Abstract
Background
Giardiasis, the most common enteric parasitic infection in the United States, causes an estimated 1.2 million episodes of illness annually. Published clinical recommendations include readily available Giardia-specific diagnostic testing and antiparasitic drugs. We investigated sequences of giardiasis diagnostic and treatment events using MarketScan, a large health insurance claims database.
Methods
We created a longitudinal cohort of 2995 persons diagnosed with giardiasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 007.1) from 2006 to 2010, and analyzed claims occurring 90 days before to 90 days after initial diagnosis. We evaluated differences in number and sequence of visits, diagnostic tests, and prescriptions by age group (children 1–17 years, adults 18–64 years) using χ2 tests and data visualization software.
Results
Among 2995 patients (212 433 claims), 18% had a Giardia-specific test followed by or concurrent with an effective antiparasitic drug, without ineffective antibiotics. Almost two-thirds of patients had an antiparasitic and 27% had an antibiotic during the study window. Compared with children, adults more often had ≥3 visits before diagnosis (19% vs 15%; P = .02). Adults were also less likely to have a Giardia-specific diagnostic test (48% vs 58%; P < .001) and more likely to have an antibiotic prescription (28% vs 25%; P = .04). When Giardia-specific tests and antiparasitic and antibiotic prescriptions were examined, pediatric clinical event sequences most frequently began with a Giardia-specific test, whereas adult sequences most frequently began with an antiparasitic prescription.
Conclusions
Giardiasis care infrequently follows all aspects of clinical recommendations. Multiple differences between pediatric and adult care, despite age-agnostic recommendations, suggest opportunities for provider education or tailored guidance.
Keywords: Giardia, MarketScan, administrative claims, data visualization
Giardiasis, the disease caused by the parasite Giardia intestinalis (also known as Giardia duodenalis or Giardia lamblia), is the most frequently reported human intestinal parasitic infection in the United States [1, 2]. With a burden of illness similar to that of nontyphoidal Salmonella infections, Giardia causes an estimated 1.2 million episodes of illness annually with the highest incidence among children aged 1–9 years [1–3]. Giardia-related hospitalizations in the United States cost an estimated $34 million per year [3]. Although giardiasis is frequently reported among travelers returning from endemic areas, only 7%–8% of US giardiasis cases are travel-associated [2, 4, 5].
Giardia parasite transmission occurs through ingestion of fecally contaminated food or water, or through person-to-person contact [6, 7]. Symptoms include prolonged diarrhea, abdominal pain, malabsorption, bloating, dehydration, and weight loss. Parasites are shed intermittently in feces, and intermittently symptomatic or asymptomatic infections occur frequently [8, 9]. Acute giardiasis is disruptive to daily living and can lead to dehydration, with children at greater risk of severe dehydration than adults [10]. Following acute infection, giardiasis might also lead to long-term chronic disease, including irritable bowel syndrome [11, 12].
Current giardiasis diagnostic and treatment recommendations include guidance on diagnostic testing and appropriate medications [13, 14]. Several stool-based assays can identify Giardia infection, including the ova and parasites microscopy test, and Giardia-specific enzyme immunoassay, indirect fluorescent assay, and direct fluorescent antibody assay. Because Giardia parasites are shed in stool only intermittently, collecting 3 stool samples on 3 different days is recommended to maximize diagnostic sensitivity [15]. Increasingly, highly sensitive molecular diagnostics are also used. Multiple antiparasitic drugs are effective against Giardia, including metronidazole, tinidazole, and nitazoxanide; metronidazole and tinidazole are the first-line treatments in the United States [14, 16].
Despite being the most common human intestinal parasitic infection in the United States, basic information on giardiasis care and treatment practices is lacking. Some previous studies suggest the occurrence of delayed diagnosis of giardiasis (measured as time from symptom onset to diagnosis) and inefective treatment with antibiotics. In a US study of 290 individuals with confirmed giardiasis from 2 states with active laboratory-based surveillance, 27% were enrolled >6 weeks after their reported symptom onset date [17]. In the same study, 10% of patients reported receiving antibiotics, such as ciprofloxacin, which are ineffective against Giardia. These data suggest the possibility that delays in giardiasis diagnosis and ineffective treatment occur widely. One explanation for these findings is low index of suspicion of giardiasis, which causes nonspecific symptoms (eg, diarrhea) common to many enteric diseases. In a survey of 1000 pediatricians, only 10% indicated they would suspect parasites in a patient with persistent diarrhea lasting more than 1–2 weeks [18]. If Giardia is suspected, specific tests must be ordered because routine bacterial stool cultures will not detect the parasite [15]. Therefore, multiple potential areas exist for improvement in the diagnosis and treatment of giardiasis in the United States.
Here, we present an analysis of clinic visits for Giardia-related symptoms and diagnoses, diagnostic tests, and drug prescriptions from 2006 to 2010 among a giardiasis patient cohort (N = 2995), created using a large US health insurance claims database.
Methods
Data Source
We used insurance claims contained in the MarketScan Commercial Claims and Encounters database (Truven Health Analytics, Ann Arbor, Michigan), from 2006 to 2010. The database contains insurance billing data for patient visits (doctors' office and emergency department), hospital stays, diagnostic tests and procedures, and prescription medication for >143 million persons in the United States covered by employer-sponsored private health insurance (employees, retirees under age 65, former employees, and spouses/partners and dependents of these individuals) [19]. Because MarketScan contains de-identified, preexisting insurance billing records, and because no interaction or intervention with human subjects occurred and no personally identifiable information was used, collected, or transmitted, this analysis was not considered human subjects research (as defined in the US Code of Federal Regulations, Title 45 Part 46), and therefore was not subject to review by the Centers for Disease Control and Prevention (CDC) institutional review board.
Cohort Construction
We constructed a cohort of persons with at least 1 outpatient visit for giardiasis, defined as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 007.1, with initial giardiasis diagnosis occurring from 1 January 2006 through 31 December 2010. Of 80 million persons enrolled during that time period, 6056 had at least 1 giardiasis diagnosis. Of these, we excluded 3061 for 1 or more of the following reasons that would lead to incomplete data: hospital stay (prescription drugs are not recorded in database during hospital stays; n = 390), gaps in enrollment in a MarketScan insurance plan (n = 374), no evidence of prescription coverage (n = 1270), and enrollments of <90 days before or after initial diagnosis (n = 1948).
The analysis was limited to claims dated from 90 days before to 90 days after each patient's first giardiasis diagnosis (ie, first clinic visit with a giardiasis diagnosis code), creating a 180-day study window for each patient.
Variable Definitions and Analytical Approach
We focused on a set of diagnosis codes, procedures codes, and prescription drugs likely to be associated with an episode of giardiasis (Supplementary Table 1). We included insurance claims for patient visits with a diagnosis code for giardiasis (ICD-9-CM code 007.1) or other gastrointestinal (GI) illnesses or problems (ICD-9-CM codes 001–009, 520–529, 787, and 792.1). We specifically identified visits with a diagnosis of Shigella, Salmonella, Campylobacter, Escherichia coli, Cryptosporidium, Clostridium difficile, or norovirus infection, to assess alternate infectious diagnoses or coinfections. We also included insurance claims for diagnostic tests used to diagnose giardiasis or other gastrointestinal illnesses or problems [20]. Use of molecular assays (eg, film array– and bead-based assays) was likely uncommon in the study time period. Among prescription drug claims, we included prescriptions for systemic antiparasitic drugs (drugs effective against Giardia: albendazole, furazolidone, metronidazole, nita-zoxanide, ornidazole, paromomycin sulfate, quinacrine, secnidazole and tinidazole) and systemic antibiotics (drugs ineffective against Giardia: cephalosporins, erythromycin and macrolides, penicillins, quinolones, sulfonamides, and miscellaneous antibiotics). The MarketScan database contains the date a prescription was filled but does not indicate which healthcare encounter was associated with the prescription. Additionally, medications can be prescribed for a variety of indications. For example, metronidazole is often prescribed for giardiasis but is also indicated for treatment of bacterial vaginosis, trichomoniasis, amebiasis, and anaerobic bacterial infections [21]. Thus, we included only prescriptions filled within the 7 days before to 30 days after a visit involving abdominal pain, diarrhea, or giardiasis. These visit-associated prescriptions [22] comprised 96% of total prescriptions during the 180-day study window.
We grouped these diagnosis codes, diagnostic test codes, and prescriptions into the following giardiasis-related “event” types: patient visits with a giardiasis diagnosis, visits for GI symptoms, Giardia-specific diagnostic tests, diagnostic tests for other GI-related illnesses or problems, antiparasitic prescriptions, and antibiotic prescriptions. We considered the timing of each event and identified the first and last date of each event type (Supplementary Table 2).
We then assessed the frequency of each event type, and because we hypothesized that giardiasis care experiences might differ for pediatric and adult patients, we stratified analyses by age at first giardiasis diagnosis (0–17 years, 18–64 years). We also evaluated differences in diagnostic testing and prescriptions by sex and US census region of residence (Northeast, South, Midwest, and West). We evaluated statistical differences in proportions using χ2 test, or Fisher exact test when expected cell counts were <5. Data management and analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, North Carolina) and R version 3.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
We then used the EventFlow data visualization tool (University of Maryland, Human-Computer Interaction Lab; http://hcil.umd.edu/eventfow) to visually inspect the data and identify clinically relevant temporal event sequences [23–25]. EventFlow aggregates longitudinal data by grouping individuals with similar sequences of events and representing these groups with color-coded vertical bars in a single graphic display that summarizes information on event order, time between events, and frequency of particular event sequences (Supplementary Figure 1). Each row in an EventFlow figure represents 1 patient's sequence of events during a period of time. The height of each bar is proportional to the number of records with that sequence, and its horizontal position is determined by the median time between events. Groups of sequences with the same preceding event are sorted by the number of records in each group. The sequence groups are shown from top to bottom in descending order of number of patients per group. A brief demonstration video illustrates the process (http://go.umd.edu/eventfow-overview). We also used EventFlow to search the patient event sequences for signatures of giardiasis care and treatment recommendations. Specifically, we queried for sequences containing a Giardia-specific test, followed by or concurrent with an antiparasitic prescription, and without any antibiotic prescription.
Results
Within the cohort of 2995 giardiasis patients, half were female, and 30% were aged ≤17 years at diagnosis (Table 1).
Table 1. Giardiasis Outpatient Cohort Characteristics (N = 2995) in the MarketScan National Insurance Claims Database, 2006–2010.
| Characteristic | No. | (%) |
|---|---|---|
| Female sex | 1499 | (50.1) |
| Age, y | ||
| 0–17 | 910 | (30.4) |
| 18–34 | 612 | (20.4) |
| 35–44 | 537 | (17.9) |
| 45–54 | 515 | (17.2) |
| 55–64 | 421 | (14.1) |
| US Census Region of residence | ||
| South | 1297 | (43.3) |
| West | 774 | (25.8) |
| Midwest | 546 | (18.2) |
| Northeast | 361 | (12.1) |
| Unknown | 17 | (0.6) |
Half of all patients (50%; n = 1496) had ≥3 clinic visits with codes for GI symptoms or giardiasis, and adults were more likely to have ≥3 visits compared with children (52% vs 46%; Table 2). Preceding their initial visit with a giardiasis diagnosis, 18% of all patients (n = 535) had ≥3 visits for GI symptoms, and adults were more likely than children (19% vs 15%) to have ≥3 GI symptom visits before receiving a diagnosis. Overall, 22% of patients (n = 657) waited >30 days from their first GI symptom visit to their first visit with a giardiasis diagnosis, and 40% (n = 1192) waited >30 days from first to last GI symptom or giardiasis diagnosis visit during the study window. These intervals did not vary by age group.
Table 2. Giardiasis Outpatient Visits for Gastrointestinal Symptoms During 180-Day Study Window (N = 2995).
| Event Type | All Outpatients (N = 2995) | Age <18 y (n = 910) | Age 18–64 y (n = 2085) | PValuea | |||
|---|---|---|---|---|---|---|---|
| Total giardiasis or GI symptom visitsb | .01 | ||||||
| 1 | 932 | (31.1) | 305 | (33.5) | 627 | (30.1) | |
| 2 | 567 | (18.9) | 190 | (20.9) | 377 | (18.1) | |
| ≥3 | 1496 | (50.0) | 415 | (45.6) | 1081 | (51.9) | |
| Median (range) | 2 | (1–31) | 2 | (1–19) | 3 | (1–31) | |
|
| |||||||
| GI symptom visits before initial giardiasis diagnosis | .02 | ||||||
| 0 | 1428 | (47.7) | 457 | (50.2) | 971 | (46.6) | |
| 1 | 591 | (19.7) | 186 | (20.4) | 405 | (19.4) | |
| 2 | 441 | (14.7) | 134 | (14.7) | 307 | (14.7) | |
| ≥3 | 535 | (17.9) | 133 | (14.6) | 402 | (19.3) | |
| Median (range) | 1 | (0–15) | 0 | (0–14) | 1 | (0–15) | |
|
| |||||||
| Days from first symptom visit until initial diagnosisc | .23 | ||||||
| Same day | 1428 | (47.7) | 457 | (50.2) | 971 | (46.6) | |
| 1–7 d | 279 | (9.3) | 79 | (8.7) | 200 | (9.6) | |
| 8–30 d | 631 | (21.1) | 191 | (21.0) | 440 | (21.1) | |
| 31–90 d | 657 | (21.9) | 183 | (20.1) | 474 | (22.7) | |
| Median (range) | 2 | (0–90) | 0 | (0–90) | 3 | (0–90) | |
|
| |||||||
| Total days from first to last giardiasis or GI symptom visit | .45 | ||||||
| Same day | 932 | (31.1) | 305 | (33.5) | 627 | (30.1) | |
| 1–7 d | 305 | (10.2) | 88 | (9.7) | 217 | (10.4) | |
| 8–30 d | 566 | (18.9) | 165 | (18.1) | 401 | (19.2) | |
| 31–90 d | 902 | (30.1) | 269 | (29.6) | 633 | (30.4) | |
| ≥91 d | 290 | (9.7) | 83 | (9.1) | 207 | (9.9) | |
| Median (range) | 17 | (0–175) | 16 | (0–159) | 17 | (0–175) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: GI, gastrointestinal.
χ2 test of age group vs event type.
Includes visits for giardiasis (International Classification of Diseases, Ninth Revision, Clinical Modification code 007.1) and any of 83 GI-related conditions or symptoms.
“Same day” denotes outpatients whose first GI-related visit included a giardiasis diagnosis, with or without additional GI symptoms.
More than half of patients (62%; n = 1853) had a diagnostic test for gastrointestinal illnesses or problems, including Giardia (Table 3). Among these, 82% (n = 1515) had a Giardia-specific test. Pediatric patients were significantly more likely than adults to have had at least 1 Giardia-specific test (58% vs 48%).
Table 3. Gastrointestinal-Related and Giardia-Specific Diagnostic Tests During 180-Day Study Window (N = 2995).
| Test | All Outpatients (N = 2995) | Age <18 y (n = 910) | Age 18–64 y (n = 2085) | PValue | |||
|---|---|---|---|---|---|---|---|
| Any GI-related testa | 1853 | (61.9) | 578 | (63.5) | 1275 | (61.2) | .22 |
| Any Giardia-specific testb | 1515 | (50.6) | 523 | (57.5) | 992 | (47.6) | <.001 |
| ≥3 Giardia-specific tests | 744 | (24.8) | 300 | (33.0) | 444 | (21.3) | <.001 |
| Any O&P microscopy testc | 1181 | (39.4) | 404 | (44.4) | 777 | (37.3) | <.001 |
| Any Giardia enzyme immunoassayd | 924 | (30.9) | 354 | (38.9) | 570 | (27.3) | <.001 |
| Any Giardia indirect fluorescent assay | 58 | (1.9) | 12 | (1.3) | 46 | (2.2) | .11 |
| Any Giardia direct fluorescent antibodye | 51 | (1.7) | 18 | (2) | 33 | (1.6) | .44 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: GI, gastrointestinal; O&P, ova and parasites.
GI-related tests include Giardia-specific tests, and are defined in Supplementary Table 1.
Giardia-specific tests are defined and listed in Supplementary Table 1.
Includes tests with or without trichrome stain.
This category includes “rapid” diagnostic tests (eg, ImmunoCard STAT).
Gold standard for Giardia diagnostic testing.
Most patients (72%; n = 2142) had prescriptions for either antiparasitic or antibiotic drugs. About two-thirds (64%; n = 1906) had an antiparasitic drug (Table 4). Adult patients were significantly more likely than pediatric patients to have an antiparasitic prescription of any kind (68% vs 53%). Metronidazole was most common overall, but pediatric patients were significantly more likely to have nitazoxanide compared with adults (23% vs 5%). Twenty-seven percent of all patients (816/2995) had a systemic antibiotic ineffective against Giardia.
Table 4. Visit-Associateda Antimicrobial Prescriptions During 180-Day Study Window (N = 2995).
| Prescription | All Outpatients (N = 2995) | Age <18 y (n = 910) | Age 18–64 y (n = 2085) | PValue | |||
|---|---|---|---|---|---|---|---|
| Patients receiving ≥1 antiparasitic prescriptionb | |||||||
| Any visit-associated antiparasitic prescriptions | 1906 | (63.6) | 480 | (52.8) | 1426 | (68.4) | <.001 |
| Metronidazole | 1516 | (50.6) | 281 | (30.9) | 1235 | (59.2) | <.001 |
| Nitazoxanide | 320 | (10.7) | 211 | (23.2) | 109 | (5.2) | <.001 |
| Tinidazole | 247 | (8.3) | 40 | (4.4) | 207 | (9.9) | <.001 |
| Albendazole | 32 | (1.1) | 3 | (0.3) | 29 | (1.4) | .01c |
| Paromomycin | 12 | (0.4) | 1 | (0.1) | 11 | (0.5) | .12c |
|
| |||||||
| Patients receiving ≥1 antibiotic prescriptiond | |||||||
| Any visit-associated antibiotic prescriptions | 816 | (27.3) | 225 | (24.7) | 591 | (28.4) | .04 |
| Quinolones | 307 | (10.3) | 6 | (0.7) | 301 | (14.4) | <.001 |
| Penicillin | 196 | (6.5) | 100 | (11.0) | 96 | (4.6) | <.001 |
| Macrolides | 187 | (6.2) | 60 | (6.6) | 127 | (6.1) | .60 |
| Cephalosporins | 123 | (4.1) | 67 | (7.4) | 56 | (2.7) | <.001 |
| Sulfonamides | 113 | (3.8) | 32 | (3.5) | 81 | (3.9) | .63 |
| Tetracyclines | 71 | (2.4) | 9 | (1.0) | 62 | (3.0) | .001 |
| Miscellaneous antibiotics | 47 | (1.6) | 4 | (0.4) | 43 | (2.1) | <.001c |
Data are presented as No. (%) unless otherwise indicated.
Prescriptions paid from 7 days before to 30 days following a visit for giardiasis or gastrointestinal symptoms.
Antiparasitic prescriptions are medications effective against Giardia. No outpatient had prescriptions for quinacrine, ornidazole, secnidazole, or furazolidone.
Fisher exact test P value.
Antibiotic prescriptions are systemic antimicrobials not effective against Giardia.
We quantified comorbid GI diagnoses and Giardia-specific tests and drugs, to evaluate the validity of the giardiasis diagnosis used to define the cohort. Comorbid diagnoses of Shigella, Salmonella, Campylobacter, E. coli, Cryptosporidium, C. diffcile, or norovirus infection were uncommon (2.2%), and most patients (83%) had either a Giardia-specific diagnostic test or a prescription appropriate for giardiasis.
The proportion of patients receiving a Giardia-specific diagnostic test varied from 44% in the South to 60% in the Northeast (P < .001), proportions of patients with antiparasitic prescriptions varied from 55% in the Northeast to 68% in the South (P < .001), and antibiotic prescriptions varied from 22% in the Northeast to 30% in the South (P = .004) (not shown).
Temporal event sequences analyzed in EventFlow revealed that the giardiasis care and treatment event sequence is variable, with 1010 (34%) unique event sequences represented among the entire cohort. In total, patients had a median of 5 events (eg, visits, tests or prescriptions; range, 1–47) during the 180-day study window. Median elapsed time from first to last event was 23 days (range, 0–196 days) (Supplementary Figure 2). The first event recorded for most patients was a visit for a GI-related symptom (61%); for 22% of patients, the first event was an antiparasitic prescription. For 28% of patients, the first event was either an antiparasitic or antibiotic prescription (SupplementaryTable 2).
Eighteen percent (n = 541) of patient event sequences included a Giardia-specific test followed by or concurrent with an antiparasitic prescription, with no antibiotic prescription, and thus were consistent with published recommendations. Event sequences differed between pediatric and adult patients. When Giardia-specific tests and antiparasitic and antibiotic prescriptions were examined, event sequences for pediatric patients most frequently began with a Giardia-specific test, whereas event sequences for adult patients most frequently began with an antiparasitic prescription (Figure 1).
Figure 1.
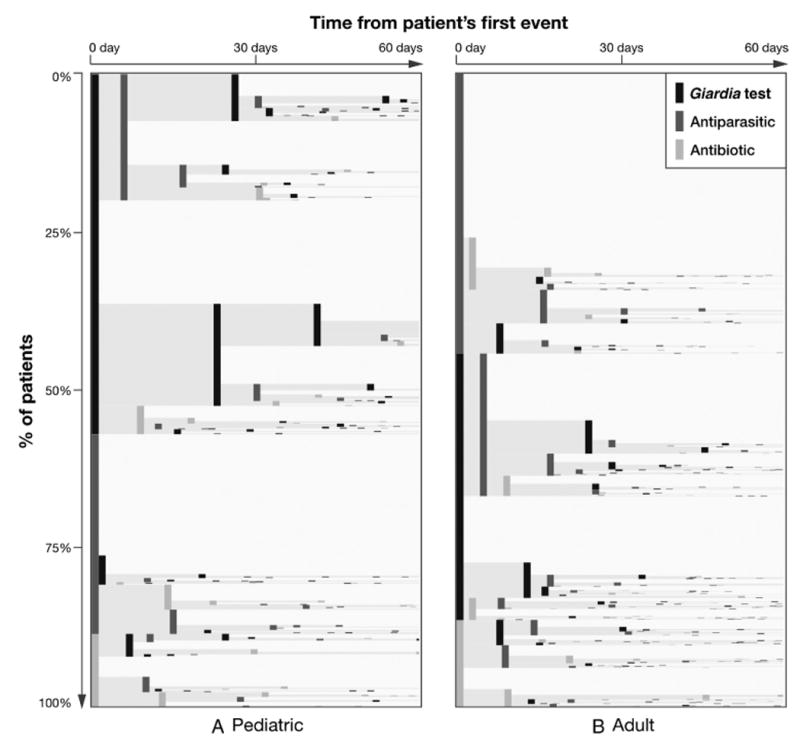
EventFlow plots of pediatric (A) and adult (B) giardiasis event sequences. All sequences (rows) are aggregated by event order, with each patient's first event during the study window represented by a vertical bar at the far left. Bar height represents the proportion of patients with a given sequence, and bar shading represents event type. Distance between bars is equivalent to median time in days between any 2 events. Three Giardia-specific event types are shown: Giardia-specific tests (black), antiparasitic prescriptions (dark gray), and antibiotic prescriptions (light gray). Most but not all cohort patients had at least 1 of these 3 events. Therefore, plots show pediatric (n = 782; 86%) and adult (n = 1808; 87%) sequences containing any of the 3 events. Time from first event (horizontal axis) is truncated to 60 days for clarity; 72% of all sequences had total elapsed time of ≤60 days. Starting from the left of the panels, we saw that more pediatric vs adult sequences started with a Giardia-specific test (black bars) and included multiple consecutive tests, whereas adult sequences were more likely to begin with an antiparasitic drug (dark gray bars).
Discussion
Using a large insurance claims database to characterize giardiasis diagnosis and treatment in the United States, we showed that the entire clinical event sequence takes >3 weeks for most patients, and often requires multiple visits, procedures, and prescriptions. Furthermore, we found that pediatric giardiasis care differs substantially from adult care, even though treatment recommendations do not differ by age group. Our results suggest that giardiasis diagnosis can be time-consuming and potentially costly for patients and clinicians, and that many patients do not have recommended diagnostics or drugs.
Our analysis also revealed that the giardiasis diagnosis and treatment process is highly variable and sometimes at odds with established guidelines. Nearly 40% of patients experienced >30 days between their first and last physician visit for GI symptoms, and 22% experienced ≥30 days before their first visit with a giardiasis diagnosis code. This finding is consistent with a previous study of laboratory-confirmed giardiasis patients in which 27% of laboratory-confirmed cases could not be enrolled until >6 weeks after their reported onset, suggesting a protracted (delayed) diagnostic process [17]. Only 18% of patients had a Giardia-specific test followed by an antiparasitic medication effective against giardiasis, without an ineffective antibiotic, a sequence we examined based on consistency with current recommendations. On the other hand, 27% of patients had an antibiotic ineffective against Giardia at some point in the study window, which is nearly 3-fold higher than a previous estimate [17]. The low consistency with recommended practice was surprising, in light of the availability of multiple sufffciently sensitive diagnostic testing options, effective antiparasitic drugs, and published guidance that suggests the use of such tests and drugs as best practices [13, 14, 26, 27]. However, the finding that 30% of patients had a visit-associated antiparasitic or antibiotic prescription as their first event suggests that, for some clinicians and patients, the presumed speed and ease of empiric treatment might outweigh the potential discomfort and expense of additional diagnostic visits. Empiric treatment carries the risk of patients taking unnecessary and ineffective medications that might contribute to the development of antibiotic resistance, and empirically treating contacts of laboratory-confirmed cases may increase the number of probable vs confirmed cases notified to CDC and could result in an underestimated national disease burden. Rapid molecular assays, while unlikely to be used in MarketScan during this study period, could increase the use and sensitivity of gastroenteritis diagnostics, and reduce apparent empirical treatment.
We found that pediatric patients had more Giardia-specific tests, but fewer prescriptions, than adults. Moreover, data visualization using EventFlow showed that age-specific event sequences had distinct hallmarks, when the subset of diagnostic tests and prescription events were analyzed: Children often had 1 or more tests preceding prescriptions, while a majority of adults began with antiparasitic or antibiotic prescriptions. Together, these observations suggest that children tend to have a more thorough workup, while adults have more frequent prescriptions without preceding diagnostic tests, suggesting empiric treatment. Although current giardiasis care recommendations are not age-specific, differences between pediatric and adult giardiasis care are not surprising. The incidence of giardiasis (and other gastroenteritis) is not uniform across the age spectrum in the United States. Giardiasis incidence is highest in children [1], and a recent study of laboratory-tested acute gastroenteritis patients showed that likelihood of detecting any GI pathogen in feces decreases significantly with age [29], suggesting that pediatric practitioners might suspect giardiasis (or other pathogenic etiologies) more often, and be more familiar with testing and treatment methods compared with adult practitioners. As expected, the majority of antiparasitic prescriptions for all patients were for metronidazole. Tat pediatric patients had more nitazoxanide prescriptions than adult patients might be explained by nitazoxanide but not metronidazole availability in an oral suspension.
Although administrative data such as insurance claims records can provide large amounts of data at comparatively low cost to investigators, we acknowledge several limitations in these data. First, our cohort represents giardiasis patients who were commercially insured for >6 months and might have different clinical experiences from the uninsured or briefly insured, or persons with Medicaid coverage. In particular, the uninsured might seek to minimize visits and diagnostic testing. Second, the structure of the medical and prescription claims databases did not allow us to precisely assign prescriptions to the disease or symptom for which they were prescribed. To restrict our analyses to prescriptions associated with giardiasis, we only included prescriptions filled from 7 days preceding to 30 days following a visit for giardiasis, abdominal pain, or diarrhea [22]. Finally, giardiasis diagnoses could not be validated with medical records, and while the database contains insurance claims for diagnostic tests, it is unknown whether separate tests involve separately collected specimens or what proportion of tests had confirmed positive results. Administrative data have been validated for irritable bowel syndrome and other diseases [30–33], but have not been used previously for giardiasis. However, an alternate infectious diagnosis was identified in only 2.2% of patients in our cohort, and 83% of patients had a Giardia-specific test or antiparasitic prescription, increasing our confidence that most patients in our cohort indeed had giardiasis.
In this comprehensive characterization of the giardiasis clinical care experience within a large cohort of commercially insured patients, we found that receiving all aspects of recommended care—including a diagnostic test, a prescription for antiparasitic medication, and no prescription for ineffective antibiotics—was relatively rare. Valid reasons may exist for these deviations from recommendations in many instances, although lack of awareness might be a factor. Qualitative studies exploring clinicians' rationale for giardiasis-related clinical decisions would be useful for planning public health messaging and clinician education. We also identified substantial differences in pediatric and adult giardiasis care, with children more often receiving diagnostic testing and adults more often starting care with a prescription. Although current clinical guidance does not differ by age, acknowledging practical reasons for age-group differences might present opportunities for revised public health guidance that better refects the unique scenarios presented by adults and children with gastrointestinal illnesses. Finally, this study contributes a greater understanding of real-world giardiasis care, which could inform future studies of the consequences of missing or ineffective treatment.
Supplementary Material
Acknowledgments
We gratefully acknowledge important contributions from Kathleen Fullerton, Jonathan Yoder, Michael Beach, Jolene Nakao, Catherine Plaisant, and Ben Shneiderman.
Footnotes
Disclaimer. The opinions expressed by authors contributing to this article do not necessarily refect the opinions of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data: Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Painter JE, Gargano JW, Collier SA, Yoder JS. Centers for Disease Control and Prevention. Giardiasis surveillance—United States, 2011–2012. MMWR Suppl. 2015;64:15–25. [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collier SA, Stockman LJ, Hicks LA, Garrison LE, Zhou FJ, Beach MJ. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;140:2003–13. doi: 10.1017/S0950268811002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chute CG, Smith RP, Baron JA. Risk factors for endemic giardiasis. Am J Public Health. 1987;77:585–7. doi: 10.2105/ajph.77.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnell K, Collier S, Derado G, Yoder J, Gargano JW. Giardiasis in the United States—an epidemiologic and geospatial analysis of county-level drinking water and sanitation data, 1993–2010. J Water Health. 2016;14:267–79. doi: 10.2166/wh.2015.283. [DOI] [PubMed] [Google Scholar]
- 6.Yoder JS, Gargano JW, Wallace RM, Beach MJ. Centers for Disease Control and Prevention. Giardiasis surveillance—United States, 2009–2010. MMWR Surveill Summ. 2012;61:13–23. [PubMed] [Google Scholar]
- 7.Adam EA, Yoder JS, Gould LH, Hlavsa MC, Gargano JW. Giardiasis outbreaks in the United States, 1971–2011. Epidemiol Infect. 2016;144:2790–801. doi: 10.1017/S0950268815003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhard M, Gabrielli A, Savioli L. Heymann DL Control of Communicable Diseases Manual. 19th. Washington, DC: American Public Health Association; 2008. Giardiasis (Giardia enteritis) pp. 258–60. [Google Scholar]
- 9.Hellard ME, Sinclair MI, Hogg GG, Fairley CK. Prevalence of enteric pathogens among community based asymptomatic individuals. J Gastroenterol Hepatol. 2000;15:290–3. doi: 10.1046/j.1440-1746.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- 10.Kliegman R, Nelson WE. Nelson Textbook of Pediatrics. 19th. Philadelphia, PA: Elsevier/Saunders; 2011. Giardiasis and balantidiasis; pp. 1180–3. [Google Scholar]
- 11.Halliez MC, Buret AG. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 2013;19:8974–85. doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanevik K, Wensaas KA, Rortveit G, Eide GE, Mørch K, Langeland N. Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study. Clin Infect Dis. 2014;59:1394–400. doi: 10.1093/cid/ciu629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramowicz M, editor. The medical letter on drugs and therapeutics. New Rochelle, NY: The Medical Letter, Inc; 2013. Drugs for parasitic infections. [Google Scholar]
- 14.Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14:114–28. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cama VA, Mathison BA. Infections by intestinal coccidia and Giardia duodenalis. Clin Lab Med. 2015;35:423–44. doi: 10.1016/j.cll.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer E, Lachish T, Schwartz E. Treatment of giardiasis after nonresponse to nitroimidazole. Emerg Infect Dis. 2014;20:1742–4. doi: 10.3201/eid2010.140073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantey PT, Roy S, Lee B, et al. Study of nonoutbreak giardiasis: novel findings and implications for research. Am J Med. 2011;124:1175 e1–8. doi: 10.1016/j.amjmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Attias E, Czinn SJ, Harro C, Munoz FM, Sockolow RE, Black JT. Emerging issues in managing pediatric parasitic infections: an assessment of clinical and epidemiological knowledge of giardiasis and cryptosporidiosis. Pediatr Therapeutics. 2015:05. [Google Scholar]
- 19.Hansen LG, Chang SH. White paper: health research data for the real world: the MarketScan databases. Ann Arbor, MI: Truven Health Analytics; 2011. [Google Scholar]
- 20.Abraham M. Current Procedural Terminology 2012. Chicago, IL: American Medical Association Press; 2011. American Medical Association. [Google Scholar]
- 21.US Food and Drug Administration. [Accessed 1 May 2016];Flagyl metronidazole tablet package insert. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/12623slr059_flagyl_lbl.pdf.
- 22.Owusu-Edusei K, Jr, Tejani MN, Gift TL, Kent CK, Tao G. Estimates of the direct cost per case and overall burden of trichomoniasis for the employer-sponsored privately insured women population in the United States, 2001 to 2005. Sex Transm Dis. 2009;36:395–9. doi: 10.1097/OLQ.0b013e318199d5fe. [DOI] [PubMed] [Google Scholar]
- 23.Monroe M, Lan R, Lee H, Plaisant C, Shneiderman B. Temporal event sequence simplification. IEEE Trans Vis Comput Graph. 2013;19:2227–36. doi: 10.1109/TVCG.2013.200. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnadóttir MV, Malik S, Onukwugha E, Gooden T, Plaisant C. Understanding adherence and prescription patterns using large-scale claims data. Pharmacoeconomics. 2016;34:169–79. doi: 10.1007/s40273-015-0333-4. [DOI] [PubMed] [Google Scholar]
- 25.University of Maryland Human-Computer Interaction Lab. EventFlow: Visual analysis of temporal event sequences. [Accessed 28 June 2016]; Available at: www.hcil.umd.edu/eventflow.
- 26.Hill DR. Giardiasis. Issues in diagnosis and management. Infect Dis Clin North Am. 1993;7:503–25. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. [Accessed 20 June 2016];Parasites—Giardia. Available at: http://www.cdc.gov/parasites/giardia/
- 28.Centers for Disease Control and Prevention. [Accessed 26 July 2016];Giardiasis 2011 case definition. Available at: https://wwwn.cdc.gov/nndss/conditions/giardiasis/case-definition/2011/
- 29.Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis. 2011;17:1381–8. doi: 10.3201/eid1708.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnahan RM. Mini-Sentinel's systematic reviews of validated methods for identifying health outcomes using administrative data: summary of findings and suggestions for future research. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):90–9. doi: 10.1002/pds.2318. [DOI] [PubMed] [Google Scholar]
- 31.Goff SL, Feld A, Andrade SE, et al. Administrative data used to identify patients with irritable bowel syndrome. J Clin Epidemiol. 2008;61:617–21. doi: 10.1016/j.jclinepi.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Sands BE, Duh MS, Cali C, et al. Algorithms to identify colonic ischemia, complications of constipation and irritable bowel syndrome in medical claims data: development and validation. Pharmacoepidemiol Drug Saf. 2006;15:47–56. doi: 10.1002/pds.1118. [DOI] [PubMed] [Google Scholar]
- 33.Townsend L, Walkup JT, Crystal S, Olfson M. A systematic review of validated methods for identifying depression using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):163–73. doi: 10.1002/pds.2310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


