Abstract
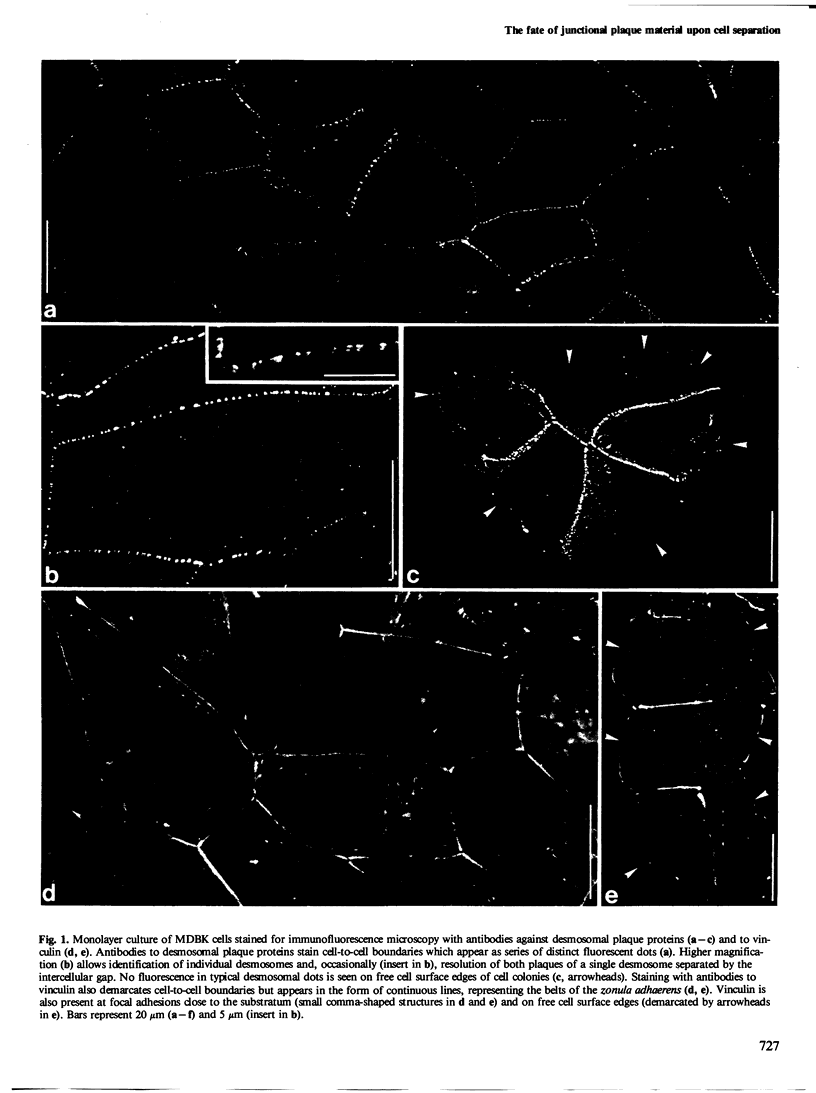
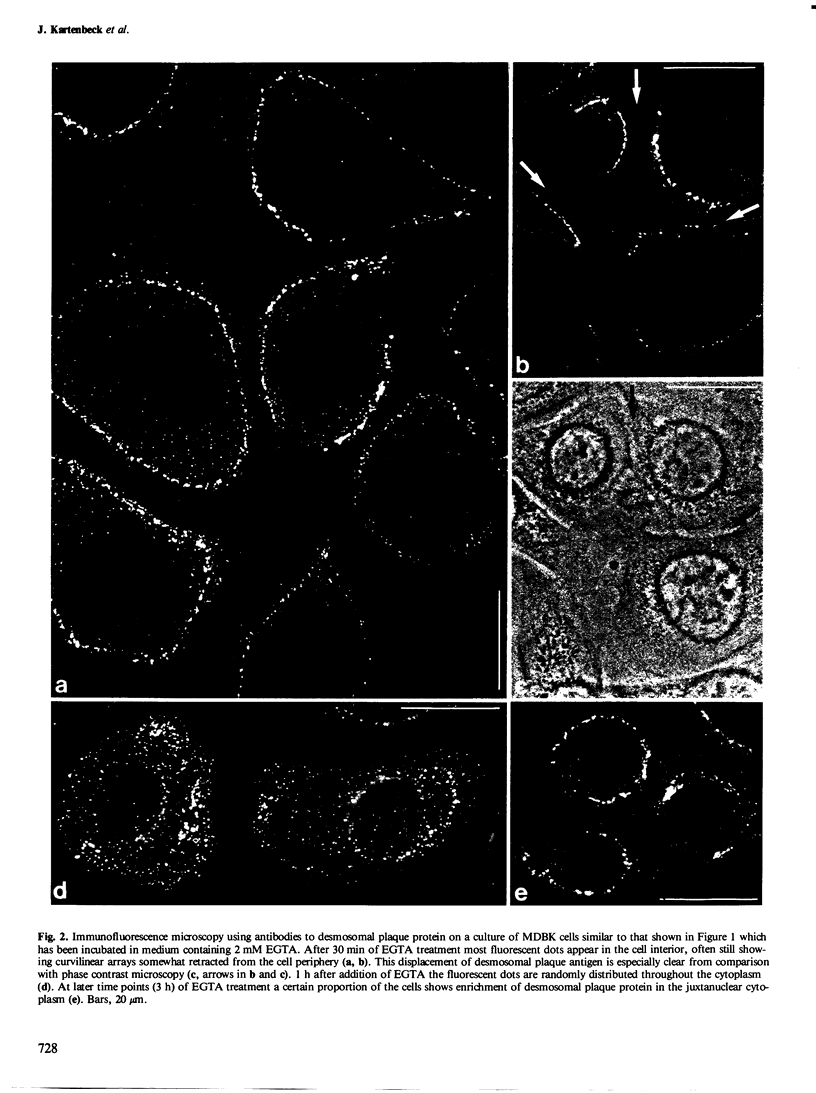
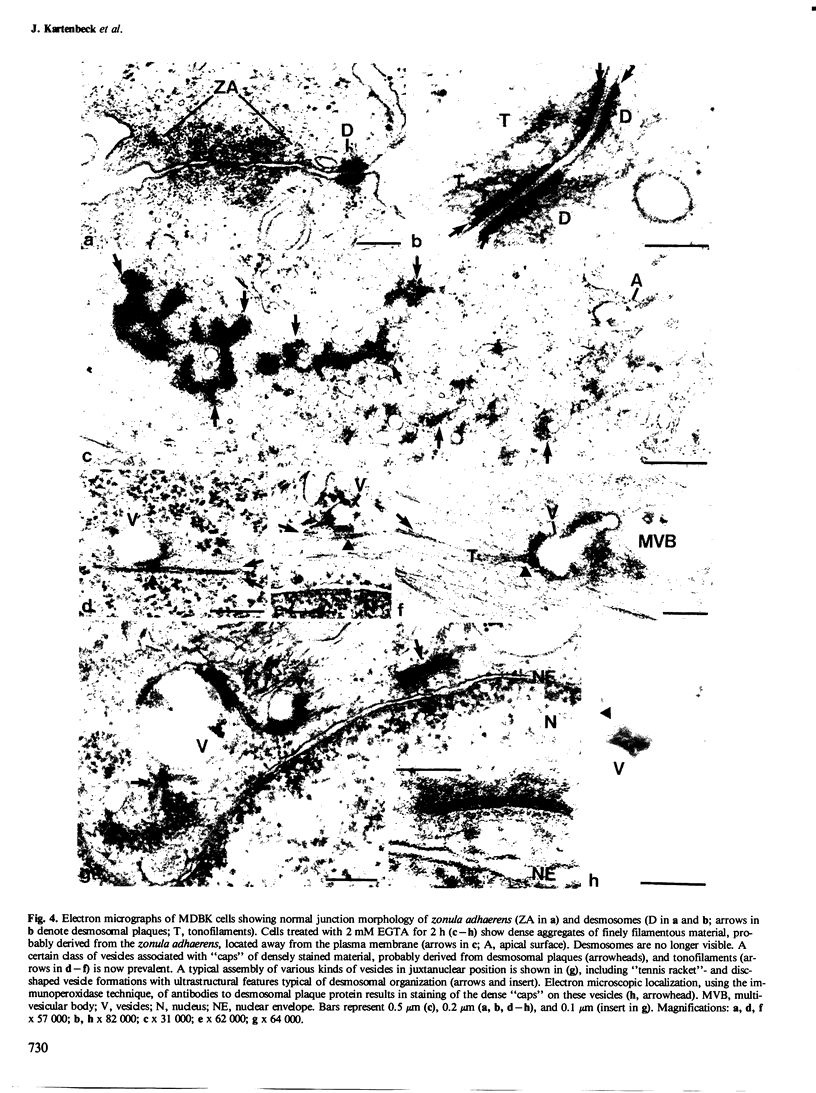
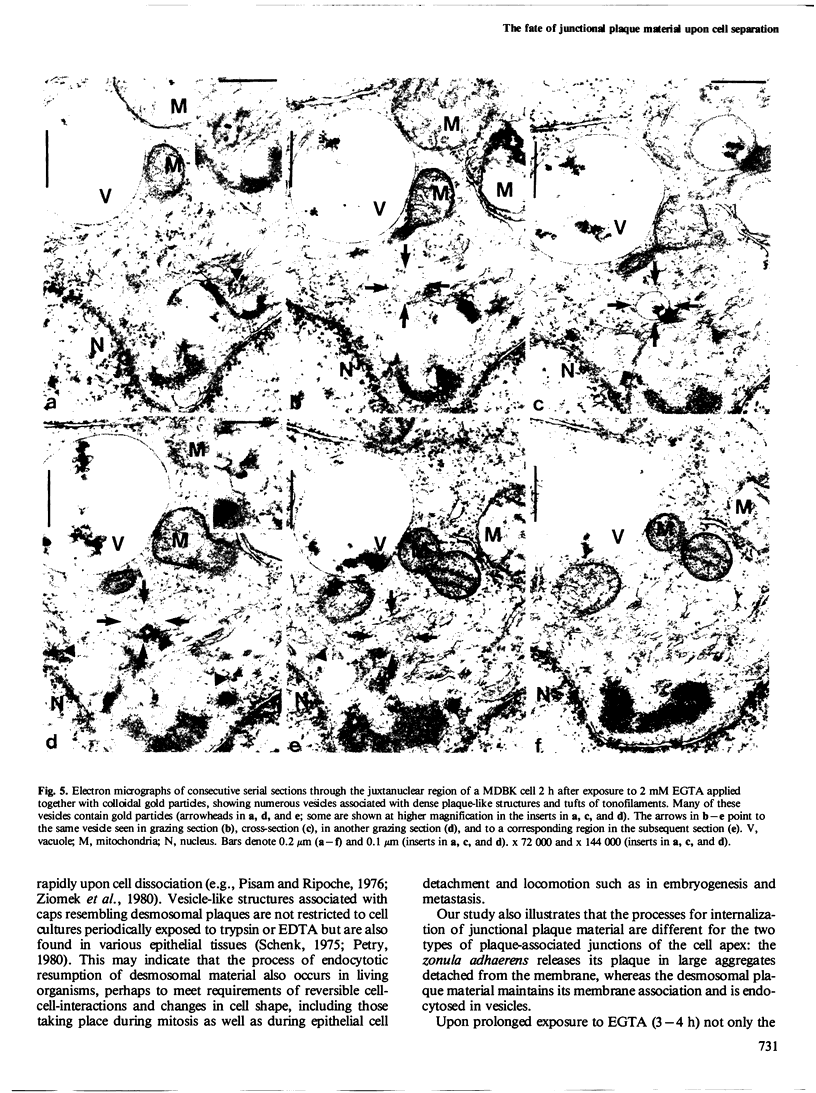
The distribution and fate of two junctional complexes, zonula adhaerens and desmosomes, after dissociation of cell-cell contacts is described in MDBK cells. Junctions were split between adjacent cells by treatment with EGTA and proteins associated with the plaques of zonulae adhaerentes and desmosomes were localized by immunological methods. Splitting of these junctions is accompanied by the dislocation of desmosomal plaque protein from the cell periphery and its distribution in punctate arrays over the whole cytoplasm. By contrast, vinculin associated with zonulae adhaerentes is still seen at early times (0.5-1 h) in a conspicuous belt-like structure which, however, is displaced from the plasma membrane. Strong vinculin staining is maintained on leading edges of free cell surfaces. Electron microscopy of EGTA-treated cells exposed to colloidal gold particles reveals the disappearance of junctional structures from the cell periphery and the concomitant appearance of a distinct class of gold particle-containing vesicles which are coated by dense plaques. These vesicle plaques react with antibodies to desmosomal plaque proteins and are associated with filaments of the cytokeratin type. In the same cells, extended dense aggregates are seen which are most probably the membrane-detached vinculin-rich material from the zonula adhaerens . The experiments show that, upon release from their junction-mediated connections with adjacent cells, major proteins associated with the cytoplasmic side of the junctions remain, for several hours, clustered within plaques displaced from the cell surface. While plaque material of adhaerens junctions containing vinculin is recovered in large belt-like aggregates, desmosomal plaque protein remains attached to membrane structures and appears on distinct vesicles endocytotically formed from half-desmosomal equivalents.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D., Dockstader J., Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982 Jan;92(1):213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysenko J. Z., Revel J. P. Experimental manipulation of desmosome structure. Am J Anat. 1973 Aug;137(4):403–421. doi: 10.1002/aja.1001370404. [DOI] [PubMed] [Google Scholar]
- Caputo R., Prandi G. Intracytoplasmic desmosomes. J Ultrastruct Res. 1972 Nov;41(3):358–368. doi: 10.1016/s0022-5320(72)90075-5. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaco C. A., Evans W. H. A biochemical dissection of the cardiac intercalated disk: isolation of subcellular fractions containing fascia adherentes and gap junctions. J Cell Sci. 1981 Dec;52:313–325. doi: 10.1242/jcs.52.1.313. [DOI] [PubMed] [Google Scholar]
- Drochmans P., Freudenstein C., Wanson J. C., Laurent L., Keenan T. W., Stadler J., Leloup R., Franke W. W. Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J Cell Biol. 1978 Nov;79(2 Pt 1):427–443. doi: 10.1083/jcb.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Heid H. W., Grund C., Winter S., Freudenstein C., Schmid E., Jarasch E. D., Keenan T. W. Antibodies to the major insoluble milk fat globule membrane-associated protein: specific location in apical regions of lactating epithelial cells. J Cell Biol. 1981 Jun;89(3):485–494. doi: 10.1083/jcb.89.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Müller H., Engelbrecht I., Moll R., Stadler J., Jarasch E. D. Antibodies to high molecular weight polypeptides of desmosomes: specific localization of a class of junctional proteins in cells and tissue. Differentiation. 1981;20(3):217–241. doi: 10.1111/j.1432-0436.1981.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Black M. M., Epstein W. L. Ultrastructural studies of newborn rat epidermis after trypsinization. J Ultrastruct Res. 1974 Feb;46(2):219–229. doi: 10.1016/s0022-5320(74)80057-2. [DOI] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B., Dutton A. H., Tokuyasu K. T., Singer S. J. Immunoelectron microscope studies of membrane-microfilament interactions: distributions of alpha-actinin, tropomyosin, and vinculin in intestinal epithelial brush border and chicken gizzard smooth muscle cells. J Cell Biol. 1981 Dec;91(3 Pt 1):614–628. doi: 10.1083/jcb.91.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Tokuyasu K. T., Dutton A. H., Singer S. J. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. F., Meiss H. K., Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981 May;89(2):230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Anderson D. J., Friedlander M., Gilula N. B. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol. 1982 Jan;92(1):53–59. doi: 10.1083/jcb.92.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi Sang U., Saier M. H., Jr, Ellisman M. H. Tight junction formation is closely linked to the polar redistribution of intramembranous particles in aggregating MDCK epithelia. Exp Cell Res. 1979 Sep;122(2):384–391. doi: 10.1016/0014-4827(79)90315-x. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Schmid E., Müller H., Franke W. W. Immunological identification and localization of clathrin and coated vesicles in cultured cells and in tissues. Exp Cell Res. 1981 May;133(1):191–211. doi: 10.1016/0014-4827(81)90369-4. [DOI] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADIN S. H., DARBY N. B., Jr Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med. 1958 Jul;98(3):574–576. doi: 10.3181/00379727-98-24111. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Castiglioni G., Parma R., Nassivera N., De Camilli P. Ca++-dependent disassembly and reassembly of occluding junctions in guinea pig pancreatic acinar cells. Effect of drugs. J Cell Biol. 1978 Oct;79(1):156–172. doi: 10.1083/jcb.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Nicholson B. J., Hunkapiller M. W., Grim L. B., Hood L. E., Revel J. P. Rat liver gap junction protein: properties and partial sequence. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7594–7598. doi: 10.1073/pnas.78.12.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton J., DeSalle R. Control of desmosome formation in aggregating embryonic chick cells. Dev Biol. 1980 Mar;75(1):168–176. doi: 10.1016/0012-1606(80)90152-9. [DOI] [PubMed] [Google Scholar]
- Overton J. Formation of junctions and cell sorting in aggregates of chick and mouse cells. Dev Biol. 1977 Jan;55(1):103–116. doi: 10.1016/0012-1606(77)90323-2. [DOI] [PubMed] [Google Scholar]
- Overton J. The fat of desmosomes in trypsinized tissue. J Exp Zool. 1968 Jun;168(2):203–214. doi: 10.1002/jez.1401680208. [DOI] [PubMed] [Google Scholar]
- Petry G. "Autodesmosomen", desmosomale Kontakte von Teilen derselben Zelle im menschlichen Chorion laeve und Amnion. Eur J Cell Biol. 1980 Dec;23(1):129–136. [PubMed] [Google Scholar]
- Pisam M., Ripoche P. Redistribution of surface macromolecules in dissociated epithelial cells. J Cell Biol. 1976 Dec;71(3):907–920. doi: 10.1083/jcb.71.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., Chuman L. M., Shaffer L., Saier M. H., Jr Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J Cell Biol. 1979 Jun;81(3):635–648. doi: 10.1083/jcb.81.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEDAR A. W., FORTE J. G. EFFECTS OF CALCIUM DEPLETION ON THE JUNCTIONAL COMPLEX BETWEEN OXYNTIC CELLS OF GASTRIC GLANDS. J Cell Biol. 1964 Jul;22:173–188. doi: 10.1083/jcb.22.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk P. Desmosomale Strukturen im Cytoplasma normaler und pathologischer Keratinocyten. Arch Dermatol Res. 1975 Aug 29;253(1):23–42. doi: 10.1007/BF00557978. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Skerrow C. J., Matoltsy A. G. Chemical characterization of isolated epidermal desmosomes. J Cell Biol. 1974 Nov;63(2 Pt 1):524–530. doi: 10.1083/jcb.63.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Ziomek C. A., Schulman S., Edidin M. Redistribution of membrane proteins in isolated mouse intestinal epithelial cells. J Cell Biol. 1980 Sep;86(3):849–857. doi: 10.1083/jcb.86.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bülow M., Klingmüller G. Eledtronenmikroskopische Untersuchungen des Keratoakanthoms. Vorkommen intracytoplasmatischer Desmosomen. Arch Dermatol Forsch. 1971;241(3):292–304. [PubMed] [Google Scholar]