Abstract
The purpose of this study was to determine the relationship between symptomatic status, transcranial Doppler (TCD) microemboli presence and plaque histopathology findings. TCD was performed on 60 subjects (37 symptomatic, 23 asymptomatic) prior to undergoing clinically indicated carotid endarterectomy. The frequency of microemboli signals was not significantly different between symptomatic and asymptomatic subject groups (p=0.88) and there were no differences observed in the macroscopic or histopathology scoring of these plaques (p-values all > 0.05). The presence of microemboli was associated with an ulceration score (regardless of symptomatic or asymptomatic status, p=0.034), with a one level increase in ulceration rating associated with an odds ratio of 5.86 (95%[CI] 1.55, 43.4). These findings suggest that both symptomatic and asymptomatic subjects may have plaque with similar features of instability and ability to create emboli. Thus, identifying new ways to measure plaque instability may provide important information for optimizing treatment to prevent future stroke.
Keywords: Ultrasound, Transcranial Doppler, Microemboli, High intensity transient signals (HITS)
Introduction
Stroke is the fifth leading cause of death in the United States and the leading cause of long term disability (Benjamin et al. 2017; Go, et al. 2013). It is estimated that for every clinically recognized stroke 5 silent strokes occur (Dempsey, et al. 2010, Rocque, et al. 2012). This results in approximately 11,000,000 silent strokes per year (Dempsey, et al. 2010, Dempsey, et al. 2017, Smith, et al. 2000, Smith, et al. 2017, Snowdon, et al.). Silent strokes are often detected on brain imaging examinations and while individuals do not present with the classic stroke symptoms (numbness, muscle weakness, difficulty speaking, ocular changes), they may demonstrate cognitive decline, especially in their executive function (Dempsey, et al. 2017, Jackson, et al. 2016, Rocque, et al. 2012). Symptomatic and asymptomatic individuals, with advanced carotid atherosclerosis, do show significant cognitive decline relative to controls (Dempsey, et al. 2017, Jackson, et al. 2016). This finding is important in that accelerated cognitive decline may be just as debilitating as clinical stroke, as it can lead to loss of independence and employment (Jackson, et al. 2016).
Current clinical examination criteria do not evaluate for cognitive decline (Dempsey, et al. 2017, Jackson, et al. 2016). Carotid atherosclerosis is thought to contribute to ischemic stroke and cognitive impairment through 1) cerebral ischemia from flow limiting lesions and/or 2) release of microemboli from vulnerable plaques (Demarin, et al. 2012, Dempsey, et al. 2010, Dempsey, et al. 2017, Stork, et al. 2002, Sztajzel, et al. 2006). Vulnerable plaque are those that are at high risk for rupture, which can result in stroke or TIA (Salem, et al. 2014). Plaque features most often associated with rupture are a thin fibrous cap, large lipid core, inflammation and intra-plaque hemorrhage (Salem, et al. 2014). The cumulative effect of the release of these small emboli from a vulnerable plaque can result in cognitive impairment and brain damage (Demarin, et al. 2012, Dempsey, et al. 2010, Purandare, et al. 2006). One method proposed to monitor the propensity of plaque to release of emboli is transcranial Doppler (TCD).
Transcranial Doppler (TCD) is able to detect high intensity transient signals (HITS) suggestive of microemboli (Altaf, et al. 2014, Babikian, et al. 1994, Markus and Brown 1993, Markus, et al. 2010, Siebler, et al. 1994, Siebler, et al. 1993, Spencer, et al. 1990). HITS as detected by transcranial Doppler (TCD), are thought to be associated with carotid plaque instability and symptoms related to motor, sensory, visual and speech deficits from stroke and/or transient ischemic attack (TIA) (Dempsey, et al. 2010, Stork, et al. 2002). The frequency of HITS in subjects with carotid stenosis varies (Mandani et al. 2011; Siebler et al. 1993) with many studies reporting that individuals with cerebrovascular symptoms have a higher incidence of HITS (Salem, et al. 2011, Stork, et al. 2002, Sztajzel, et al. 2006, Tegos, et al. 2001). Such cerebrovascular symptoms may include, stroke, which may present with visual deficits (amarousis fugax), sensory deficits (numbness of an extremity), motor deficits (paralysis, muscle weakness), speech deficits (difficulty speaking) or transient ischemic attack (Dempsey, et al. 2010, Shintani, et al. 2000, Wu, et al. 2014).
Carotid plaque morphology also plays a role in plaque stability, especially if ulceration is present (Sztajzel, et al. 2006). Plaque ulceration refers to an uneven plaque surface caused by a defect in the endothelium. Clots can adhere to the irregular surface of the plaque, causing the release of microemboli or thrombosis (Miskolczi, et al. 1996, Svindland and Torvik 1988). Ulceration is more prevalent in persons with symptomatic cerebrovascular disease compared to asymptomatic individuals (Sitzer et al. 1995, Stork et al. 2002), however, asymptomatic patients have findings, such as silent stroke (Dempsey, et al. 2010, Rocque, et al. 2012) and cognitive decline (Dempsey, et al. 2017). These asymptomatic individuals, therefore may have plaques that are unstable or not as stable as previously described. Furthermore, the standard clinical examination does not assess the sequeli of repeated small emboli and probable differences between symptomatic and asymptomatic plaques. The purpose of this study was to determine the relationship between symptoms, the presence of microemboli and plaque histopathology findings.
Methods
Participants
Sixty (60) patients scheduled for carotid endarterectomy, were recruited for participation in the “Structural Stability of Carotid Plaque and Symptomatology” (NIH funded study: R01 NS064034) study from 2010–2016, and underwent TCD examination as part of the research study protocol. All participants met criteria for clinically indicated carotid endarterectomy (>60% stenosis of the carotid artery based on the North American Symptomatic Carotid Endarterectomy Trials (NASCET 1991) criteria (North American Symptomatic Carotid Endarterectomy Trial 1991) and Asymptomatic Carotid Artery Stenosis (ACAS 1995) (Asymptomatic Carotid Atherosclerosis Study 1995). This study was approved by the University of Wisconsin Health Sciences Institutional Review Boards and all subjects provided informed consent.
Transcranial Doppler
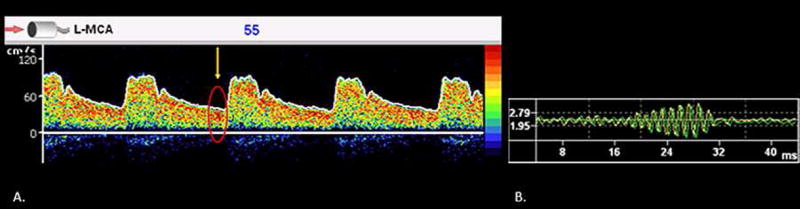
The SONARA Digital Bilateral Systems Transcranial Doppler system (Natus, formerly CareFusion, Middleton, WI) was utilized to perform all TCD examinations. The right and left middle cerebral arteries were isonated at a depth of 45–62 mm from the trans-temporal window. Two, 2.0 MHz pulsed-wave transducers were used simultaneously to record the Doppler signals. Emboli detection software on the SONARA Digital Bilateral Systems Transcranial Doppler was used to identify high intensity transient signals (HITS) suggestive of microemboli. The following criteria, as defined by the Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium were used to differentiate HITS from artifacts; 1) high intensity signal (detected by the system), 2) unidirectional signal within the Doppler velocity spectrum, 3) a short duration signal (<300 ms), and 4) the presence of an audible noise (heard as a crackle, thud, chirp, moan) (Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium 1995). In addition, we also utilized the complex mode to identify high frequency oscillations associated with a moving embolus (see Figure 1). All subjects had at least one HIT satisfying four out of the five criteria present to distinguish a HIT suggestive of a microemboli from an artifact. A physician and two observers reviewed all HITSs based on the aforementioned criteria to distinguish HITS suggestive of microemboli from artifacts (Berman, et al. 2015, Dempsey, et al. 2017). The exam was considered positive for the presence of microemboli if one or more HITSs were identified during the TCD monitoring period (Berman, et al. 2015, Dempsey, et al. 2017).
Figure 1.
Panel A demonstrates the presence of a high intensity transient signal on TCD examination (yellow arrow and red circle). Panel B demonstrates the change in complex associated with the high intensity transient signal.
Surgical Ulceration Scoring
All carotid endarterectomies and ulceration scores were provided by a board-certified surgeon (Mitchell et al. 2017). At the time of carotid endarterectomy, the surgeon scored the plaque on an ordinal scale of 1 to 4. The score is derived based on the depth and extent of the ulcer. An ulceration score of 1 represents an ulcer visible with the microscope, a score of 2 represents an ulcer just visible without magnification, a score of 3 represents an ulcer that is at an intermediate depth and a score of 4 represents an ulcer that is deeper than 3mm and extensively involves the media layer of the arterial wall. A dissecting microscope was used for macroscopic scoring of cholesterol, thrombus, calcium and ulceration.
Histopathologic Examination
Paraffin-embedded plaque sections were stained using standard hematoxylin and eosin methods (Mitchell, et al. 2017). Sections were assessed for the percent calcification, cholesterol content, hemorrhage, hemosiderin content, and inflammatory cell infiltration by a single pathologist blinded to subject characteristics. Histologic classification of plaques was made using the updated classification of atherosclerotic plaques recommended by the American Heart Association (Tureyen, et al. 2006). Atheromatous plaques were scored for hemorrhage, hemosiderin, inflammation, percent cholesterol and calcium. An ordinal scale (0–3) was used for scoring the presence of hemosiderin and inflammation, with 0 representing no hemosiderin or inflammation, 1 minimal, 2 moderate and 3 representing extensive hemosiderin/inflammation Mitchell et al. 2017).
Statistical Analysis
Statistical analysis was completed with R Core Team (R Development Core Team 2015). Pearson’s chi-squared test was used to assess the frequency of surgical side microemboli between symptomatic and asymptomatic patients. For each operative (macroscopic) and histopathology plaque finding, relationship with the presence of surgical side microemboli was analyzed using logistic regression with age as a covariate. Each operative (macroscopic) and histopathology plaque finding was fitted as a continuous predictor in the model.
Results
Participants
Sixty patients scheduled for clinically indicated carotid endarterectomy underwent 60 minutes of TCD monitoring for HITS suggestive of microemboli. The mean (standard deviation) age for asymptomatic subjects was 68.0 (6.73) years, 39.13% female. The mean age for symptomatic subjects was 69.11 (11.46) years, 35.14% female. 23 patients were asymptomatic and 37 were symptomatic. Symptomatic status was defined as previous history of stroke (classical symptoms of motor and/or speech deficits) or transient ischemic attack. Asymptomatic patients did not present with classic symptoms but may have evidence of silent stroke present on imaging (See Table I.).
Table 1.
Subject Demographics, TCD, Macroscopic and Histopathology Findings
| Variable | Asymptomatic | Symptomatic | p-value | |
|---|---|---|---|---|
| N | 23 | 37 | ||
|
| ||||
| Gender, women n (%) | 9 (39.13) | 13 (35.14) | ||
|
| ||||
| Age mean (SD) | 68 (6.73) | 69.11 (11.46) | ||
|
| ||||
| Transcranial Doppler | ||||
| HITS Present n (%) | 5 (21.7) | 10 (27) | 0.88 | |
|
| ||||
| Macroscopic | Asymptomatic | Symptomatic | p-value | |
| n (%) | n (%) | |||
| Ulceration Rating | 0.964 | |||
| 1 | 1 (5) | 4 (11) | ||
| 2 | 2 (10) | 3 (8) | ||
| 3 | 8 (38) | 8 (22) | ||
| 4 | 10 (48) | 22 (59) | ||
| Cholesterol Rating | 0.381 | |||
| 1 | 2 (10) | 2 (5) | ||
| 2 | 9 (43) | 15 (41) | ||
| 3 | 8 (38) | 13 (35) | ||
| 4 | 2 (10) | 7 (10) | ||
| Calcification Rating | 0.931 | |||
| 1 | 6 (29) | 6 (16) | ||
| 2 | 3 (14) | 8 (22) | ||
| 3 | 1 (5) | 9 (24) | ||
| 4 | 11 (52) | 14 (38) | ||
| Thrombus Rating | 0.141 | |||
| 1 | 11 (52) | 14 (38) | ||
| 2 | 8 (38) | 13 (35) | ||
| 3 | 0 (0) | 2 (5) | ||
| 4 | 2 (10) | 8 (22) | ||
|
| ||||
| Histopathology | Asymptomatic | Symptomatic | p-value | |
|
| ||||
| Hemosiderin | n (%) | n (%) | 0.913 | |
| 0 | 13 (65) | 20 (61) | ||
| 1 | 3(15) | 9 (27) | ||
| 2 | 4 (20) | 2 (6) | ||
| 3 | 0 (0) | 2 (6) | ||
| Inflammation | 0.549 | |||
| 0 | 3 (15) | 3 (9) | ||
| 1 | 12 (60) | 18 (55) | ||
| 2 | 2 (10) | 8 (24) | ||
| 3 | 3 (15) | 4 (12) | ||
| Percent Cholesterol | 0.369 | |||
| Mean (SD) | 53.25 (25.77) | 58.94 (20.34) | ||
| Range | 10–90 | 20–90 | ||
| Percent Calcium | 0.109 | |||
| Mean (SD) | 26.00 (22.22) | 17.27 (16.06) | ||
| Range | 0–65 | 0–60 | ||
| Percent hemorrhage | 0.169 | |||
| Mean (SD) | 2.25 (2.55) | 6.06 (11.51) | ||
| Range | 0–5 | 0–60 | ||
Microemboli Detection
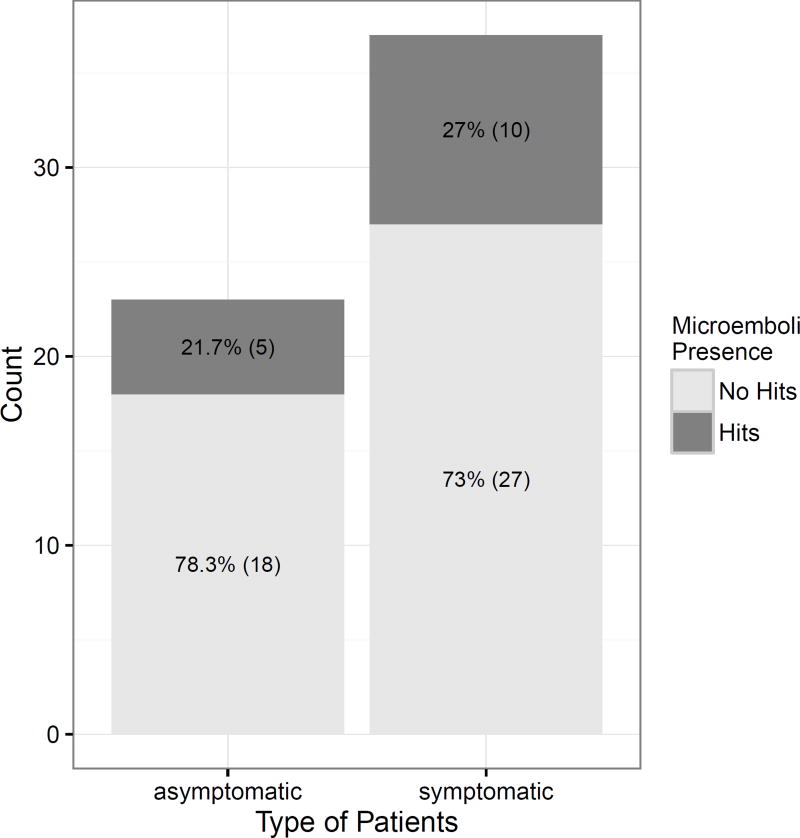
High intensity transient signals (HITS) suggestive of microemboli were detected in 15 of the 60 (25%) participants, (five of the 23 [21.7%] asymptomatic participants and ten of the 37 [27%] symptomatic patients). The frequency of HITS was not statistically significant between the symptomatic and asymptomatic groups, Pearson’s chi-squared test (p=0.88) (see Figure 2).
Figure 2.
Presence of HITS in asymptomatic and symptomatic patients. No significant difference is noted between the two groups (p=0.88).
Macroscopic Plaque Evaluation
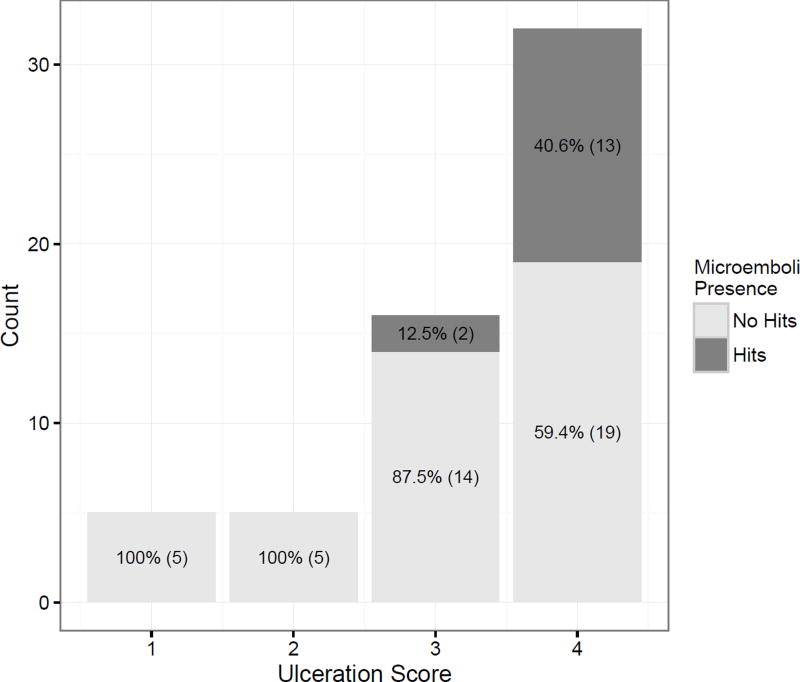
No difference was observed in the macroscopic scoring of these plaques for ulceration, cholesterol, calcium nor thrombus (p-values all > 0.05) (see Table 1). Microemboli presence was associated with an ulceration score (regardless of symptomatic or asymptomatic status, p=0.034), with a one level increase in ulceration rating associated with an odds ratio of 5.86 (95% [CI] 1.55, 43.4) (see figure 3). Thus, for every one unit increase in ulceration rating, the odds of microemboli presence can be expected to increase by a factor of 5.86. An odds ratio of 5.86 can be considered a relatively large effect.
Figure 3.
Presences of HITS are associated with higher ulceration score.
Plaque characteristics at histopathologic examination
Logistic regression revealed no statistically significant differences between asymptomatic and symptomatic patient histopathology plaque scoring for inflammation, hemosiderin, percent calcium, cholesterol nor hemorrhage (p-values all > 0.05) (see Table I).
Discussion
This study demonstrated that 1) the presence of surgical side HITS on TCD was associated with increased macroscopic ulceration score, and 2) there were no differences between symptomatic and asymptomatic subject plaques at histology examination. In addition, patients with HITS were more likely to have ulcerated plaques. Our findings are similar to the findings of others (Mandani, et al. 2011, Sitzer, et al. 1995) in that HITS identified with TCD are associated with plaque ulceration (Sitzer, et al. 1995). Ulcerated plaque, as seen on angiography, ultrasound imaging and histopathology (Mandani, et al. 2011, Sitzer, et al. 1995, Valton, et al. 1998) has been associated with the presence of HITS. HITS have also been associated with other characteristics of plaque vulnerability, such as larger lipid content and thrombus, at histology examination (Van Lammeren, et al. 2014). Thus, in our study, the finding of the presence of HITS and the an association with macroscopic ulceration score seem to support the hypothesis that HITS are associated with the feature of ulceration and that vulnerable or unstable plaque can produce microemboli. Furthermore, no difference was seen in the presence of plaque, characteristics as assessed macroscopically and microscopically.
Our finding of no difference between symptomatic and asymptomatic status differ from others (Salem, et al. 2011, Siebler, et al. 1994, Sun, et al. 2014, Tegos, et al. 2001) in which microemboli were more prevalent in symptomatic patients. Our findings also differ from others (Sitzer, et al. 1995) in that we did not see any macroscopic nor microscopic differences in plaque between the symptomatic and asymptomatic groups. While others (Sitzer, et al. 1995) have reported differences in the frequency of HITS and plaque characteristics at histopathology examination between symptomatic and asymptomatic groups, HITS have been reported in asymptomatic patients (Mandani, et al. 2011). In the Asymptomatic Carotid Emboli Study (ACES) (Topakian, et al. 2011), visual rating of carotid plaque echogenicity and monitoring for HITS were used to assess how these two parameters could be used to assess risk for transient ischemic attack (Topakian, et al. 2011). ACES demonstrated that lower level grayscale plaques (darker plaques) as seen on ultrasound, and the presence of HITS, could be used to further risk stratify patients for risk of stroke (Topakian, et al. 2011). Other imaging findings that are associated with TCD HITS include, low grayscale median values at the surface of the plaque with ultrasound imaging, (Sztajzel, et al. 2006) and higher total white matter hyperintensity lesion volume with magnetic resonance imaging (Berman, et al. 2015).
There are several reasons why our results may differ from those previously reported. First, we monitored our subjects for 60 minutes, longer than several of the other studies, which only monitored patients for 30 minutes. (Salem, et al. 2011, Stork, et al. 2002, Sun, et al. 2014, Tegos, et al. 2001). It is possible that additional HITS suggestive of microemboli were observed in the longer TCD monitoring period. Second, many studies performed TCD on symptomatic participants between 48 hours and 14 days after the occurrence of an event (Sun, et al. 2014); Salem, et al. 2011, and asymptomatic participants underwent TCD at the time of pre-assessment. Studies have shown that microemboli signals peak within the first week after the event, therefore, there may have been a propensity to find more emboli in symptomatic patients when TCD examination was performed close in time to the event (Giles and Rothwell 2007, Salem, et al. 2011). Finally, we do not know if our asymptomatic participants with HITS suggestive of microemboli and high ulceration scores would have gone on to become symptomatic if not treated with carotid endarterectomy. Mandani et al. (Mandani, et al. 2011) demonstrated, in asymptomatic patients with carotid stenosis, that individuals with microembolic signals on TCD examination or those with more than 3 ulcers identified with 3D ultrasound were more likely to suffer a stroke or death within a 3 year follow up time period than those without the presence of microembolic signals and 3 ulcers.
Our findings are significant in that they indicate that in these participants microemboli were associated equally with asymptomatic and symptomatic status, further indicating that it may be time to reframe how we view symptomatic patients, and that asymptomatic patients can have plaque that is throwing microemboli.
Limitations
In this study we only monitored patients for 60 minutes and recorded HITS during this time period. Patients could be having HITS outside this timeframe and this information would not be accounted for. In addition, we do not have information on co-morbidities, such as other health issues that might cause emboli (e.g. atrial fibrillation, patent foramen ovale, cardiac thrombi etc.) which may also explain the presence of HITS.
Conclusion
This study, unlike much of the literature, demonstrates that in participants with advanced carotid atherosclerosis, there is no difference between symptomatic and asymptomatic subjects in HITS frequency or plaque composition. This study, similar to others, did demonstrate that higher macroscopic ulceration scores are associated with the presence of microemboli signals on TCD. These findings suggest that both symptomatic and asymptomatic subjects (with clinical indications for carotid endarterectomy) may have plaque with similar features of instability and the ability to create emboli. Thus, identifying new ways to measure plaque instability may provide important information for optimizing treatment to prevent future stroke. It also suggests that present clinical exam may not be sensitive enough to detect the sequeli of small emboli.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (NIH) Grant: R01 NS064034.
This work was supported by the National Institutes of Health, under Ruth L. Kirschstein National Research Service Award T32 HL-007936 from the National Heart Lung and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center.
C.C. Mitchell: Other; Davies Publishing, Inc, authorship for two echocardiography textbooks, currently under review, may have future royalties. Elsevier, Wolters Kluwer, author textbook chapters, may have future royalties.
T. Varghese: Other; Siemens Ultrasound, Research Agreement for use of Ultrasound Research Interface.
T.D. Cook: Consultant/Advisory Board; GlaxoSmithKline, Bristol-Myers Squibb, Merck, Mast Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
S.M. Wilbrand: None.
C.N. Steffel: None.
T. Varghese: No financial benefit.
N. H. Meshram: None.
G. Li: None.
S. Salamat: None.
R.J. Dempsey: None.
References
- Altaf N, Kandiyil N, Hossseini A, Mehta R, MacSweeney S, Auer D. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: A comparative study of carotid plaque morphology, microemboli assessment and the European Carotid Surgery Trial Risk Model. J Am Heart Assoc. 2014;3:e000173. doi: 10.1161/JAHA.113.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asymptomatic Carotid Atherosclerosis Study A. Endarterectomy fo rasymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–28. [PubMed] [Google Scholar]
- Babikian VL, Hyde C, Pochay V, winter MR. Clinical correlates of high-intensity transient signals detected on transcranial doppler sonography in patients with cerebrovascular disease. Stroke. 1994;25:1570–73. doi: 10.1161/01.str.25.8.1570. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Glaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Flooyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Titchey M, Rodriquez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SE, Wang X, Mitchell CC, Kundu B, Jackson DC, Wilbrand SM, Varghese T, Hermann BP, Rowley HA, Johnson SC, Dempsey RJ. The relationship between carotid artery plaque stability and white matter ischemic injury. NeuroImage: Clinical. 2015;9:216–22. doi: 10.1016/j.nicl.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium. Basic identification criteria of Doppler microembolic signals. Stroke. 1995;26:1123. [PubMed] [Google Scholar]
- Demarin V, Zavoreo I, Kes VB. Carotid artery disease and cognitive impairment. J Neurol Sci. 2012;322:107–11. doi: 10.1016/j.jns.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Dempsey R, Vemuganti R, Varghese T, Hermann B. A review of carotid atherosclerosis and vascular cognitive decline: A new understanding of the keys to symptomology. Neurosurgery. 2010;67:484–94. doi: 10.1227/01.NEU.0000371730.11404.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Varghese T, Jackson DC, Wang X, Meshram NH, Mitchell CC, Hermann BP, Johnson SC, Berman SE, Wilbrand SM. Carotid atherosclerotic plaque instability and cognition determined by ultrasound-measured plaque strain in asymptomatic patients with significant stenosis. J Neurosurg. 2017 doi: 10.3171/2016.10.JNS161299. Published online March 10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic reiew and meta-analysis. Lancet Neurol. 2007;6:1063–72. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics-2013 Update A Report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DC, Sandoval-Garcia C, Rocque BG, Wilbrand SM, Mitchell CC, Hermann BP, Dempsey RJ. Cognitive deficints in symptomatic and asymptomatic carotid endarterectomy surgical candidates. Archives of Clinical Neuropshycology. 2016;31:1–7. doi: 10.1093/arclin/acv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandani A, Bleletsky v, Tamayo A, Munoz C, Spence JD. High-risk asymptomatic carotid stenosis ulceration on 3D ultrasound vs TCD microemboli. Neurology. 2011:744–50. doi: 10.1212/WNL.0b013e31822b0090. [DOI] [PubMed] [Google Scholar]
- Markus HS, Brown MM. Differentiation between different pathological cerebral embolic materials using transcranial Doppler in an in vitro model. Stroke. 1993;24:1–5. doi: 10.1161/01.str.24.1.1. [DOI] [PubMed] [Google Scholar]
- Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, Bornstein NM, Schaafsma A. Asymptomatic embolisation for prediction of stroke in Asymptomatic Carotid Emboli Sudy (ACES): a prospecitve observational study. Lancet Neurol. 2010;9:663–71. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN. Depiction of carotid plaque ulceration and othe rplaque-related disorders by intravascular sonography: A flow chamber study. Am J Neuroradiol. 1996;17:1881–90. [PMC free article] [PubMed] [Google Scholar]
- Mitchell CC, Stein JH, Cook TD, Salamat S, Wang X, Varghese T, Jackson DC, Sandoval Garcia C, Wilbrand SM, Dempsey RJ. Histopathologic Validation of Grayscale Carotid Plaque Characteristics Related to Plaque Vulnerability. Ultrasound in Med & Biol. 2017;43:129–37. doi: 10.1016/j.ultrasmedbio.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial N. North American Symptomatic Carotid Endarterectomy Trial: Methods, Patient Characteristics, and Progress. Stroke. 1991;22:711–20. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- Purandare N, Burns A, Daly KJ, Hardicre J, Moriris J, Macfarlane G, McCollum C. Cerebral emboli as a potential cause of Alzheimer's disease and vascular dementia: Case-control study. BMJ. 2006;332:1119–24. doi: 10.1136/bmj.38814.696493.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing; 2015. [Google Scholar]
- Rocque B, Jackson D, Varghese T, Hermann B, McCormick M, Kliewer M, Mitchell CRJD. Impaired cognitive function in patients with atherosclerotic carotid stenosis and correlation with ultrasound strain measurements. J. Neurol. Sci. 2012;322:20–24. doi: 10.1016/j.jns.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem MK, Bown MJ, Sayers RD, West K, Moore D, Nicolaides A, Robinson TG, Naylor AR. Identification of patients with a histologically unstable carotid plaque using ultrasonic plaque image analysis. Eur J Vasc Endovasc Surg. 2014;48:118–25. doi: 10.1016/j.ejvs.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Salem MK, Butt HZ, Watts APW, Sayers RD, Bown MJ, Naylor AR. Spontaneous cerebral embolisation in asymptomatic and recently symptomatic patients with TIA/Minor stroke. Eur J Vasc Endovasc Surg. 2011;41:720–25. doi: 10.1016/j.ejvs.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Shintani S, Tsuruoka S, Shiigai T. Pure senory stroke caused by a cerebral hemorrhage: Clinical-radiologic correlations in seven patients. Am J Neuroradiol. 2000;21:515–20. [PMC free article] [PubMed] [Google Scholar]
- Siebler M, Kleinschmidt A, Sitzer M, Steinmetz H, Freund HJ. Cerebral microembolism in symptomatic and asymptomatic high-grade internal carotid artery stenosis. Neurology. 1994:615–18. doi: 10.1212/wnl.44.4.615. [DOI] [PubMed] [Google Scholar]
- Siebler M, Seitzer M, rose G, bendfeldt D, Steinmetz H. Silent cerebral embolism caused by neurologically symptomatic high-grade carotid stenosis: event rates before and after carotid endarterectomy. Brain. 1993;116:1005–15. doi: 10.1093/brain/116.5.1005. [DOI] [PubMed] [Google Scholar]
- Sitzer M, Muller W, Siebler M, Hort W, Kniemeyer HW, Jancke L, Steinmetz H. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke. 1995;26:1231–3. doi: 10.1161/01.str.26.7.1231. [DOI] [PubMed] [Google Scholar]
- Smith CD, Snowdon DA, Whang H, Markesbery WR. White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology. 2000;54:838–42. doi: 10.1212/wnl.54.4.838. [DOI] [PubMed] [Google Scholar]
- Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, KAsner SE, Seshadri S. Prevention of stroke in patients with silent cerbrovascular disease scientific statement for healthcare professionals from teh American Heart Association/American Stroke Association. Sttroke. 2017;48:000–00. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 277:813–17. [PubMed] [Google Scholar]
- Spencer MP, thomas gI, Nicholls SC, Sauvage LR. Detection of middle cerebral artery emboli during carotid endarterectomy using transcranial Doppler ultrasonography. Stroke. 1990;21:415–23. doi: 10.1161/01.str.21.3.415. [DOI] [PubMed] [Google Scholar]
- Stork JL, Kimura K, Levi CR, Chambers BR, Abbott AL, Donnan GA. Source of microembolic signals in patients with high-grade carotid stenosis. Stroke. 2002;33:2014–18. doi: 10.1161/01.str.0000021002.17394.7f. [DOI] [PubMed] [Google Scholar]
- Sun DJ, Xhuang AX, Zeng QH, Jiang YL, Jiang JD, Feng SQ, Zhang Y, Huang HM, Nie HX, Liu L. A study of microemboli monitoring of atherosclerotic thrombotic cerebral infarction and artery stenosis. Genet. Mol. Res. 2014;13:6734–45. doi: 10.4238/2014.August.28.17. [DOI] [PubMed] [Google Scholar]
- Svindland A, Torvik A. Atheroscleroitc carotid disease in asymptomatic individuals an histological study of 53 cases. Acta Neurol. Scand. 1988;78:506–17. doi: 10.1111/j.1600-0404.1988.tb03694.x. [DOI] [PubMed] [Google Scholar]
- Sztajzel R, Momjian-Mayor I, Commelli M, Momjian S. Correlation of cerebrovascular symptoms and microembolic signals with stratified gray-scale median analysis and color mapping of the carotid plaque. Stroke. 2006;37:824–29. doi: 10.1161/01.STR.0000204277.86466.f0. [DOI] [PubMed] [Google Scholar]
- Tegos TJ, Sabetai MM, Nicolaides AN, Robless P, Kalodiki E, Elatrozy TS, Ramaswami G, Dhanjil S. Correlates of embolic events detected by means of transcranial Doppler in patients with carotid atheroma. J Vasc Surg. 2001;33:131–8. doi: 10.1067/mva.2001.109746. [DOI] [PubMed] [Google Scholar]
- Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS. Ultrasonic plaque echolucency and emoli signals predict stroke in asymptomatic carotid stenosis. Neruology. 2011;77:751–58. doi: 10.1212/WNL.0b013e31822b00a6. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and agniogenic gene expression incarotid artery plaques from symptomatic stroke patients. Neurosurgery. 2006;58:971–77. doi: 10.1227/01.NEU.0000210246.61817.FE. [DOI] [PubMed] [Google Scholar]
- Valton L, Larrue V, le Traon AP, Massabuau P, Geraud G. Microembolic signals and risk of early recurrence in patients with stroke or transient ischemic attack. Stroke. 1998;29:2125–28. doi: 10.1161/01.str.29.10.2125. [DOI] [PubMed] [Google Scholar]
- Van Lammeren GW, Van De Mortel RH, Visscher M, Pasterkamp G, De Borst GJ, Moll fL, Vink A, Tromp SC, DeVries JP. Spontaneous preoperative microemboic signals detected with th etranscranial Doppler are associated with vulnerable carotid plaque characteristics. J Cardiovasc Surg. 2014;55:375–80. [PubMed] [Google Scholar]
- Wu X, Zhang H, Liu H, Xing Y, Liu K. Microembolic signals detected with transcranial Doppler sonography differ between symptomatic and asymptomatic middle cerebral artery stenoses in Northeast China. PLoS One. 2014;9:e88986. doi: 10.1371/journal.pone.0088986. [DOI] [PMC free article] [PubMed] [Google Scholar]