX2− ureteric tip cells, but not in scattered FOXD1+ renal cortical stroma cells around SIX2+ cells (Fig 1E–H). At 15.5 dpc, PAX2 is expressed in SIX2+ cap mesenchyme cells and a subset of differentiating nephron cells, but not surrounding FOXD1+ renal cortical stroma cells (Fig 1I–L) as previously shown (Hatini et al., 1996). We did not detect SIX2+ FOXD1+ double positive cells using confocal immunofluorescence throughout kidney development except for very rare cells (data not shown, see below). Although a recent single cell RNA-seq analysis identified rare cells expressing both Six2 and Foxd1 mRNA, the Foxd1 mRNA was partially degraded in these Six2+ Foxd1+ cells (Brunskill et al., 2014), which indicates that our observations are consistent.
Figure 1. Inactivation of Pax2 in the cap mesenchyme of the developing kidney.
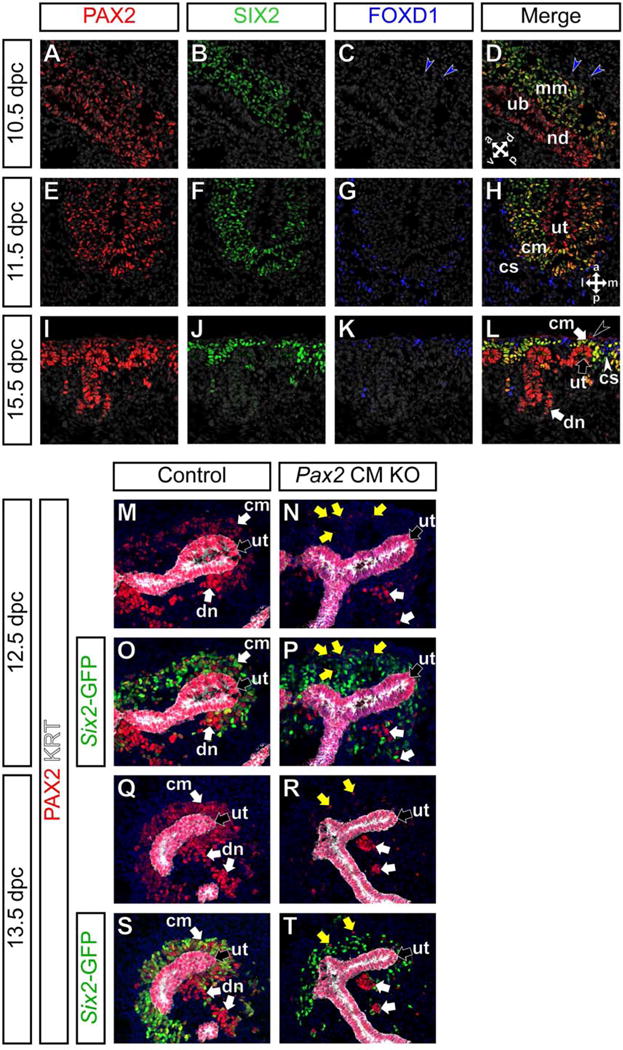
(A–H) Confocal immunofluorescence of wild-type kidneys at 10.5 (A–D), 11.5 (E–H) and 15.5 dpc (I–L) with PAX2 (red), SIX2 (green) and FOXD1 (blue) and Hoechst (gray) staining. Double arrows in D and H indicate anterio-posterior, dorso-ventral or medio-lateral axes. Blue arrowheads in C,D indicate cells expressing FOXD1 at low levels. A black arrowhead in L indicates non-specific (non-PAX2) staining from an anti-mouse IgG secondary antibody, between connective tissue and renal capsule cells. (M–T) Confocal immunofluorescence of the nephrogenic zone of kidneys from Six2-eGFPCretg/+; Pax2flox/+ control (M,O,Q,S) and Six2-eGFPCretg/+; Pax2flox/del cap mesenchyme-specific Pax2 knock-out (Pax2 CM KO) (N,P,R,T) mutant mice with PAX2 (red), Six2-GFP (cap mesenchyme; green), cytokeratin (KRT, ureteric tip; white) and Hoechst (nucleus; blue) staining at 12.5 dpc (M–P) and 13.5 dpc (Q–T). PAX2 expression is reduced in the cap mesenchyme surrounding the ureteric tip. Note that the Six2-eGFPCre BAC transgene is mosaic in the cap mesenchyme, as previously observed (Kobayashi et al., 2008). Yellow and white arrows in N,P,R,T indicate PAX2+ cells that escaped Cre recombination in the cap mesenchyme and differentiating nephron, respectively. cm, cap mesenchyme; cs, renal cortical stroma; dn, differentiating nephron; mm, metanephric mesenchyme; nd, nephric duct; ub, ureteric bud; ut, ureteric tip.
Generation of a conditional Pax2-null allele in the mouse
Because Pax2 is required to maintain the nephric (Wolffian) duct, there is no induction of kidney (metanephros) development in Pax2-null mutant mice (Torres et al., 1995). To examine Pax2 function at later stages in the nephron progenitor population, we first generated a Cre/loxP-dependent conditional Pax2-null mouse allele where a conditional removal of exon 2 results in a frame-shift mutation with a premature termination, producing a transcript encoding only the first 14 amino acids of a normal 393 amino acid PAX2 protein (Dressler, 2011) (Fig S1A,B). This predicted null allele is designated as Pax2del ; the conditional allele (with selection cassette removed) is referred to as Pax2flox (Fig S1A).
To validate the Pax2flox allele, Pax2flox/+ mice were mated with an epiblast-specific Sox2-Cre mice (Hayashi et al., 2002) to generate Pax2 deleted (Pax2del/+) mice (Fig S1C). While Pax2flox/flox embryos were phenotypically normal at 18.5 dpc (Fig S1D), Pax2del/del embryos showed identical abnormalities to those of Pax2-null (Pax2−/−) mutant mice (Torres et al., 1995), including a complete absence of the kidney, ureter and reproductive tracts (Fig S1E), which validates the conditional Pax2-null mouse allele.
Inactivation of Pax2 in the cap mesenchyme of the developing kidney
To investigate Pax2 function in the cap mesenchyme, we inactivated Pax2 using the Six2-eGFPCre BAC transgenic (Six2-eGFPCretg/+) mice (Kobayashi et al., 2008). To confirm Pax2 inactivation by the Six2-eGFPCre transgene, we examined PAX2 expression in Pax2 cap mesenchyme-specific knock-out mutant (Pax2 CM KO) kidneys from Six2-eGFPCretg/+; Pax2flox/del embryos using an anti-mouse PAX2 antibody against amino acids 188–385, which are absent from the translated product of the Pax2del allele. In kidneys from Six2-eGFPCretg/+; Pax2flox/+ control littermates at 12.5 dpc, PAX2 expression was detected in the cap mesenchyme, differentiating nephrons and cytokeratin+ (KRT+) developing collecting duct system including the ureteric tip (Fig 1M), while Six2-GFP expression was detected strongly in the cap mesenchyme and weakly in the immature nephron precursors (Fig 1O). In Pax2 CM mutant kidneys at the same stage, PAX2 expression was greatly reduced in the Six2-GFP+ cap mesenchyme cells (Fig 1N,P). PAX2 expression was observed in some Six2-GFP negative (Six2-GFP−) cells in the cap mesenchyme and differentiating nephrons (yellow and white arrows in Fig 1N,P, respectively), most likely due to delayed onset of the mosaic Six2-eGFPCre transgene expression (Kobayashi et al., 2008; Park et al., 2007). At 13.5 dpc, PAX2 expression was not detected in the cap mesenchyme of Pax2 CM mutant kidneys, except for a few rare cells (Fig 1Q–T). These data suggest that Pax2 was specifically inactivated in the majority of cap mesenchyme cells during early stages of kidney organogenesis.
Pax2 function is required for development of the cap mesenchyme
To determine the effect of Pax2 inactivation in the cap mesenchyme, we examined phenotypes of the urogenital system. We found that Pax2 CM mutants die within 24 hours of birth, around 19 dpc. Unlike Pax2-null (Pax2−/−) mutant neonates at birth (Torres et al., 1995), Pax2 CM mutant mice showed a vestigial kidney structure connected to a ureter and reproductive tracts including the epididymis and vas deferens in males (Fig 2A–D, S1E). Histological analysis revealed that the Pax2 CM mutant kidneys lacked the cap mesenchyme and all but a few nephrons that were PAX2+ (Fig 2C–F and data not shown), reflecting early escape from Cre recombination probably due to the delayed onset of the Six2-eGFPCre transgene. Thus, Pax2 function is required for development of the cap mesenchyme in the developing kidney.
Figure 2. Pax2 is required for development of the cap mesenchyme.
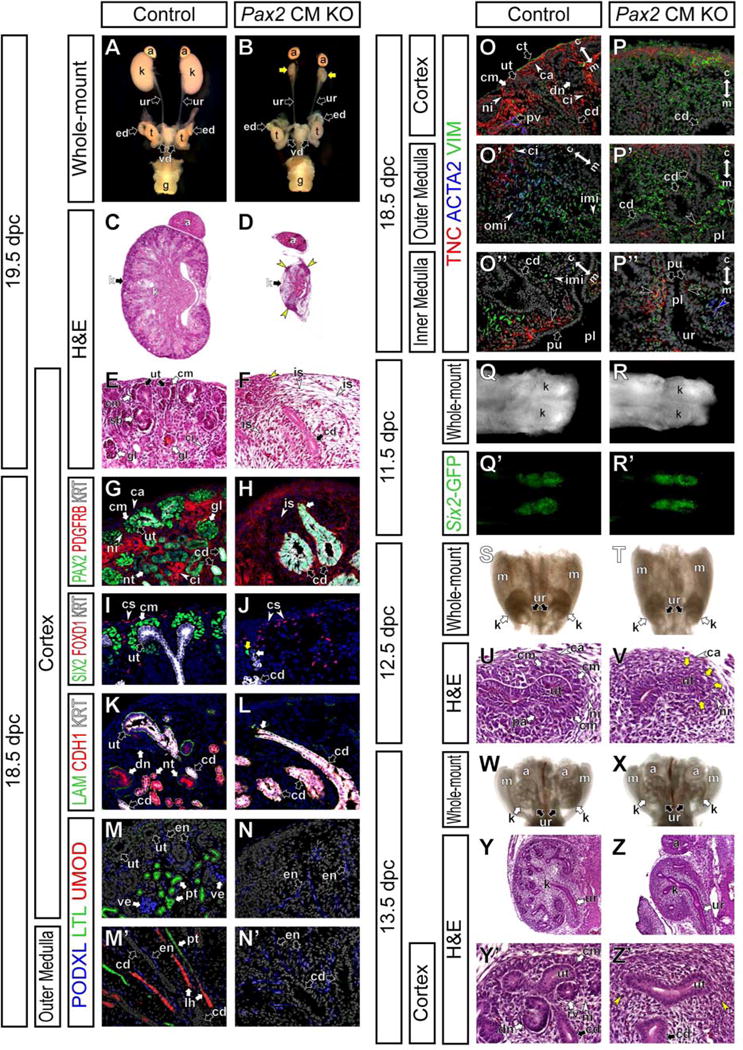
Six2-eGFPCretg/+; Pax2flox/+ control mice (Control) and Six2-eGFPCretg/+; Pax2flox/del cap mesenchyme-specific Pax2 mutant mice (Pax2 CM KO). (A and B) Whole-mount view of the urogenital system at 19.5 dpc. Yellow arrows in B indicate hypoplastic kidneys. (C and D) H&E stained sections of kidneys at 19.5 dpc. (E and F) High magnification of cortical regions in C and D, respectively. Yellow arrowheads in D and F indicate focal thickened tissues around rare ureteric tips. (G–P″) Confocal immunofluorescence of the renal cortex (G–N,O,P), outer renal medulla (M′,N′,O′,P′) and inner renal medulla (O″,P″) of kidneys at 18.5 dpc. (G and H) PAX2 (green), PDGFRB (red) and cytokeratin (KRT; white). (I and J) SIX2 (green), FOXD1 (red) and cytokeratin (KRT; white). Yellow and white arrows in J indicate a rare SIX2+ cell and a rare ureteric tip, respectively. (K and L) Epithelial markers; laminin (LAM; green), E-cadherin (CDH1; red) and cytokeratin (KRT; white). (M–N′) Nephron segment markers; podocalyxin (PODXL, podocytes and endothelium; green), Lotus Tetragonolobus Lectin (LTL, proximal tubule; red) and Uromodulin (UMOD, loop of Henle; red). (O–P″) Interstitial layer markers; tenascin C (TNC; green), α-smooth muscle actin (ACTA2; blue) and Vimentin (VIM; green). Double arrows indicate the cortico-medullary axis of the kidney. Black arrowheads in O″ and P″ indicate the TNC+ most inner renal medullary interstitium adjacent to the pelvic urothelial lining (pu). The blue arrowhead in P″ indicates ACTA2+ cells in the ureter mesenchyme. (Q–R′) Dorsal view of the whole-mount posterior urogenital system at 11.5 dpc. (Q and R) Bright view. (Q′ and R′) GFP expression in Q and R, respectively. (S and T) Dorsal view of the whole-mount urogenital system at 12.5 dpc. (U and V) H&E stained sections of the nephrogenic zone of kidneys at 12.5 dpc. Yellow arrows in V indicate less condensed cap mesenchyme cells. (W and X) Dorsal view of the whole-mount urogenital system at 13.5 dpc. (Y–Z′) H&E stained sections of kidneys at 13.5 dpc. (Y and Z) Whole kidneys. (Y′ and Z′) Higher magnification of the nephrogenic zone in Y and Z, respectively. Yellow arrowheads in Z′ indicate interstitium-like tissues directly surrounding the ureteric tip. a, adrenal gland; ca, renal capsule; cd, collecting duct; ci, renal cortical interstitium; cm, cap mesenchyme; dn, differentiating nephron; en, endothelium; ed, epididymis; g, genitalia; gl, glomerulus; is, renal interstitium; imi, inner renal medullary interstitium; k, kidney; lh, loop of Henle; m, mesonephros; ni, nephrogenic interstitium; nt, nephron tubule; omi, outer renal medullary interstitium; pl, pelvis; pt, proximal tubule, pu, pelvic urothelial lining; pv, peri-vasculature; rv, renal vesicle; sb, S-shaped body; t, testis; ur, ureter; ut, ureteric tip; ve, visceral epithelium (podocytes); vd, vas deferens.
In control kidneys at 18.5 dpc, PAX2 was expressed in the cap mesenchyme, differentiating nephron epithelium, KRT+ ureteric tip and KRThigh collecting duct, but not in the PDGFRB+ renal interstitium (Fig 2G). In Pax2 CM mutant kidneys at this stage, PAX2 expression was observed only in the KRThigh collecting duct (white arrow in Fig 2H). Most cells outside of the KRThigh collecting duct were PDGFRB+ renal interstitial cells (Fig 2H). While FOXD1 expression was normally detected in the cortical region, SIX2 expression was absent (Fig 2I,J, S2A–B and data not shown), except for in rare PAX2+ cells adjacent to the rare ureteric tip in Pax2 CM mutants (yellow arrow in Fig 2J and data not shown). All the other cap mesenchyme markers examined, including CITED1 and N-cadherin (CDH2), were not detected (see below), suggesting that the cap mesenchyme may fail to undergo normal development in Pax2 CM mutants.
We also examined nephron differentiation in Pax2 CM mutant kidneys. In control kidneys at 18.5 dpc, a KRT− or low Laminin+ (LAM+) E-cadherin+ (CDH1+) differentiating nephron epithelium was observed together with a KRT+ LAM+ CDH1+ ureteric tip and KRThigh LAM+ CDH1+ collecting duct (Fig 2K). In Pax2 CM mutant kidneys, no KRT− or low LAM+ CDH1+ nephron epithelium was detected, except for a few nephrons that escaped Cre recombination (Fig 2L). Segmental nephron organization in control kidneys was evident by examining Podocalyxin (PODXL) and Podocin (NPHS2) (visceral epithelium, podocytes), Lotus Tetragonolobus Lectin (LTL) (proximal tubule), Uromodulin (UMOD; also known as Tamm-Horsfall Glycoprotein) (loop of Henle) and SLC12A3 (also known as NCC) (distal tubule) (Fig 2M,M′ and data not shown). In contrast, none of the nephron segment markers were detected in Pax2 CM mutant kidneys (Fig 2N,N′). Low PODXL expression was observed in the endothelium (Fig 2M–N′). These molecular marker data confirm that Pax2 inactivation in the cap mesenchyme results in loss of the nephron epithelium.
In the interstitium of control kidneys at 18.5 dpc, Tenascin C (TNC) expression was observed in the renal capsule, nephrogenic/renal cortical interstitium and innermost medullary interstitium adjacent to the pelvic urothelial lining, Vimentin (VIM) expression in the renal cortical and medullary interstitium, and α-smooth muscle actin (ACTA2) expression in the outer medullary interstitium (Fig 2O–O″). In the Pax2 CM mutants, TNC and VIM expression domains were observed along a cortico-medullary axis of the kidney, but strong ACTA2 expression was not observed (Fig 2O′,P′). These data suggest that renal interstitium differentiation along the cortico-medullary axis of the kidney was largely normal even without nephron formation in Pax2 CM mutants, although there was likely some secondary effect that results in a failure of development of ACTA2+ outer renal medullary interstitial cells.
In Pax2 CM mutants, focal dense tissues were observed around the rare ureteric tips on the surface of the kidney (yellow arrowheads in Fig 2D,F). Molecular marker analysis at 18.5 dpc showed a few Six2-GFP− SIX2+ PAX2+ cells surrounding rare ureteric tips (Fig S2A–B″‴), indicating that the dense tissues contain some cap mesenchyme cells that escaped Pax2 inactivation probably due to the mosaic Six2- eGFPCre expression. LIV2 was detected in the connective tissue surrounding the kidney and adrenal gland, and renal capsule in control kidneys (Fig S2E) (Kobayashi et al., 2014). Molecular marker analysis revealed that the dense tissues are a heterogeneous population containing LIV2+ FOXD1− PDGFRB− CDH2+ TNC− VIM+ connective tissue cells and LIV2+ FOXD1+ PDGFRBlow CDH2low TNC+ VIM− renal capsule cells (Fig S2C–L). These data suggest that the dense tissues formed by expansion of both the connective tissue and renal capsule around the rare ureteric tips surrounded by some PAX2+ cap mesenchyme cells.
Pax2 function is required to maintain the cap mesenchyme
At the T-shaped ureteric stage at 11.5 dpc, high Six2-GFP expression was observed in the cap mesenchyme surrounding the ureteric tips in both control and Pax2 CM mutant kidneys, indicating that the cap mesenchyme is formed in both control and mutant kidneys initially (Fig 2Q–R′). As the ureteric tips continue to grow and branch, cap mesenchyme cells remained tightly condensed around the ureteric tips, surrounded by the renal interstitial progenitor cells in control kidneys (Fig 2S,U,W,Y,Y′). The developing nephrons, including the pretubular aggregate, renal vesicles, and comma- and S-shaped bodies, were observed (Fig 2U,Y′ and data not shown). In contrast, in Pax2 CM mutant kidneys at 12.5 dpc, a tightly packed cap mesenchyme was less evident (Fig 2T,V). In Pax2 CM mutant kidneys at 13.5 dpc, the cap mesenchyme and differentiating nephrons were not observed and the ureteric tip was directly surrounded by interstitium-like cells (Fig 2X,Z,Z′).
These results show that in Pax2 CM mutants, the early cap mesenchyme properly drives cap mesenchyme-dependent ureteric bud branching. Nevertheless, Pax2 function is essential to maintain the cap mesenchyme nephron progenitor pool after its initial specification from 11.5 dpc. Whether Pax2 plays an earlier role in specifying this population at 10.0–10.5 dpc cannot be addressed in this genetic model due to the delayed onset of expression of the Six2-eGFPCre transgene.
Pax2 function maintains the cell status of cap mesenchyme cells
Next, we examined how Pax2 function maintains cap mesenchyme cells. One possibility is that Pax2 is required for survival of cap mesenchyme cells and loss of Pax2 results in death of cap mesenchyme cells. Another possibility is that Pax2 function maintains the cellular identity of cap mesenchyme cells and Pax2-deficient cap mesenchyme cells undergo a cell fate switch.
To address these potential outcomes, we visualized the fate of cap mesenchyme cells by incorporating a Rosa26-lacZ Cre reporter allele (Soriano, 1999) into Pax2 CM mutants. In Six2-eGFPCretg/+; Pax2flox/del; R26RlacZ/+ mice, all Pax2-deficient descendant cells derived from the cap mesenchyme express β-gal. Six2-eGFPCretg/+; Pax2flox/+; R26RlacZ/+ littermates were used for controls. In control kidneys, we observed the contribution of β-gal+ cells into differentiating cap mesenchyme-derivatives but not to renal interstitial progenitors or their derivatives as expected (Fig 3A,C,E,G). However, in Pax2 CM mutant kidneys at 13.5 dpc (Fig 3B,D), some β-gal+ cells showed a round cell shape around the ureteric tip, while other β-gal+ cells became dispersed away from the ureteric tip within the renal interstitium (yellow arrows and arrowheads in Fig 3D, respectively). β-gal+ cells persisted within the renal interstitium of the rudimentary mutant kidney at 18.5 dpc (yellow arrowheads in Fig 3F,H), suggesting that Pax2-deficient nephron progenitor cells may adopt ectopic renal interstitial cell fates (see below).
Figure 3. Some Pax2-deficient cap mesenchyme cells can persist throughout kidney development.
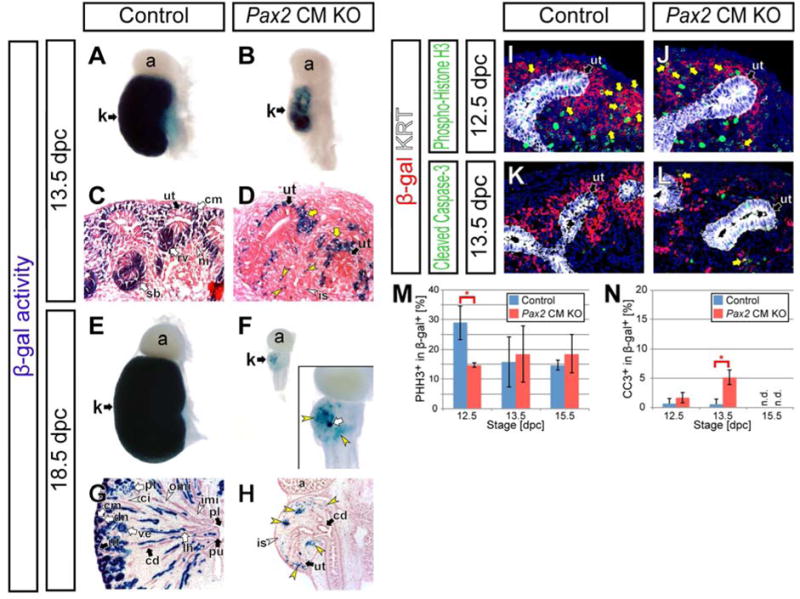
(A–H) Kidneys from Six2-eGFPCretg/+; Pax2flox/+; R26RlacZ/+ control mice (Control) and Six2-eGFPCretg/+; Pax2flox/del; R26RlacZ/+ cap mesenchyme-specific Pax2 mutant mice (Pax2 CM KO). X-gal staining of whole-mount tissues (A,B,E,F) and sections counter-stained with eosin (C,D,G,H) at 13.5 dpc (A–D) and 18.5 dpc (E–H). Yellow arrows and yellow arrowheads in D indicate β-gal+ cap mesenchyme-derived cells around the ureteric tip with less columnar cell shape and spreading away from the ureteric tip, respectively. The inset in F shows a high magnification of a hypoplastic kidney in Pax2 CM mutants. Yellow arrowheads and white arrow in F and H indicate β-gal+ cap mesenchyme-derived cells in the interstitium and in a few rare nephrons, respectively. (I–L) Confocal immunofluorescence of the nephrogenic zone of kidneys from Control mice (I,K) and Pax2 CM KO mice (J,L) with β-gal (red) and cytokeratin (KRT; ureteric tip, white) staining. (I,J) Phospho-histone H3 (green) staining at 12.5 dpc. Yellow arrows indicate β-gal+ phospho-histone H3+ cells. (K,L) Cleaved caspase-3 staining at 13.5 dpc. Yellow arrows in M indicate β-gal+ cleaved caspase-3+ cells. (M and N) Quantification of phospho-histone H3+ (PHH3+) (M) and cleaved caspase-3+ (CC3+) (N) cells in β-gal+ cap mesenchyme-derived cells. Data are represented as mean ± SD. Asterisks indicate p<0.05 with unpaired t-test. n.d., not detected. Abbreviations as in Figure 2.
We examined cell proliferation and apoptosis in β-gal+ cap mesenchyme-derived cells in the nephrogenic zone at 12.5, 13.5 and 15.5 dpc using phospho-histone H3 (PHH3) and cleaved caspase-3 (CC3) (Fig 3I–L). A significant reduction of PHH3+ cells among β-gal+ cells was observed at 12.5 dpc (0.51 fold; 29.0 ± 5.63 % in controls and 14.7± 0.73 % in Pax2 CM KO) (Fig 3M). Subsequently, a significant increase in CC3+ β-gal+ cells was observed in the nephrogenic zone at 13.5 dpc (8.95 fold; 0.58 ± 0.90 % in controls and 5.18 ± 0.58 % in Pax2 CM KO) (Fig 3N). These data suggest that Pax2 function is also required for normal proliferation and survival of a subset of cap mesenchyme cells and their descendant cells during early stages of kidney development, although we cannot exclude the possibility that the partial defects in cell proliferation and survival are secondary effects caused by the ectopic cell fate change.
Cap mesenchyme cells lacking Pax2 function can transdifferentiate into renal interstitial progenitors and inner renal medullary interstitial cells
To determine cell types of the Pax2-deficient cap mesenchyme-derived cells, we examined cell type marker expression in β-gal+ cells in Pax2 CM mutant kidneys at 18.5 dpc (Fig 4A,B). All cap mesenchyme markers examined, including SIX2, CITED1, CDH2 (N-cadherin) and ITGA8 (integrin α8), were not detected in the cortical region of Pax2 CM mutant kidneys (Fig 4C–F,S3A–H), suggesting that Pax2-deficient cap mesenchyme cells failed to maintain the cap mesenchyme status. Further, no KRT− or low LAM+ CDH1+ differentiating nephron epithelial cells were detected in Pax2 CM mutants (Fig 4G,H and data not shown), indicating a failure of the expected mesenchymal to epithelial transition (MET) of differentiating cap mesenchyme-derived cells during nephron differentiation.
Figure 4. Cap mesenchyme cells lacking Pax2 function can differentiate into nephrogenic and inner renal medullary interstitial cells.
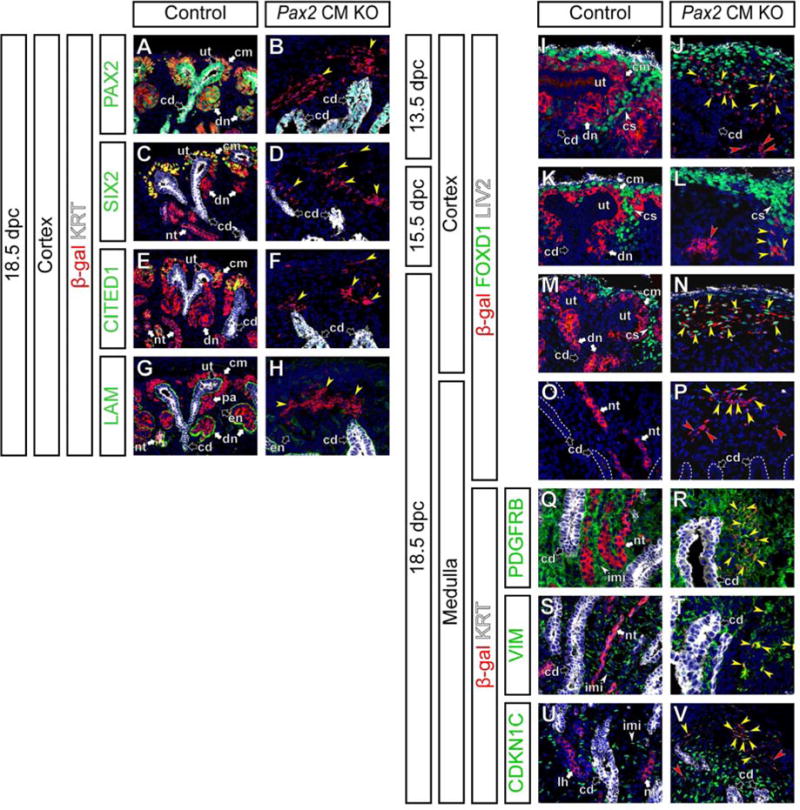
Confocal immunofluorescence of kidneys from Control and Pax2 CM KO mice with β-gal (cap mesenchyme-derived cells; red) and Hoechst (nucleus; blue) staining. (A–H) Cortical regions at 18.5 dpc with cytokeratin (KRT, ureteric tip and collecting duct; white) with PAX2 (A,B), SIX2 (C,D), CITED1 (E,F) and Laminin (LAM) (G,H) staining in green. Yellow arrowheads in B,D,F,H indicate β-gal+ cells without PAX2 expression (B), cap mesenchyme markers (SIX2 in D and CITED1 in F) or nephron tubule makers (LAM+ KRT− or low in H) in Pax2 CM mutants. (I–P) Cortical (I–N) and medullary (O,P) regions at 13.5 dpc (I,J), 15.5 dpc (K,L) and 18.5 dpc (M–P) with FOXD1 (cortical stroma; green) and LIV2 (connective tissue and renal capsule; white) staining. Yellow and red arrows in J,L,N,P indicate β-gal+ FOXD1+ (β-gal in the cytoplasm and FOXD1 in the nucleus) and β-gal+ FOXD1− cells, respectively. (Q–V) Medullary regions at 18.5 dpc with cytokeratin (KRT, collecting duct; white) staining with PDGFRB (Q,R), Vimentin (VIM) (S,T) and CDKN1C (also known as P57KIP2) (U,V) staining in green. Yellow arrowheads in R,T,V indicate cytoplasmic β-gal+ cells expressing a interstitial marker (membranous PDGFRB in R, cytoplasmic VIM in T and nuclear CDKN1C in V). Red arrowheads in V indicate β-gal+ CDKN1C− cells. Note that not all inner renal interstitial cells express CDKN1C in controls (U). Abbreviations as in Figure 2 and cs, renal cortical stroma; pa, pretubular aggregate.
Analysis of FOXD1 as a marker of renal interstitial progenitor cells showed some Pax2-deficient cap mesenchyme-derived cells expressing cytoplasmic β-gal also ectopically express FOXD1 in the nucleus at a low level at 13.5 and 15.5 dpc (Fig 4I–L). At 18.5 dpc in Pax2 CM mutant kidneys, some β-gal+ cap mesenchyme-derived cells showed higher FOXD1 in the cortical region (Fig 4M,N) and some maintain lower FOXD1 expression in the medullary region (Fig 4O,P). These β-gal+ FOXD1+ cells in Pax2 CM mutants also expressed PDGFRB at low levels (Fig S3I–J″), indicating that these cells are not FOXD1+ PDGFRB− podocytes (Brunskill et al., 2011; Kobayashi et al., 2014). Thus, our data suggest that Pax2-deficient cap mesenchyme cells can differentiate into FOXD1+ PDGFRBlow renal cortical stroma cells.
Some of the β-gal+ interstitial cells were FOXD1− at each stage examined suggesting this population may represent a distinct interstitial cell fate (red arrowheads in Figure 4J,L,P and data not shown). At 18.5 dpc, PDGFRB was expressed broadly in the renal interstitium of control kidneys (Fig 2G,4Q), VIM was expressed in the renal capsule, renal cortical and medullary interstitium (Fig 2O,O′,O″,4S), ITGA8 was highly expressed in the cap mesenchyme in the nephrogenic zone as well as the inner renal medullary interstitium (Fig S3E,G), and CDKN1C (also known as P57KIP2) was observed in a subset of inner renal medullary interstitial cells around the collecting duct system (Fig 4U) and maturing podocytes. In the medullary region of control kidneys, β-gal+ cap mesenchyme-derived cells contributed to the nephron epithelia, but did not contribute to the renal interstitium, including the PDGFRB+, VIM+, ITGA8+ CDKN1C+ or − inner renal medullary interstitium (Fig 4Q,S,U,S3G). In Pax2 CM mutant kidneys at 18.5 dpc, β-gal+ cap mesenchyme-derived cells contributed to PDGFRB+, VIM+, ITGA8+ CDKN1C+ or − inner renal medullary interstitial cells (Fig 4R,T,V,S3H). Further, PDGFRB, VIM and CDKN1C expression was already observed in some β-gal+ cap mesenchyme-derived cells in Pax2 CM mutants at 15.5 dpc (Fig S3K–P). Taken together, these observations suggest that some Pax2-deficient cap mesenchyme cells can change cell fate into renal interstitial cell types expressing markers for the cortical stroma and inner renal medullary interstitium.
Transdifferentiation of Pax2-deficient cap mesenchyme cells into renal interstitial cells is mediated through transient appearance of a SIX2+ FOXD1+ cell type
We next asked what cellular mechanisms mediate the transdifferentiation of Pax2-deficient cap mesenchyme cells into renal interstitial cell types. SIX2+ cap mesenchyme and FOXD1+cortical stroma cells may derive from common progenitors, although there is no definitive evidence due to technical difficulties for clonal analysis of limited number of nephrogenic mesenchyme cells at early stages (Mugford et al., 2008). Therefore, one possibility is that SIX2+ FOXD1− cap mesenchyme cells de-differentiate into SIX2− FOXD1− nephrogenic mesenchyme cells followed by re-differentiation into SIX2− FOXD1+ cortical stroma cells. If this is the case, it is expected that SIX2 and FOXD1 will not be co-expressed in a transdifferentiating cell. Alternatively, a process that is not frequently observed during normal kidney development may mediate the transdifferentiation.
Therefore, we examined SIX2 and FOXD1 expression in β-gal+ cap mesenchyme-derived cells in Pax2 CM mutants. In control kidneys at 13.5 dpc, detection of a SIX2+ FOXD1+ cells was a very rare event (Fig 5A,C,E) and tracing of almost all β-gal+ cap mesenchyme-derived cells did not detect a contribution of the SIX2+ derivatives to FOXD1+ cortical stroma (Fig 5G). In Pax2 CM mutant kidneys at the same stage, SIX2+ FOXD1+ double positive cells were abundantly observed (Fig 5B,D,F). All of these SIX2+ FOXD1+ cells were β-gal+ (Fig 5H), indicating their cap mesenchyme origin. These cap mesenchyme-derived SIX2+ FOXD1+ double positive cells were also observed in Pax2 CM mutants at 15.5 dpc (Fig S3Q–R‴). These data suggest that a cellular pathway that is not frequently seen in normal development mediates the transdifferentiation of Pax2-deficient cap mesenchyme cells into renal interstitial cells through transient SIX2+ FOXD1+ double positive cells.
Figure 5. Pax2-deficient cap mesenchyme cells transiently become SIX2+ FOXD1+ double positive cells.
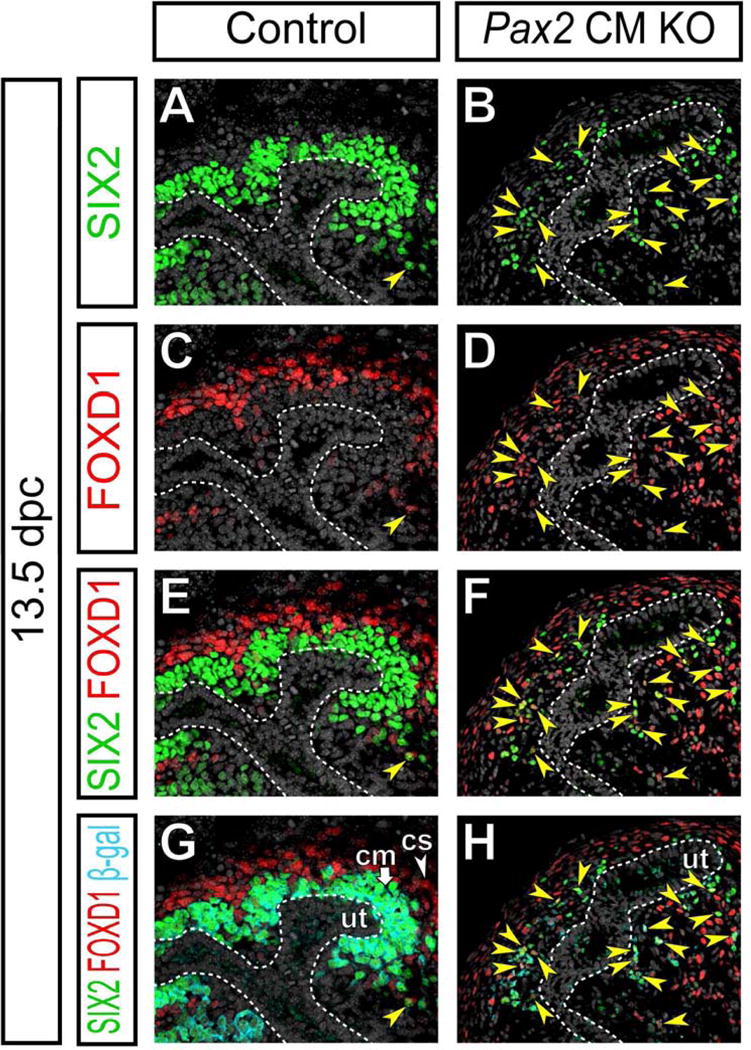
Confocal immunofluorescence of the cortical region of kidneys from Control and Pax2 CM KO mice at 13.5 dpc with SIX2 (green), FOXD1 (red), β-gal (cap mesenchyme-derived cells; cyan) and Hoechst (nucleus; gray) staining. Yellow arrowheads and white dotted lines indicate SIX2+ FOXD1+ β-gal+ cells and the collecting duct system, respectively. Abbreviations as in Figure 2.
Pax2 function cell autonomously represses renal interstitial cell fates in cap mesenchyme cells throughout kidney development
Although SIX2 expression is largely lost in Pax2 CM mutant kidneys at 18.5 dpc (Fig 2I,J,4C,D), we observed Six2-GFP expression remained at low levels (Fig S4). One possibility is that the half-life of CreGFP is longer than that of SIX2. It is also possible that Pax2-deficient cap mesenchyme-derived cells repressed endogenous SIX2 expression, but not GFP expression from the Six2-eGFPCre BAC transgene efficiently. Alternatively, the Six2-eGFPCre transgene is activated ectopically in the Six2− renal interstitium of Pax2 CM mutant kidneys, which would invalidate the fate map analysis of the cap mesenchyme. To exclude the latter possibility, we performed fate map analysis of Pax2-deficient cap mesenchyme cells using the inducible Six2-eGFPCreERT2 mouse allele, which faithfully recapitulates the endogenous Six2 expression (Kobayashi et al., 2008). Because induction of eGFPCreERT2 by tamoxifen often results in mosaic recombination, this approach also allows mosaic analysis for Pax2 function in the cap mesenchyme (Fig 6A). If Pax2 function is required cell-autonomously in cap mesenchyme cells, Pax2-deficient cells may transdifferentiate into renal interstitial cell types. If Pax2 function is non cell-autonomously required, loss of Pax2 in some cap mesenchyme will be compensated by surrounding Pax2+ cells, resulting in retention of the cap mesenchyme identity in Pax2-deficient cells.
Figure 6. Pax2 function in cap mesenchyme cells is required cell-autonomously to repress transdifferentiation into renal interstitial cells at a later stage of kidney development.
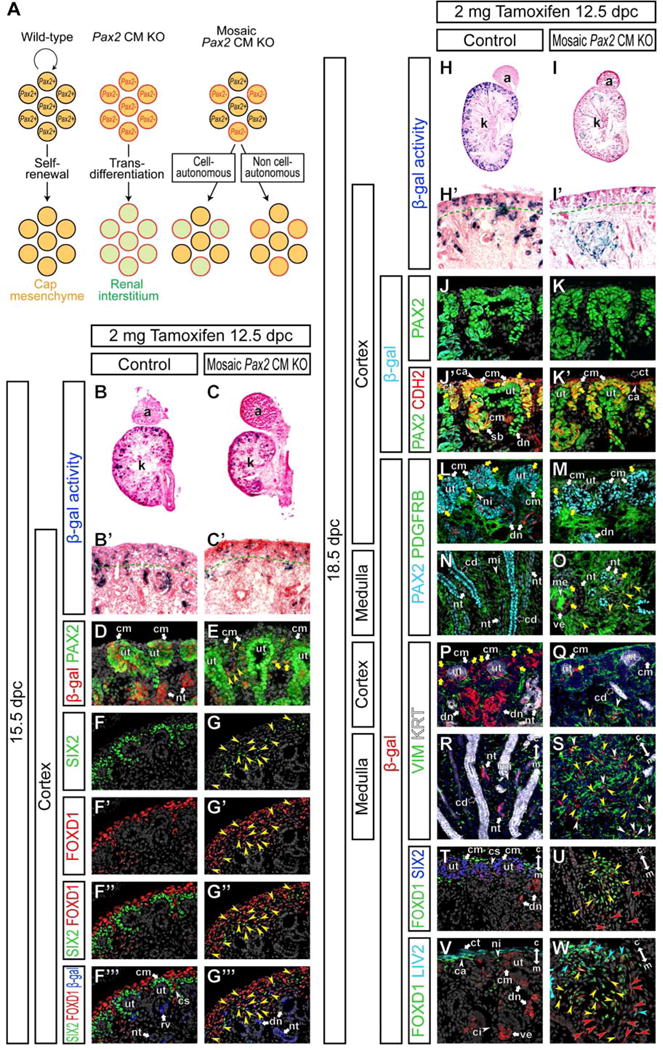
(A) Schematic illustration of mosaic analysis for Pax2 function in cap mesenchyme cells. In wild-type kidneys, Pax2 wild-type (black perimeter) cap mesenchyme cells (orange fill) can self-renew. Pax2-deficient (red perimeter) cap mesenchyme cells transdifferentiate into renal interstitial cell types (green fill) in the Pax2 CM mutants. In mosaic cap mesenchyme-specific Pax2 (mosaic Pax2 CM) mutants, some cap mesenchyme cells remain Pax2+, while the others become Pax2−. If Pax2 function is cell-autonomously required to repress renal interstitial cell fates (1), Pax2− cells will transdifferentiate into renal interstitial cells and may become excluded from the cap mesenchyme. If Pax2 function is required non cell-autonomously by regulating secreted factors or membrane-bound molecules signaling extracellularly (2), defects in Pax2− cells will be compensated by surrounding Pax2+ cells. Therefore, Pax2− cells will be able to remain as cap mesenchyme cells. (B–W) Kidneys from Six2eGFPCreERT2/+; Pax2flox/+; R26RlacZ/+ control mice (Control) and Six2eGFPCreERT2/+; Pax2flox/del; R26RlacZ/+ mosaic cap mesenchyme-specific Pax2 mutant mice (Mosaic Pax2 CM KO) after injection of 2 mg of tamoxifen into dams at 12.5 dpc. (B and C) X-gal (blue) stained section counter-stained with eosin (pink) at 15.5 dpc. (B′ and C′) High magnification of cortical regions in B and C, respectively. Green dotted lines indicate the boundary between the nephrogenic zone and renal cortex. (D and E) Confocal immunofluorescence of the cortical region of the kidney at 15.5 dpc with β-gal (cap mesenchyme-derived cells; red), PAX2 (green) and Hoechst (nucleus; gray) staining. Yellow arrowheads and yellow arrows in E indicate β-gal+ cells with reduced and normal PAX2 expression levels, respectively. (F–G‴) Confocal immunofluorescence of the cortical region of the kidney at 15.5 dpc with SIX2 (green), FOXD1 (red), β-gal (cap mesenchyme-derived cells; blue) and Hoechst (nucleus; gray) staining. Yellow arrowheads indicate SIX2+ FOXD1+ β-gal+ cells. (H and I) X-gal (blue) stained sections counter-stained with eosin (pink) at 18.5 dpc. (H′ and I′) High magnification of cortical regions in H and I, respectively. Green dotted lines indicate the boundary between the nephrogenic zone and renal cortex. (J–W) Confocal immunofluorescence of the kidney at 18.5 dpc with β-gal (cap mesenchyme-derived cells; cyan in J–K′ and red in L–W) and Hoechst (nucleus; gray in J–O,T–W and blue in P–S) staining. (J–K′) The cortical region with PAX2 (green) and N-cadherin (CDH2; red) staining. Yellow arrows in J′,K′ indicate β-gal+ PAX2+ cells in the CDH2+ cap mesenchyme. (L–O) The cortical (L,M) and medullary (N,O) regions with PAX2 (cyan) and PDGFRB (green) staining. Yellow arrows in L,M indicate β-gal+ PAX2+ cells in the cap mesenchyme. Yellow arrowheads and yellow arrows in O indicate β-gal+ PDGFRB+ cells and β-gal+ cells in the nephron epithelium, respectively. (P–S) The cortical (P,Q) and medullary (R,S) regions with VIM (green) and cytokeratin (KRT; white) staining. Yellow arrows in P,Q, yellow arrowheads and white arrowheads in Q,S indicate β-gal+ cells in the cap mesenchyme, and β-gal+ VIM− and β-gal+ VIM+ cells in interstitial aggregates in mosaic Pax2 CM mutants, respectively. (T–W) Cortical regions (T,V) and interstitial aggregates (U,W) with FOXD1 (green) and SIX2 (blue) (T,U) or LIV2 (cyan) (V,W) staining. Yellow arrowheads in U, β-gal+ FOXD1+ cells; red arrowheads in U, β-gal+ FOXD1− cells. Cyan arrowheads in W, β-gal+ FOXD1+ LIV2+ cells; yellow arrowheads in W; β-gal+ FOXD1+ LIV2− cells; red arrowheads in W, β-gal+ FOXD1− LIV2− cells. Double arrows in R–W indicate the cortico-medullary axis of the kidney. Abbreviations as in Figure 2 and cs, renal cortical stroma.
We injected 2 mg of tamoxifen into dams at 12.5 dpc and examined β-gal activity in Six2eGFPCreERT2/+; Pax2flox/del; R26RlacZ/+ mutant embryos (mosaic Pax2 CM KO) at 15.5 dpc; Six2eGFPCreERT2/+; Pax2flox/+; R26RlacZ/+ littermates were used as controls. At this stage, β-gal+ cells were observed in a subset of cap mesenchyme cells in the nephrogenic zone of both controls and mosaic Pax2 CM mutant kidneys at 15.5 dpc (Fig 6B–C′). Although there was variation between embryos, we observed around 30–50% of cap mesenchyme cells were β-gal+ in both controls and mosaic Pax2 CM mutants under these conditions (Fig 6D,E and data not shown). In mosaic Pax2 CM mutants, most of β-gal+ cells showed reduced PAX2 expression and some started to detach from the ureteric tip at this stage (yellow arrowheads in Fig 6E). A small subset of β-gal+ cells maintained the normal PAX2 expression level (yellow arrows in Figure 6E), most likely due to unlinked recombination of the Pax2 flox and Rosa26-lacZ alleles.
At the same stage, while SIX2 and FOXD1 expression patterns were largely mutually exclusive in control kidneys, SIX2+ FOXD1+ double positive cells were abundantly observed in mosaic Pax2 CM mutant kidneys (Fig 6F–G″). Most of these SIX2+ FOXD1+ cells in mosaic Pax2 CM mutants also expressed β-gal (Fig 6F‴,G‴), indicating their cap mesenchyme origin.
Cell tracing at 18.5 dpc showed that β-gal+ cells persisted in mosaic Pax2 CM mutants (Fig 6H,I) but in contrast to control kidneys where β-gal+ cells were maintained in the nephrogenic zone of control kidneys, β-gal+ cells were reduced in the nephrogenic zone of mosaic Pax2 CM mutants (Fig 6H′,I′). No PAX2− cells were detected in the CDH2+ cap mesenchyme of mosaic Pax2 CM mutant kidneys (Fig 6J–K′), suggesting that Pax2 function is cell-autonomously required for cap mesenchyme development. All β-gal+ cells in the CDH2+ cap mesenchyme also expressed PAX2 in mosaic Pax2 CM mutants (yellow arrow in Fig 6K′), indicating that the Pax2 flox allele was not recombined in these β-gal+ cap mesenchyme cells due to the unlinked recombination.
In mosaic Pax2 CM mutants, large aggregates of β-gal+ cap mesenchyme-derived cells were observed within the renal interstitium around the border of the renal cortex and outer renal medulla (Fig 6H–I′). All β-gal+ cap mesenchyme-derived cells localized in the renal interstitium expressed PDGFRB (Fig 6L–O), suggesting transdifferentiation into renal interstitial cell types. As above, rare β-gal+ cells observed in the cap mesenchyme and nephron epithelia that were PDGFRB− resulted from a partial recombination and retain PAX2 expression in PAX2+ tissues (Fig 6M,O).
In the interstitial aggregates in mosaic Pax2 CM mutants at 18.5 dpc, a subset of β-gal+ cells expressed VIM (Fig 6P–S), indicating that Pax2-deficient cap mesenchyme-derived cells differentiated into multiple cell types. While these β-gal+ cells did not express a nephron progenitor marker SIX2, a renal interstitial progenitor marker FOXD1 was ectopically expressed in a subset of β-gal+ cells within the interstitial aggregates (Fig 6T,U). Examination of LIV2 expression together with FOXD1 further divided the β-gal+ FOXD1+ cells into sub populations; LIV2+ FOXD1+ and LIV2− FOXD1+ cells (Fig 6V,W). Although boundaries were not strict, there was tendency for LIV2+ FOXD1+, LIV2− FOXD1+ and LIV2− FOXD1− cells to be localized along the cortico-medullary axis within the interstitial aggregate (blue, yellow and red arrowheads in Fig 6W, respectively). These expression patterns in a interstitial aggregate in mosaic Pax2 CM mutants are reminiscent of interstitial layers in the cortical region of the kidney; the LIV2+ FOXD1+ VIM− SIX2− renal capsule, LIV2− FOXD1+ VIMlow SIX2− nephrogenic interstitium and LIV2− FOXD1− VIM+ SIX2− renal cortical interstitium along the cortico-medullary axis of the kidney (Fig 6P,T,V). In controls, no β-gal+ cell was observed in the interstitium (Fig 6L,N,P,R,T,V). This cell-autonomous Pax2 function to maintain cap mesenchyme cells by repressing renal interstitial cell fates is required continuously at a later stage of kidney development, 14.5 dpc (Fig S5).
Single cell RNA-seq profiling revealed repression of nephron programs, but activation of renal interstitial programs in Pax2-deficient cap mesenchyme-derived cells
The nephron epithelium and renal interstitium are heterogeneous populations containing molecularly distinct cell types. To compare global gene expression profiles of transdifferentiating Pax2-deficient cap mesenchyme cells to those in the nephron and interstitial lineages, we performed single cell RNA-seq analysis. By using a single cell strategy we could better define intermediate stages in the transdifferentiation process as well as better characterize the multiple cell types produced by the Pax2 mutant cells. Using FACS analysis, tdTomato+ Pax2-deficient cap mesenchyme-derived cells were isolated from Six2-eGFPCretg/+; Pax2flox/del; R26R-CAGGStdTomato/+ mutant kidneys at 13.5 and 18.5 dpc. Control cells were collected from Six2-eGFPCretg/+; Pax2flox/+; R26R-CAGGStdTomato/+ kidneys at the same stages. For renal interstitial cells, tdTomato+ cells were isolated from Foxd1eGFPCreERT2/+; Pax2flox/+; R26R-CAGGStdTomato/+ kidneys at 18.5 dpc following an injection of 2 mg tamoxifen at 11.5 dpc, which labels all cell types in the renal interstitium (Kobayashi et al., 2014).
The single cell RNA-seq data was first used to provide a global view of the altered gene expression profiles of the Pax2 CM mutant cells. We were particularly interested in their potentially altered cap mesenchyme versus interstitial character. A list of 792 genes that distinguish wild type interstitial cells from wild type cap mesenchyme and its descendant cells at 18.5 dpc was derived (P < 0.05, FC > 3) and used for hierarchical clustering (Pearson centered, Wards linkage rule) (Fig 7A). At 13.5 dpc the Pax2-deficient cap mesenchyme-derived cells showed significant heterogeneity. Most clustered with the control cap mesenchyme-derived cells, showing that they had not yet acquired significant interstitial gene expression. Other cells, however, clustered among the interstitial cells and showed repression of cap mesenchyme markers and activation of interstitial specific genes (asterisk in Fig 7A). At 18.5 dpc almost all of the Pax2-deficient cap mesenchyme-derived cells showed a convincing interstitial gene expression signature. Strongly down regulated genes in the Pax2-deficient cap mesenchyme-derived cells at 18.5 dpc included Six2, Crym, and Eya1, markers of the cap mesenchyme, while strongly up regulated genes included Meis1, Anxa2, and Foxd1, markers of the interstitium. These results provide a comprehensive validation of the striking change in character of the Pax2-deficient cap mesenchyme-derived cells, diverging to an interstitial cell type.
Figure 7. Gene expression profiles for Pax2-mutant cap mesenchyme-derived cells are similar to those in renal interstitial cells than nephron epithelial cells, but distinct.
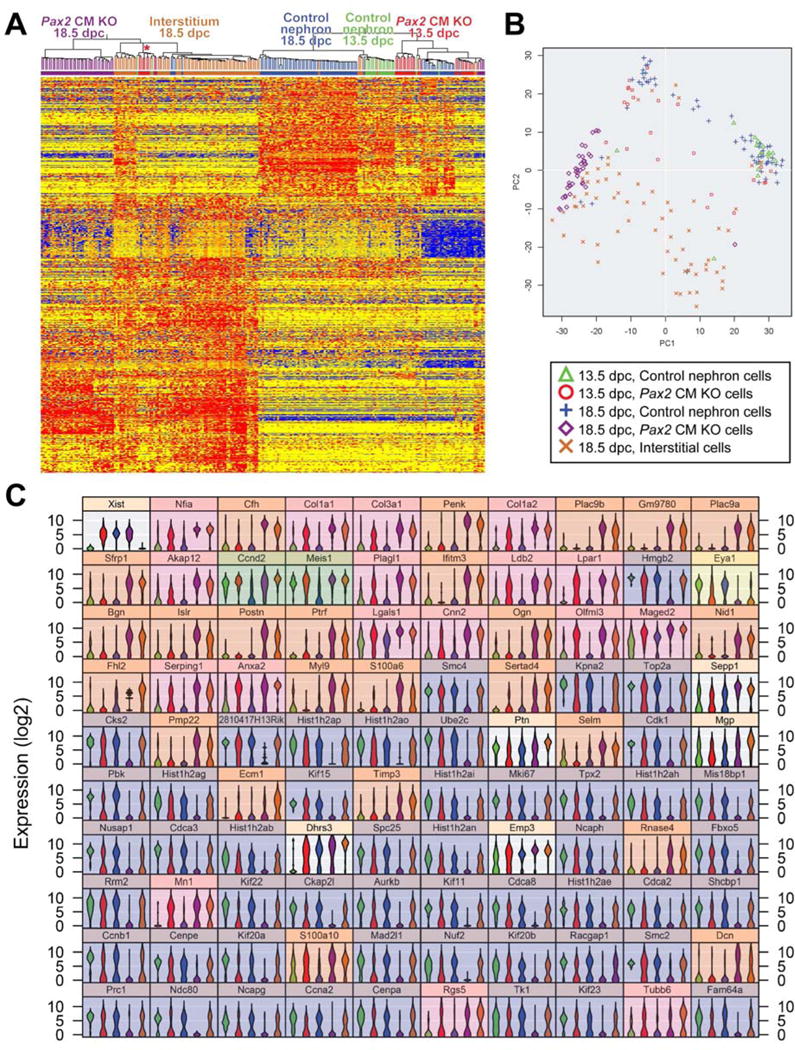
(A) Heat map and unsupervised hierarchical clustering by GeneSpring for single cell RNA-seq analysis of Pax2-mutant cap mesenchyme-derived cells in Six2-eGFPCretg/+; Pax2flox/del; R26R-CAGGStdTomato/+ mutant kidneys at 13.5 and 18.5 dpc, control nephron cells from Six2-eGFPCretg/+; Pax2flox/+; R26R-CAGGStdTomato/+ kidneys at 13.5 and 18.5 dpc and renal interstitial cells from Foxd1eGFPCreERT2/+; Pax2flox/+; R26R-CAGGStdTomato/+ kidneys at 18.5 dpc following 2 mg tamoxifen injection at 11.5 dpc. The red asterisk indicates Pax2 mutant cells expressing interstitial genes at 13.5 dpc. (B) PCA plot by AltAnalyze. (C) Violin plot for the top 100 discriminating genes by AltAnalyze.
Principle components analysis of the single cell expression data provided further insight. A PCA plot showed that most control cap mesenchyme-derived cells at 13.5 dpc had similar gene expression profiles (Fig 7B). Through differentiation into diverse cell types in the nephron epithelium, control cells at 18.5 dpc exhibit showed more diverse gene expression profiles (Fig 7B). Renal interstitial cells at 18.5 dpc had distinct gene expression profiles from control cells in the nephron lineage at 13.5 or 18.5 dpc (Fig 7B). Interestingly, at 18.5 dpc, renal interstitial cells showed larger diversity than the control nephron cells (Fig 7B), indicating that the renal interstitium is a highly heterogeneous population at this stage.
Many Pax2-deficient cap mesenchyme-derived cells at 13.5 dpc showed gene expression profiles intermediate between the nephron and renal interstitial cells (Fig 7B). This PCA plot verified that Pax2-deficient cells at 18.5 dpc exhibited gene expression profiles much more similar to renal interstitial cells than nephron cells (Fig 7B). Nevertheless, the Pax2 mutant cells did not show the full heterogeneity of signatures associated with the wild type interstitium, suggesting that they might represent a specific subtype. Of interest, the single cell RNA-seq data showed robust expression of several interstitial type marker genes in the Pax2-deficient cap mesenchyme-derived cells. For example, 27 of the top 36 Vim+ cells in this study, including wild type interstitial cells, were Pax2-deficient cap mesenchyme-derived cells. Likewise, ten of the top sixteen Foxd1+ cells were Pax2 mutant, with the other six being wild type interstitial cells. The top five cells with the strongest Acta2+ were all Pax2 mutant. Nevertheless, as measured by global gene expression patterns, the Pax2 mutant cells did not clearly fall within any of the wild-type interstitial Foxd1+, Vim+, Tnc+ or Acta2+ subtypes (data not shown).
The top 100 genes that discriminate the multiple single cell sample types were identified using AltAnalyze (Salomonis et al., 2010) (Fig 7C). The most highly differentially expressed gene was Xist, an X-chromosome inactivation gene (Fig 7C), simply reflecting the different sexes of embryos used. The remaining genes were largely classified into two major groups. The most discriminating category contained genes up-regulated in Pax2-mutant cells compared to control cells and also highly expressed in the interstitial cells (41 genes out of the 99 genes; 41.4%) (Fig 7C); 17 genes (17.2%) and 24 genes (24.2%) were up-regulated in Pax2-mutant cells by 13.5 and 18.5 dpc, respectively (highlighted in pink and orange in Fig 7C). Among these genes, high-quality expression data were available for 35 genes in the GenePaint, Euroexpress and GUDMAP databases (Diez-Roux et al., 2011; Harding et al., 2011; Visel et al., 2004). These expression data confirmed expression patterns in the renal interstitium for 33 genes (94.3%) including collagens (Col1a1, Col3a1 and Col1a2), Anxa2 and Dcn (decorin) (Fig S6A,B) (Brunskill et al., 2014; Fetting et al., 2014). These data indicate that renal interstitial genes were up-regulated in the Pax2-mutant cells.
Expression of some genes (Ccnd2 and Meis1) was down-regulated in the control cells but high in the interstitial cells at 18.5 dpc (highlighted in green in Fig 7C). Expression of these genes was maintained in the Pax2 mutant cells at 13.5 and 18.5 dpc (Fig 7C), indicating that the repression of their expression observed during nephron differentiation did not occur in Pax2-deficient cap mesenchyme cells.
Eya1 is specifically expressed in the nephron lineage, but not in the renal interstitium (Xu et al., 2014). Interestingly, Eya1 expression is lost at 13.5 dpc in mutants (highlighted in yellow in Fig 7C), indicating that Pax2 function is required for Eya1 expression in nephron progenitor cells.
The second major category in the top 99 genes contained 50 genes (50.5%) that were down-regulated in Pax2 mutants at 13.5 dpc and highly repressed at 18.5 dpc compared with high expression in both the control and interstitial cells (highlighted in blue in Fig 7C). In this category, 39 genes (78.0%) were associated with components involved in cell division; cell cycle regulation (Cks2, Ube2c, Cdk1, Cdca3, Cdca8, Cdca2, Ccnb1, Prc1 and Ccna2) (Bertoli et al., 2013; Jiang et al., 1998; Lara-Gonzalez et al., 2012; Walker, 2001), histone structure (Hist cluster genes), condensins (Smc4, Top2a, Ncaph, Smc2 and Ncapg) (Hirano, 2012), centromere assembly (Mis18bp1, Cenpe and Cenpa) (McKinley and Cheeseman, 2016), mitotic spindle assembly checkpoint at the kinetochore (Spc25, Mad2l1, Nuf2 and Ndc80) (Cheeseman and Desai, 2008; Foley and Kapoor, 2013; Lara-Gonzalez et al., 2012), attachment of the mitotic spindle to the centromere (Aurkb) (Carmena et al., 2009), spindle microtubule organization (Tpx2 and Nusap1) (Neumayer et al., 2014; Raemaekers et al., 2003), transport along the microtubule (Kif15, Kif22, Kif11, Kif20a, Kif20b and Kif23) (Hirokawa et al., 2009), and cell proliferation as an antigen identified by antibody Ki-67 (Mki67). Among the 50 genes, the public databases contained expression patterns for 36 genes and all (100.0%) were highly expressed in the nephrogenic zone (Fig S6C), where cell proliferation is the most active in the developing kidney (Saifudeen et al., 2002).
As described above, in the cap mesenchyme-specific Pax2 mutants, loss of the cap mesenchyme causes loss of the nephrogenic zone, leading to arrest of kidney growth around 12.5 dpc (Fig 2A–F), consistent with the reduced cell proliferation at the same stage (Fig 3M). Reduced cell division partially explains the differences in the gene expression profiles for the Pax2-mutant cells and renal interstitial cells. It is clear, however, at the global level, the Pax2-deficient cap mesenchyme-derived cells undergo a dramatic shift, transforming towards an interstitial identity (Fig 7). Taken together, our observations suggest that Pax2 function represses a renal interstitial cell program in nephron progenitor cells.
DISCUSSION
Pax2 function in nephron progenitor cells
Pax2 is expressed in multiple tissues in the developing kidney and has been regarded as a key regulator of kidney development since its cloning 25 years ago (Dressler et al., 1990). Consistent with this view, Pax2 is required for nephric (Wolffian) duct development prior to the onset of kidney (metanephros) development (Bouchard et al., 2002; Torres et al., 1995). Further, human mutations in PAX2 are associated with kidney anomalies (Sanyanusin et al., 1995). However, despite extensive molecular studies on Pax2 function, there has been no study specifically addressing Pax2’s possible role within the nephron progenitor pool. The conditional Pax2 null allele reported here gives new insights into Pax2 function by providing a useful genetic model for broadly assessing the temporal and spatial action of Pax2 in potentially any organ system.
Our previous cell fate analysis highlighted specific multipotent progenitor populations for the nephron epithelium and renal interstitium within the ureteric branch tip niche that drives kidney assembly: Six2+ cap mesenchyme defines a self-renewing nephron progenitor pool and the more peripherally positioned Foxd1+ population defines a self-renewing renal interstitial progenitor pool (Kobayashi et al., 2014; Kobayashi et al., 2008). Fate mapping also demonstrated that these are mutually exclusive cell populations shortly after the initiation of metanephric kidney organogenesis, indicating a lineage boundary between nephron forming epithelia and renal interstitial compartments of the developing kidney (Kobayashi et al., 2014). Our findings herein suggest that Pax2 function in nephron progenitor cells is required to maintain the nephron epithelia-interstitium lineage boundary by repressing an alternative renal interstitium-forming pathway cell-autonomously (Fig S7). Thus, Pax2 is a key transcriptional factor in nephron specification, most likely acting in conjunction with several other transcriptional regulators critical for the nephron progenitor state (Hendry et al., 2011; O’Brien and McMahon, 2014). With kidney disease, the nephrons are lost while the renal interstitium expands (Humphreys et al., 2010). Modulating the action of Pax2 in interstitial cell lineages could have value in developing regenerative strategies to treat kidney disease by overcoming the lineage boundary segregating the nephron and renal interstitial compartments.
Maintenance of the nephron progenitor status
While there are several factors regulating proliferation and survival of nephron progenitor cells (Barak et al., 2012; Blank et al., 2009; Couillard and Trudel, 2009; Kanda et al., 2014), only a few factors are known to maintain the nephron progenitor status. The Six2 gene cell-autonomously maintains cap mesenchyme cells by repressing precocious differentiation into mature nephron epithelial cell types (Fig S7) (Kobayashi et al., 2008; Park et al., 2012; Self et al., 2006). Recently, it was shown that EYA1 interacts with SIX2 in nephron progenitor cells during this process (Xu et al., 2014). Inactivation of Six2 or Eya1 in the cap mesenchyme results in ectopic formation of nephrons on the cortical side of ureteric tip (Kobayashi et al., 2008; Self et al., 2006; Xu et al., 2014). Although Pax2 also maintains the nephron progenitor status, its genetic mechanism is novel. Pax2 function maintains nephron progenitor cells mainly by suppressing transdifferentiation into renal interstitial cell types whereas Six2 predominantly acts to block commitment to mature nephron cell types (Fig S7).
Pax2-deficient cap mesenchyme cells lose cap mesenchyme status as they detach from the ureteric tip. It is thought that the ureteric tip provides a niche for nephron progenitor cells (Barak et al., 2012; Karner et al., 2011). One possibility is that Pax2 directly regulates genes essential for the nephron progenitor state, loss of which may cause the detachment from the ureteric tip. Alternatively, Pax2 function in nephron progenitor cells may regulate cell adhesion to the ureteric tip niche. It is also possible that Pax2 represses activation of genes specific to interstitial cells, which are located distantly from the ureteric tip. Our studies cannot address whether PAX2 acts as a transcriptional activator and/or repressor in nephron progenitor cells (Abraham et al., 2015). Future studies will distinguish these alternative mechanisms.
Transdifferentiation of Pax2-deficient nephron progenitor cells into renal interstitial cell types
Transdifferentiation can be observed in some genetic mutants and during regeneration after injury. Some transdifferentiation processes are mediated by de-differentiation followed by re-differentiation, and some by artificial events (Jopling et al., 2011). Our observations suggest that transdifferentiation of Pax2-deficient nephron progenitor cells into renal interstitium-like cells unfolds in a process involving a transient population of SIX2+ FOXD1+ cells that are not frequently observed during normal kidney development, although possible involvement of partial de- and re-differentiation cannot be excluded.
Interestingly, during mammalian development, mis-regulation of some Pax genes can cause transdifferentiation from one lineage to another in various progenitor cells, including Pax4 function for α and β/γ cell lineages during pancreas development (Collombat et al., 2009; Sosa-Pineda et al., 1997), Pax5 for B and T cell lineages during lymphocyte development (Cobaleda et al., 2007) and Pax6 for multiple neuroretinal lineages from the amacrine cell lineage during eye development (Marquardt et al., 2001). One of the common roles for Pax genes might be cell lineage specification in progenitor cells during mammalian development, including Pax2 in nephron progenitor cells during kidney development as we described. Our single cell RNA-seq data suggested that transdifferentiated renal interstitium-like cells derived from Pax2-null nephron progenitor cells had somewhat different gene expression profiles from those in endogenous renal interstitial cells, probably because ectopically localized Pax2-mutant cells in an abnormal kidney cannot proliferate normally and/or fully differentiated into the mature renal interstitial cells.
Heterogeneity during formation of renal interstitial cell types
Molecular marker analysis showed considerable heterogeneity in the renal interstitium. In Pax2 CM mutants, not all renal interstitial cell types were observed, likely suggesting that loss of nephrons and arrested ureteric branching may affect renal interstitial differentiation.
An ectopic interstitial aggregate in mosaic Pax2 CM mutants contains both β-gal+ and β-gal− cells, suggesting that multiple cells aggregated when surrounded by more medullary interstitial cell types. It is possible that different renal interstitial cell types have distinctive cell adhesion properties, which may contribute to formation of interstitial layers along the cortico-medullary axis during kidney development.
Currently, it is unclear why Pax2-deficient cap mesenchyme cells transdifferentiated into a subset of the renal interstitium. When Pax2 is inactivated around ~11.0 dpc by the Six2-eGFPCre BAC transgene, Pax2-deficient cap mesenchyme cells transdifferentiated mainly into renal interstitial cells expressing markers for the nephrogenic and inner renal medullary interstitium. When Pax2 is inactivated at 12.5 dpc by the Six2-eGFPCreERT2 knock-in allele, Pax2-deficient cap mesenchyme cells transdifferentiated into renal interstitial cells expressing more cortical renal interstitial markers for the renal capsule, nephrogenic interstitium and renal cortical interstitium. We previously showed that the medullary interstitium starts to differentiate at early stages of kidney development and the cortical interstitium differentiation initiates at later stages (Kobayashi et al., 2014). It is possible that renal interstitial cell precursors derived from FOXD1+ cortical stroma progenitors remain multipotent initially. The niche for renal interstitial cell precursors may be dynamic and directs different renal interstitial cell fates in a stage-dependent manner during kidney development. However, the cortico-medulla axis is correctly maintained in vestigial kidneys with arrested development in Pax2 CM mutants and in an ectopic interstitial aggregate in mosaic Pax2 CM mutant kidneys. It is also possible that there may be an external positional signal regulating the cortico-medulla axis in the renal interstitium. Further investigation should clarify how multiple renal interstitial cell types are specified and organized in the cortico-medullary layers during kidney development.
STAR★METHODS
KEY RESOURCES TABLE
Please see the separately uploaded file.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Akio Kobayashi (akiok@uw.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse strains
All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by Institutional Animal Care and Use Committees at Harvard Medical School and the University of Washington. All mice were housed in specific pathogen free (SPF) colonies within vivaria.
Six2-eGFPCre BAC transgenic mice (Kobayashi et al., 2008) and Six2-eGFPCreERT2 knock-in mice (Kobayashi et al., 2008) were maintained on a CD-1 × Swiss Webster (Taconic) × C57BL/6J (Jackson Laboratory) mixed background and a 129/Sv × C57BL/6J mixed background, respectively, as previously described (Kobayashi et al., 2008). Rosa26R-lacZ Cre reporter (R26R-lacZ) mice (Soriano, 1999) were purchased from Jackson Laboratory and maintained on a C57BL/6J congenic background using a previously described genotyping methods (Kobayashi et al., 2008). Rosa26-Flpe mice (Farley et al., 2000) were purchased from Jackson Laboratory and maintained on a C57BL/6J × 129/Sv mixed background using a genotyping method with the following primers; Flpe-Fw4: AGCATCATGTGCTGCTGAACTAACCTA, Flpe-Rv3: GCTTATGATAGTATTATAGCTCATGAA, R26-Fw11: CTCCCAAAGTCGCTCTGAGTTGTTATCAGT and R26-Rv12: CTCGGGTGAGCATGTCTTTAATCTACCT, which gives a 103-bp band for the Rosa26-Flpe allele (Flpe-Fw4 and Flpe-Rv3) and a 484-bp band for the Rosa26 wild-type allele (R26-Fw11 and R26-Rv12). Sox2-Cre mice (Hayashi et al., 2002) were maintained on a C57BL/6J × CBA/J mixed background using a genotyping method with the following primers; Cre-Fw13; GGGGCAATGGTGCGCCTGCTGGAAGAT and rbglobin-pA-Rv1; CCTTTATTAGCCAGAAGTCAGATGCTCA, which gives a 220-bp band for the Sox2-Cre allele (Cre-Fw13 and rbglobin-pA-Rv1).
The Pax2 flox mouse allele was generated as follows. A Pax2 targeting vector was constructed using sequence-confirmed homologous arms sub-cloned by PCR from a BAC clone RP23-228G11 containing the Pax2 locus. loxP sites were introduced in PCR primers. An FRT-PGKneobpA-FRT selection cassette (Kobayashi et al., 2008) was introduced following the second loxP site. Detailed description of the targeting strategy is available upon a request. Gene targeting and blastocyst injection were performed as previously described (Behringer et al., 2013; Kobayashi et al., 2008). The FRT-PGKneobpA-FRT selection cassette was removed by breeding with Rosa26-Flpe mice (Farley et al., 2000). Pax2 flox mice were maintained on a 129/Sv × C57BL/6J mixed background. Pax2 flox mice were crossed with R26R-lacZ and maintained on a 129/Sv × C57BL/6J mixed background. To generate Pax2del allele, Pax2 flox mice were crossed with Sox2-Cretg/tg mice (Hayashi et al., 2002) maintained on a CBA × C57BL/6J mixed background.
The Pax2 flox and del mouse allele was genotyped using the following primers; mPax2-Fw29: GGCGCAGGCGGGTTTCTAGTCCGCAGCAGT, mPax2-Rv30: GTGGCTTGGTGGGCGGTCCGGATAGAGGAT, and mPax2-Rv31: ATACCTCCTTTTCTGAATGTAGCTGCCTT, which gives a 288-bp band for the Pax2 wild-type allele (mPax2-Fw29 and mPax2-Rv30), a 334-bp band for the Pax2 flox allele (mPax2-Fw29 and mPax2-Rv30) and a 403-bp band for the Pax2 del allele (mPax2-Fw29 and mPax2-Rv31).
To inactivate Pax2 in the cap mesenchyme, Pax2flox/flox or Pax2flox/flox; R26RlacZ/lacZ females were mated with Six2-eGFPCretg/+; Pax2del/+ or Six2eGFPCreERT2/+; Pax2del/+ males. Littermates were used for controls. Mice were bred using timed-mating, noon on the day of vaginal plug detection considered 0.5 day post coitum (0.5 dpc). For induction of the eGFPCreERT2 protein, tamoxifen (Sigma-Aldrich, T5648) was dissolved in corn oil (Sigma-Aldrich, C8267; Integra Chemical, C819.31) and administrated to dams by intraperitioneal (IP) injection (Danielian et al., 1998).
METHOD DETAILS
Histology
Histology of kidneys was performed as described previously (Kobayashi et al., 2008). Briefly, dissected kidneys were fixed in 4% paraformaldehyde for 1 hr at 4 °C and soaked in 30% sucrose overnight at 4 °C. After embedding in OCT (Sakura, 4583), cryosections were generated at 16 μm using a Leica CM1510 cryostat. Cryosections were blocked with anti-mouse IgG antibody (Jackson ImmunoResearch, 715-007-003) prior to immunostaining to reduce backgrounds from secondary anti-mouse IgG antibodies.
X-gal staining
X-gal staining was performed as described previously (Kobayashi et al., 2005; Kobayashi et al., 2004). Cryosections were stained with X-gal at 37 °C overnight and counter-stained with 0.2% Eosin-Y (Fisher, 23-245-658). Whole-mount kidneys were fixed in 4% paraformaldehyde for 1 hr at 4 °C and stained at 37 °C overnight for embryonic samples or at 4 °C for 2–3 days for neonate samples.
Immunofluorescence
Sections were incubated with primary antibodies against β-gal (MP Biomedicals, 08559761; Abcam, ab9361), CDH1 (E-cadherin) (Thermo Fisher Scientific, 13-1900), CDH2 (N-cadherin) (BD Biosciences, 610920), CDKN1C (P57KIP2) (Abcam, ab4058), CITED1 (Thermo Fisher Scientific, RB-9219-P0), cleaved caspase-3 (Cell Signaling Technology, 9661S), FOXD1 (Santa Cruz Biotechnology; sc-47585, Lot # A1107 and C2310), GFP (Aves labs, GFP-1020; Abcam, ab290), ITGA8 (integrin α8) (R&D Systems, AF4076), KRT (cytokeratin) (Sigma-Aldrich, C2562; DSHB TROMA-I and TROMA-III), LAM (Laminin) (Sigma-Aldrich, L9393), LIV2 (MBL International, D118-3), LTL (Lotus tetragonolobus lectin) (Vector Laboratories, FL-1321), PAX2 (Abcam, ab37129; Abnova, H00005076-M01), PDGFRB (eBiosciences, 14-1402-82), phospho histone H3 (EMD Millipore, 06-570), PODXL (podocalyxin) (R&D Systems, MAB1556), SIX2 (Proteintech, 11562-1-AP), ACTA2 (α-smooth muscle actin) (Sigma-Aldrich, A5228), TNC (tenascin C) (Sigma-Aldrich, T3413), UMOD (Uromodulin, Tamm-Horsfall glycoprotein) (R&D Systems, MAB5175) and VIM (Vimentin) (EMD Millipore, AB5733), and detected by the secondary antibodies with DyLight 488, 549 and 649 (Jackson ImmunoResearch). Sections were stained with Hoechst (Thermo Fisher Scientific, H3570) prior to mounting with Immu-Mount (Fisher, 9990412). Fluorescent images were photographed on a Nikon Eclipse C1si confocal with an 80i fluorescent microscope and a Leica TCS SPE-II confocal system with a DMI 4000 fluorescent microscope.
Single cell RNA-seq
Single cell suspension from embryonic kidneys was prepared as previously described (Kobayashi et al., 2008). FACS purification of tdTomato+ cells was performed using a BD FACSAria II. Following the Fluidigm C1 capture, the individual chambers were microscopically examined for the presence of single cells (Brunskill et al., 2014). The cells were robotically lysed and a series of biochemical reactions will be carried out using the Clontech UltraLow SMARTer amplification chemistry and Illumina/Nextera tagmentation-barcoding, as per Fluidigm recommended protocols. Quality of cDNAs was confirmed using an Agilent 2100 Bioanalyzer. The harvested products were then sequenced and paired end, 75 base reads, by an Illumina HiSeq 2500. The resulting data were analyzed using a combination of GeneSpring (Agilent Technologies), AltAnalyze, ToppGene (Chen et al., 2009), TopCluser (Chen et al., 2013), Cytoscape (Shannon et al., 2003) and STRand (Toonen and Hughes, 2001) programs.
QUANTIFICATION AND STATISTICAL ANALYSIS
For quantification of phospho-histone H3+ (PHH3+) and cleaved caspase-3+ (CC3+) cells in β-gal+ cap mesenchyme-derived cells, data were represented as mean ± SD. Significance was defined using unpaired t-test. Values of n represent number of animals.
DATA AND SOFTWARE AVAILABILITY
The single-cell RNA-seq datasets have been deposited in Gene Expression Omnibus (GEO) under accession number GSE79137.
Supplementary Material
HIGHLIGHTS.
Pax2 represses interstitial fates in nephron progenitor cells of developing kidneys
Pax2-null cells adapt hybrid status of nephron and interstitium progenitor cells
Nephron progenitor cells lacking Pax2 differentiate into renal interstitial cells
Acknowledgments
We thank Dr. Kevin Eggan for v6.5 ES cells and Andrew S. Potter for data analysis by AltAnalyze. Work in A.P.M.’s laboratory was supported by a grant from the NIH DK054364. Work in the A.K.’s laboratory was supported by grants from NIH DK094933 and OD021437, Basil O’Connor Starter Scholar Research Award from March of Dimes, Carl W. Gottschalk Research Scholar Grant from American Society of Nephrology, and grants from the Harvard Stem Cell Institute and John H. Tietze Stem Cell Scientist Research Award from the Institute for Stem Cell and Regenerative Medicine at University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, A.K.; Methodology, S.S.P. and A.K.; Investigation, N.N., K.F., M.F., S.S.P. and A.K.; Resources, M.T.V. and A.P.M.; Writing – Original Draft, A.K.; Writing – Review & Editing, M.T.V., S.S.P., A.P.M. and A.K.; Funding Acquisition, A.P.M. and A.K.
References
- Abraham S, Paknikar R, Bhumbra S, Luan D, Garg R, Dressler GR, Patel SR. The Groucho-associated phosphatase PPM1B displaces Pax transactivation domain interacting protein (PTIP) to switch the transcription factor Pax2 from a transcriptional activator to a repressor. The Journal of biological chemistry. 2015;290:7185–7194. doi: 10.1074/jbc.M114.607424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn D, Maric C, McCausland J. Development of the renal interstitium. Pediatr Nephrol. 1999;13:347–354. doi: 10.1007/s004670050624. [DOI] [PubMed] [Google Scholar]
- Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, et al. FGF9 and FGF20 Maintain the Stemness of Nephron Progenitors in Mice and Man. Developmental cell. 2012;22:1191–1207. doi: 10.1016/j.devcel.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R, Gertsenstein M, Vintersten Nagy K, Nagy A, Nagy KV. Manipulating the mouse embryo : A laboratory manual. Fourth. Cold Spring Harbor Laboratory Press; 2013. [Google Scholar]
- Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram JF, Cullen-McEwen LA, Egan GF, Gretz N, Baldelomar E, Beeman SC, Bennett KM. Why and how we determine nephron number. Pediatr Nephrol. 2014;29:575–580. doi: 10.1007/s00467-013-2600-y. [DOI] [PubMed] [Google Scholar]
- Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L. BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development (Cambridge, England) 2009;136:3557–3566. doi: 10.1242/dev.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubüser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes & development. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreER(T2) mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Developmental biology. 2007 doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SC, Liu Z, Kopan R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development (Cambridge, England) 2014;141:346–354. doi: 10.1242/dev.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy P, Ostrom L, Lang K, Dressler G. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development (Cambridge, England) 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS. Defining the molecular character of the developing and adult kidney podocyte. PloS one. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill EW, Park JS, Chung E, Chen F, Magella B, Potter SS. Single cell dissection of early kidney development: multilineage priming. Development (Cambridge, England) 2014;141:3093–3101. doi: 10.1242/dev.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrian C, Borodo K, Charles N, Herzlinger DA. Morphometric index of the developing murine kidney. Dev Dyn. 2004;231:601–608. doi: 10.1002/dvdy.20143. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Chen G, Wu Y, Wu J, Zheng W. TopCluster: A hybrid cluster model to support dynamic deployment in Grid. Journal of Computer and System Sciences. 2013;79:201–215. [Google Scholar]
- Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic acids research. 2009;37:W305–311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol. 2012;1:693–713. doi: 10.1002/wdev.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Developmental cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard M, Trudel M. C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn. 2009;238:405–414. doi: 10.1002/dvdy.21841. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. Patterning and early cell lineage decisions in the developing kidney: the role of Pax genes. Pediatr Nephrol. 2011;26:1387–1394. doi: 10.1007/s00467-010-1749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development (Cambridge, England) 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development (Cambridge, England) 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, et al. The GUDMAP database–an online resource for genitourinary research. Development (Cambridge, England) 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman H, Lai H, Patterson L. Cessation of renal morphogenesis in mice. Developmental biology. 2007 doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Huh S, Herzlinger D, Soares V, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes & development. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mechanisms of development. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Hendry C, Rumballe B, Moritz K, Little MH. Defining and redefining the nephron progenitor population. Pediatr Nephrol. 2011;26:1395–1406. doi: 10.1007/s00467-010-1750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, Little MH. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol. 2013;24:1424–1434. doi: 10.1681/ASN.2012121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D, Hurtado R. Patterning the renal vascular bed. Seminars in cell & developmental biology. 2014;36:50–56. doi: 10.1016/j.semcdb.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Condensins: universal organizers of chromosomes with diverse functions. Genes & development. 2012;26:1659–1678. doi: 10.1101/gad.194746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Humphreys B, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic Epithelial Cells Repair the Kidney after Injury. Cell stem cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jimenez G, Wells NJ, Hope TJ, Wahl GM, Hunter T, Fukunaga R. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Molecular cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Kanda S, Tanigawa S, Ohmori T, Taguchi A, Kudo K, Suzuki Y, Sato Y, Hino S, Sander M, Perantoni AO, et al. Sall1 maintains nephron progenitors and nascent nephrons by acting as both an activator and a repressor. J Am Soc Nephrol. 2014;25:2584–2595. doi: 10.1681/ASN.2013080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development (Cambridge, England) 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development (Cambridge, England) 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem cell reports. 2014;3:650–662. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer R. Requirement of Lim1 for female reproductive tract development. Development (Cambridge, England) 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–980. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nature medicine. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney international. 1991;39:370–381. doi: 10.1038/ki.1991.49. [DOI] [PubMed] [Google Scholar]
- Levinson R, Mendelsohn C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Seminars in cell & developmental biology. 2003;14:225–231. doi: 10.1016/s1084-9521(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Lin EE, Sequeira-Lopez ML, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol. 2014;306:F249–258. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, et al. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007;7:680–699. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, McMahon AP. Mammalian kidney development: principles, progress and projections. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nature reviews Nephrology. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- McKinley KL, Cheeseman IM. The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol. 2016;17:16–29. doi: 10.1038/nrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Developmental biology. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayer G, Belzil C, Gruss OJ, Nguyen MD. TPX2: of spindle assembly, DNA damage response, and cancer. Cell Mol Life Sci. 2014;71:3027–3047. doi: 10.1007/s00018-014-1582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LL, McMahon AP. Induction and patterning of the metanephric nephron. Seminars in cell & developmental biology. 2014;36:31–38. doi: 10.1016/j.semcdb.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Valerius M, McMahon A. Wnt/{beta}-catenin signaling regulates nephron induction during mouse kidney development. Development (Cambridge, England) 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Developmental cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. The American journal of pathology. 2013;183:542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development (Cambridge, England) 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- Raemaekers T, Ribbeck K, Beaudouin J, Annaert W, Van Camp M, Stockmans I, Smets N, Bouillon R, Ellenberg J, Carmeliet G. NuSAP, a novel microtubule-associated protein involved in mitotic spindle organization. J Cell Biol. 2003;162:1017–1029. doi: 10.1083/jcb.200302129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranghini EJ, Dressler GR. Evidence for intermediate mesoderm and kidney progenitor cell specification by Pax2 and PTIP dependent mechanisms. Developmental biology. 2015;399:296–305. doi: 10.1016/j.ydbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nature reviews Nephrology. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development (Cambridge, England) 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Saifudeen Z, Marks J, Du H, El-Dahr SS. Spatial repression of PCNA by p53 during kidney development. Am J Physiol Renal Physiol. 2002;283:F727–733. doi: 10.1152/ajprenal.00114.2002. [DOI] [PubMed] [Google Scholar]
- Salomonis N, Schlieve CR, Pereira L, Wahlquist C, Colas A, Zambon AC, Vranizan K, Spindler MJ, Pico AR, Cline MS, et al. Alternative splicing regulates mouse embryonic stem cell pluripotency and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10514–10519. doi: 10.1073/pnas.0912260107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nature genetics. 1995;9:358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the Kidney. New York: Cambridge University Press; 1987. [Google Scholar]
- Self M, Lagutin O, Bowling B, Hendrix J, Cai Y, Dressler G, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. The EMBO journal. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira Lopez ML, Gomez RA. Development of the renal arterioles. J Am Soc Nephrol. 2011;22:2156–2165. doi: 10.1681/ASN.2011080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Stewart K, Bouchard M. Coordinated cell behaviours in early urogenital system morphogenesis. Seminars in cell & developmental biology. 2014;36:13–20. doi: 10.1016/j.semcdb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell stem cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- Toonen RJ, Hughes S. Increased throughput for fragment analysis on an ABI PRISM 377 automated sequencer using a membrane comb and STRand software. Biotechniques. 2001;31:1320–1324. [PubMed] [Google Scholar]
- Torres M, Gómez-Pardo E, Dressler G, Gruss P. Pax-2 controls multiple steps of urogenital development. Development (Cambridge, England) 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic acids research. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MG. Drug target discovery by gene expression analysis: cell cycle genes. Curr Cancer Drug Targets. 2001;1:73–83. doi: 10.2174/1568009013334241. [DOI] [PubMed] [Google Scholar]
- Xu J, Wong EY, Cheng C, Li J, Sharkar MT, Xu CY, Chen B, Sun J, Jing D, Xu PX. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Developmental cell. 2014;31:434–447. doi: 10.1016/j.devcel.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


