Abstract
We report two rare cases of inflammatory reactions with multiple subcutaneous facial painful collections after Hyaluronic acid injections, expose their management and discuss aetiologic hypothesis. Due to unfavourable evolution despite antibiotic treatment, surgical drainage was performed. Immune-mediated delayed hypersensitivity reactions were the most probable cause.
Keywords: Hyaluronic acid, hypersensitivity, complications, risk factors
Introduction
Hyaluronic acid (HA) represents 90% of the filler market with a growing demand [1]. Even if HA is considered as the safest synthetic filler, the number of complications after HA injection will also increase, particularly severe complications requiring aggressive treatment and leaving permanent sequelae. We present two rare and unusual cases of inflammatory reactions with multiple subcutaneous painful collections following facial hyaluronic acid injections, where the immune-mediated delayed hypersensitivity reactions were the most probable cause.
Case reports
Case 1
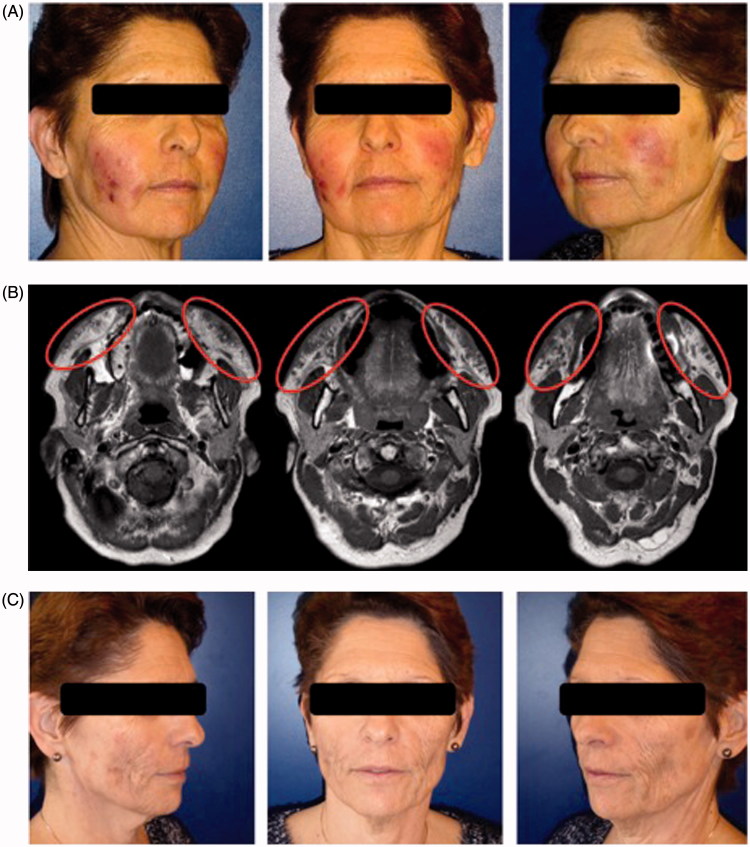
A 60-year-old woman was addressed to our department for multiple inflammatory collections on the face 14 days after HA injections by her plastic surgeon in February 2016. She had received 1 ml of HA (Volbella®, Allergan, Dublin, Ireland) injections on each cheek for the treatment of wrinkles. It was her third HA injection and she reported satisfactory results without adverse events in the past. The patient was in good health and not known for any type of allergies. At day 1, the patient presented a sore throat without fever. At day 10, she observed pain, redness and oedema at multiple injection sites of the right cheek and consulted her injector physician. A swab test confirmed a streptococcal throat infection and the patient received a first treatment of amoxicillin and clavulanic acid. After 24h, the same inflammatory signs appeared on the left cheek and pustules were noted on the right cheek, allowing a bacterial sample analysis. At day 12, after 72h of antibiotic treatment, the patient developed multiple pustules over the entire body. An allergic reaction was suspected and the antibiotic regimen was switched to azithromycin. She was referred to our consultation at day 14 following worsening erythema and oedema of the face without fever. Physical examination showed warm and swollen cheeks with multiple painful indurations without collection. The antibiotic treatment was switched again to clindamycin following consultation with an infectiologist. Due to a worsening status with multiple palpable collections in both cheeks, the patient was hospitalised on day 16 (Figure 1(A)). The white blood cell count was 17.9 G/l and C-reactive protein was 29.1 mg/l. Incision and drainage performed at the bedside showed coagulated blood mixed with a sticky liquid like pus. Treatment with intravenous amoxicillin and clavulanic acid was started once again. Due to the occurrence of new collections, magnetic resonance imaging was performed and showed a diffuse infiltration of cutaneous and subcutaneous tissues with confluent subcutaneous collections in both cheeks (Figure 1(B)). Drainage under general anaesthesia was performed on day 20. The collections exuded approximately 5 ml of coagulated blood with a greyish viscous material. She presented other lesions that were indurated without collection at the time of operation. However, these lesions transformed to collections a few days later. Daily bedside collection drainage and antiseptic rinsing and antibiotic treatment were continued. All bacterial analysis and culture were negative. The clinical and biological evolution was favourable and the patient returned home after 11 days of hospitalisation. However, during the first 2 weeks backed to home, she continued to have new collections that needed drainage of the same type of liquid. After this period, she presented post-inflammatory induration on both cheeks that improved with daily physiotherapy and intense pulsed light therapy once a week. At 6 months, the patient presented aesthetic sequelae (Figure 1(C)).
Figure 1.
Case 1: A 60-year-old woman who received 1 ml of hyaluronic acid (Volbella®, Allergan, Dublin, Ireland) injections on each cheek to improve wrinkles. (A). Day 16: warm and swollen cheeks with multiple painful indurations and collections. (B). Magnetic resonance imaging at day 20: diffuse infiltration of cutaneous and subcutaneous tissues with confluent subcutaneous collections in both cheeks, the largest measuring 35 × 11 × 11 mm in the left cheek. (C). At 6 months, the patient presented aesthetic sequelae with inflammatory induration on both cheeks improved after daily physiotherapy and intense pulsed light therapy once a week.
Case 2
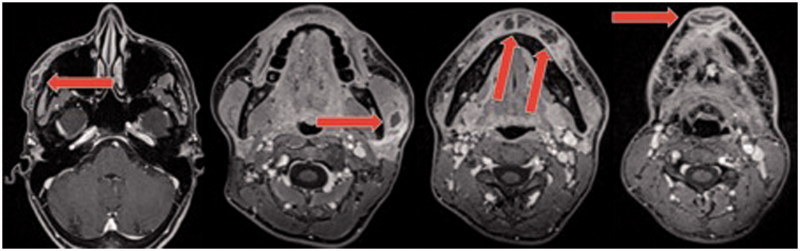
In May 2016, a 30-year-old woman was addressed to our department for multiple inflammatory collections on the face 18 days following HA injections by her dermatologist. She was in good health and not known for allergy. Five years previously, she had received HA injection in the chin. To improve the facial contour, she received HA injections: 0,6 ml of Voluma® (Allergan) and 0.8 ml of Volift® (Allergan) in the cheeks, mandible and chin. She also presented a sore throat the following day and received amoxicillin treatment. Treatment was switched to clarithromycin and ciprofloxacin at day 4 due to the occurrence of multiple small pustules over the entire body. At day 10, the patient presented erythema and swelling of the white inferior lip and the chin. The patient received a prednisone regimen of 60 mg/day for 5 days with temporary improvement. At day 16, the patient presented erythema and swelling at different injection sites. An echography showed subcutaneous collections at the chin. A puncture showed the presence of pus and a sample was sent for bacterial analysis and culture. The patient was referred to our department at day 18 after HA injection. She presented with erythema, swelling and painful palpable subcutaneous collections at the chin, over the right cheekbone and the left angle of the mandible, without fever. The lower white lip was indurated and painful. The white blood cell count was 12.9 G/l and C-reactive protein was 15.2 mg/l. Magnetic resonance imaging confirmed subcutaneous facial collections in different localisations (Figure 2). The patient was hospitalised and incision and drainage were performed under general anaesthesia that showed a greyish viscous discharge with coagulated blood, mainly in the chin (∼4 ml) and over the right cheekbone (∼1 ml). Bedside drainage and antiseptic rinsing were continued twice daily and then daily until necessary for 10 days. The patient was treated with intravenous piperacillin tazobactam for 6 days post-operatively. Due to the occurrence of multiple pustules over the entire body, the antibiotic therapy was switched to oral ciprofloxacin and clindamycin for one month. The clinical and biological evolution was favourable and the patient went home after 9 days of hospitalisation. Bacterial analysis and culture were negative. She presented an early recurrence of the subcutaneous collection at the chin, necessitating drainage and antiseptic rinsing for one additional week. The patient presented a chronic palmoplantar pustolosis 10 days after the last antibiotic treatment, which was finally diagnosed as a psoriasis variant and treated with ultraviolet therapy. At three months, she did not present any aesthetic sequelae, but the lower white lip was still indurated at palpation. An acute generalised exanthematous pustulosis hypersensitivity reaction to aminopenicillins or HA was suspected, but both were negative following patch skin and intradermal testing. In vitro lymphoproliferation test was not interpretable due to a non-specific immunological reaction against HA.
Figure 2.
MRI Case 2: From left to right: 12 × 8 mm collection at the right temporal region, one of 16 × 12 mm facing the left angle of the mandible, tree collections in the lower white lip (two of 7 mm and one of 13 × 9 mm), one collection at the chin (22 × 8 mm).
Discussion
Technical aesthetic adverse events are common with HA [2] but complications due to the product itself are rare. We presented two cases of subacute reaction to HA injections leading to subcutaneous collections. The differential diagnosis included infection, foreign body granulomatous reaction and immune-mediated delayed hypersensitivity reactions.
Infection could have been caused by contamination during treatment or by the dissemination of infection through bacteraemia as both patients had a sore throat the day following HA injection. Infection after filler injection has been reported when a dental infection was present [3]. Among the few proven cases of infection, group A Streptococcus was the most represented pathogen [4]. In our cases, the infection diagnosis is questionable for different reasons. First, the clinical evolution was not improved by the antibiotic treatment, and even in one of the two patients a good response was obtained with corticoids. Second, there was a recurrence of the inflammatory reactions up to four weeks after HA injections with the development of new delayed collections. Finally, all bacteria samples were negative, but it has been taking in consideration that both patients had received antibiotics for at least 72h before a sample could be sent for analysis.
Considering the hypothesis of a granulomatous reaction, this is a rare event after HA injection (0.02–0.4%) and usually occurs at least two to three months after filler injections [5,6]. In our patients, the diagnosis of granulomatous is mostly improbable as the first collections appeared only few days after HA injection.
The last diagnosis hypothesis is immune-mediated delayed hypersensitivity reactions. In both cases, the patients developed in addition to inflammatory collections on the injected site, an acute pharyngitis and delayed generalised exanthematous pustulosis reaction. The explanation could be an immunological reaction against HA fillers. HA molecules contented in all fillers are the same polysaccharide molecules that compose a major part of our skin. Therefore, HA molecule itself is not usually considered immunogen. However, as suggested by Bitterman-Deutsch et al., in some conditions, glycosaminoglycan (HA) could trigger directly a specific immune response without the primary phase of inflammation, like a ‘superantigen’ [7]. Other components that are added to stabilise HA molecules (e.g. crosslinking, conserving) could also be immunogenic. Interestingly, both of our patients developed these reactions after the injection of new HA products using a new crosslinking technique: Vycross technique used in Volbella®, Voluma®, Volift® (Allergan). Such reactions have never been seen in our department, and a literature review did not permit to find similar type of reactions in the past. To verify this hypothesis, an allergic work-up was performed in the second patient with the same HA that was used. Even if the results were negative, a hypersensitivity reaction cannot be excluded; because the sensitivity of these tests are limited in acute generalised exanthemous pustulosis [8–10].
An immunologic interaction between aminopenicillin and an infection (i.e. sore throat) could be another factor. It is known for example that a concomitant aminopenicillin treatment and Ebstein–Barr viral infection can induce maculopapular exanthems [11]. The exact mechanism behind them is unclear. It is not well explained yet, whether a true allergic drug reaction, infectious-dependent eruption or transient loss of drug tolerance due to the infection is responsible for the symptoms [12]. HA, like viral infection, is known to activate in vitro T-lymphocytes via CD44 [13]. Therefore, HA could be considered as a risk factor for the development of hypersensitivity reactions when an aminopenicillin treatment is introduced. As both cases reported here developed a generalised exanthematous pustulosis 72 hours after the beginning of aminopenicllin treatment, it makes this a more plausible cause of the generalised reaction. Therefore, as long as the mechanism of this reaction is not fully understood, caution should be exercised in the use of penicillin when an HA infection is suspected; and another type of antibiotics such as cephalosporin should be considered.
Conclusions
As the demand grows for rejuvenation treatment, complications will also increase. Complications are probably underreported, thus contributing to an adding difficulty regarding an understanding of their causes, mechanism and optimal treatment. All complications should be reported to obtain epidemiologic data and elicit proper guidelines. Unfortunately, in the case of inflammation after dermal filler injection, antibiotics are mostly prescribed before any sample has been analysed. Therefore, if any infection is suspected, a bacteria sample should always be sent for culture before any antibiotic treatment is started. Of note, aminopenicillins should be used with caution after HA injection as generalised skin reactions may occur.
Disclosure statement
The authors report no conflicts of interest.
Funding
No funding was received for the study.
References
- 1.Rzany B, DeLorenzi C.. Understanding, avoiding, and managing severe filler complications. Plast Reconstr Surg. 2015;136:196S–203S. [DOI] [PubMed] [Google Scholar]
- 2.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575. [DOI] [PubMed] [Google Scholar]
- 3.Ramzi AA, Kassim M, George JV, et al. Dental procedures: is it a risk factor for injectable dermal fillers? J Maxillofac Oral Surg. 2015;14:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chader H, Bosc R, Hersant B, et al. [Infectious cellulitis of the face complicating injection for esthetic nasolabial sulcus by hyaluronic acid: report of seven cases]. Annales De Chirurgie Plastique Et Esthetique. 2013;58:680–683. (Dermohypodermite infectieuse de la face compliquant l'injection a visee esthetique des sillons nasogeniens par de l'acide hyaluronique: a propos de sept cas.) [DOI] [PubMed] [Google Scholar]
- 5.Friedman PM, Mafong EA, Kauvar AN, et al. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494. [DOI] [PubMed] [Google Scholar]
- 6.Lemperle G, Gauthier-Hazan N, Wolters M, et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123:1842–1863.. [DOI] [PubMed] [Google Scholar]
- 7.Bitterman-Deutsch O, Kogan L, Nasser F.. Delayed immune mediated adverse effects to hyaluronic acid fillers: report of five cases and review of the literature. Dermatol Report. 2015;7:5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolkenstein P, Chosidow O, Flechet ML, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Derm. 1996;35:234–236. [DOI] [PubMed] [Google Scholar]
- 9.Britschgi M, Steiner UC, Schmid S, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padial MA, Alvarez-Ferreira J, Tapia B, et al. Acute generalized exanthematous pustulosis associated with pseudoephedrine. Br J Dermatol. 2004;150:139–142. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Delgado P, Blanes M, Soriano V, et al. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76–78. [DOI] [PubMed] [Google Scholar]
- 12.Onodi-Nagy K, Kinyo A, Meszes A, et al. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan AR, Racine RR, Hennig MJ, et al. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. 2015;6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]