Abstract
Background
LADA is probably the most prevalent form of autoimmune diabetes. Nevertheless, there are few data about cardiovascular disease in this group of patients. The aim of this study was to investigate the frequency of carotid atherosclerotic plaques in patients with LADA as compared with patients with classic type 1 diabetes and type 2 diabetes.
Methods
Patients with LADA were matched for age and gender in different proportions to patients with type 2 diabetes, and classic type 1 diabetes. None of the patients had clinical cardiovascular disease. All subjects underwent B-mode carotid ultrasound to detect atheroma plaques. Demographics were obtained from all subjects.
Results
We included 71 patients with LADA, 191 patients with type 2 diabetes and 116 patients with type 1 diabetes. Carotid atherosclerosis was more frequent in patients with LADA compared with type 2 diabetes (73.2% vs. 56.9%, P = 0.0018) and classic type 1 diabetes (57.1%, P = 0.026); these changes occurred despite healthier macrovascular risk profiles in the former. Age (P < 0.001), smoking (P = 0.003) and hypertension (P = 0.019) were independently associated with carotid atherosclerosis. Multiple plaques were also more frequent in patients with LADA as compared with classic type 1 diabetes and type 2 diabetes (45.1% and 33.6% vs. 27.2%, respectively, P = 0.022). The frequency of carotid plaques increased with increasing diabetes duration in LADA patients compared with type 2 diabetes (85.7% vs. 58.8%, inverse OR 5.72 [1.5–21.8]; P = 0.009).
Conclusions
LADA patients do not present with less carotid atherosclerosis than patients with type 1 and type 2 diabetes. Their macrovascular risk occurs despite a healthier macrovascular risk profile than those patients with type 2 diabetes.
Keywords: Carotid plaque, Atherosclerosis, Late onset autoimmune diabetes, LADA, Type 1 diabetes, Type 2 diabetes
Background
Macrovascular disease is the leading cause of morbidity and mortality in patients with both type 1 diabetes and type 2 diabetes [1]. Latent autoimmune diabetes of the adults (LADA), that is patients with adult-onset autoimmune diabetes who do not initially require insulin, represent 4–14% of subjects previously diagnosed with type 2 diabetes [2]. LADA can be estimated to have a higher prevalence than classic type 1 diabetes in both children and adults. Notwithstanding its clinical relevance, data about cardiovascular events and mortality in patients with LADA are limited. It could be anticipated that patients with LADA would be less likely to develop macrovascular disease than patients with type 2 diabetes, as they have a reduced risk of metabolic syndrome [3–5]. However, most studies to date, despite their limited size, population heterogeneity and variable disease duration, have failed to show such a reduced risk, and indeed many find an increased risk [6–9].
Preclinical atherosclerosis can be detected and quantified non-invasively by carotid ultrasound, which is a strong predictor of cardiovascular events [10–16]. Although there is still controversy in this field, established carotid atherosclerosis (carotid plaques), detected by ultrasound imaging, improves risk stratification when added to traditional risk factors. Indeed, individuals with carotid plaques are considered a very-high risk category according to the most recent guidelines, and carotid artery plaque assessment using ultrasonography may be considered to be a valid risk modifier in cardiovascular prediction [17–19]. Patients with diabetes mellitus are at high risk of cardiovascular events; therefore, the added potential of carotid plaque detection in risk assessment would be different from those cases without diabetes [20–22]. However, primary prevention targets are often not reached in patients with diabetes, and carotid plaque detection could focus more intensive preventive strategies in these cases, especially in those patients without known organ damage or important associated cardiovascular risk factors [18, 19, 23].
The current study was initially designed to assess the difference in preclinical carotid plaque burden in patients with LADA, as compared to type 2 diabetes. After the results of the initial study, we extended the comparison to age and gender matched patients with classic onset type 1 diabetes.
Methods
This was a cross-sectional study to evaluate carotid atherosclerotic disease in patients with LADA in comparison with patients with type 2 diabetes and classic onset type 1 diabetes.
Study subjects
All adult patients attending local diabetes outpatient clinics in the province of Lleida, Spain, who were screened for diabetes-associated autoantibodies and defined as LADA if they had diabetes diagnosed over 30 years of age, with positive glutamic acid decarboxylase (GAD) antibodies and without need of insulin treatment in the first 6 months after diagnosis [24]. From this cohort of 80 LADA patients, we invited all the patients that fulfilled the inclusion criteria (n = 71, acceptance rate: 100%): absence of clinical cardiovascular disease and without established diabetic nephropathy [urine albumin-to-creatinine ratio (UACR) < 300 mg/g and estimated (MDRD4) glomerular filtration rate (eGFR) > 60 ml/min/1.73 m2].
Age- and sex-matched subjects with type 2 diabetes were randomly selected to a 3:1 proportion and for classic onset type 1 diabetes on a 2:1 proportion from the same local cohort using the same cardiovascular and renal inclusion criteria. All patients selected with type 2 diabetes were negative for GAD antibodies.
The absence of clinical macrovascular disease in all subjects was confirmed by clinical assessment and review of patients’ medical records to verify absence of heart failure, cerebrovascular disease, coronary heart disease, or peripheral arterial disease (all of them clinically assessed, including any form of diabetic foot disease).
All the participants signed an informed consent form and the Ethics Committee of both participant centers approved the study.
Clinical assessment
For each subject, age, sex, weight, height, body mass index and waist circumference were measured by standardized methods. Blood pressure (mean of 2 measurements 5 min apart) was measured using a blood pressure monitor (HEM-7001E, Omron, Spain) after 10 min in the seated position. Patients were specifically interviewed about the treatment of diabetes and smoking habit. Former smokers were those who had quit at least for 1 year before enrollment. A patient was arbitrarily considered to have previous hypertension or dyslipidemia if she/he was taking medication for the given condition.
Fasting blood and urine samples were collected and analyzed locally, using standardized assays to measure glucose, glycosylated haemoglobin, the lipid profile [including high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides] creatinine and the albumin-to-creatinine ratio. Glomerular filtration rate was estimated by the modification of diet in renal disease formula (MDRD4). GAD antibodies were measured with a commercially available ELISA kit (DRG Diagnostic, Marburg, Germany), as previously described [25]. Optimal cut-off value for positivity was set at 5 U/ml. The assay showed good performance when tested in the Diabetes Antibody Standardization Program (DASP) Workshops. In DASP 2007, sensitivities and specificities for GAD antibodies were 94 and 97% and in DASP 2009 were 82 and 95%, respectively.
Carotid ultrasound imaging
All the study participants underwent the same carotid ultrasound protocol. Carotid ultrasound imaging was performed using a high resolution B-mode ultrasound (Sequoia 512, Siemens, North Rhine-Westphalia, Germany) equipped with a 15-Mhz linear array probe. A standardized imaging protocol was performed to evaluate intima-media-adventitia thickness (IMT) as has been described before [26]. The analysis of the presence of atheromatous plaques was performed indistinctly by two readers in a non-blinded fashion. Plaques were identified using B-mode and color Doppler examinations in both the longitudinal and transverse planes to consider circumferential asymmetry. Plaques were defined according to the Mannheim consensus [27]. We examined bilateral carotid arteries (common, bifurcation and internal) to evaluate the presence of plaques. Subclinical carotid atherosclerosis was defined as the presence of at least one plaque in any of the carotid territories explored. The presence of multiple plaques, defined by the presence of plaques in more than one of the explored territories, was considered to reflect more severe atherosclerotic disease [28, 29].
Sample size
For the comparison of LADA and classic type 1 diabetes with type 2 diabetes, we hypothesized a carotid plaque frequency of 50% for the former two and 70%, for the latter; the latter was based on the known frequency in our local cohort, and the former on a presumed reduced frequency. We calculated that with LADA (n = 62) and type 2 diabetes (n = 186) we would have 80% power, at P < 0.05 to detect differences. Using the 2:1 proportion for classic type 1 diabetes we calculated a minimum sample size of 104 subjects (80% power, P < 0.05).
Statistical analysis
A comparison of the characteristics between groups was performed expressing the quantitative variables as mean and standard deviation, and qualitative variables as frequencies and percentages. The statistical significance of differences between groups was assessed using the Chi square test for comparison of proportions, and analysis of variance (ANOVA) for comparison of means. Multiple pairwise comparisons were performed using Bonferroni correction. To assess whether the frequency of plaque and/or multiple plaque was associated with the type of diabetes the crude Odds Ratio was calculated and subsequently adjusted with the respective confidence intervals at 95%. The adjustment variables, besides the type of diabetes, were duration of diabetes, age, sex, smoking, hypertension, dyslipidemia and retinopathy at the time of inclusion. Age and duration of diabetes were categorized according to tertiles, and adjusted models were introduced by functions smoothing splines, with a focus on automatic smoothing parameter selection. The adjusted ORs were estimated using generalized additive models with logit link with package “gam” [30, 31] from R 3.2.1 statistical software [32]. The goodness of fit of the models was assessed with the Hosmer–Lemeshow test. P values less than 0.05 were considered statistically significant.
Results
We recruited 71 patients with LADA, 191 patients with type 2 diabetes and 116 patients with type 1 diabetes. The clinical characteristics of the study groups are shown in Table 1. Patients with LADA, classic type 1, and type 2 diabetes were similar in age, gender distribution, current smoking status, use of antihypertensive drugs, and HbA1c concentrations. As patients were matched by age, the diabetes duration was longer in patients with type 1 diabetes than in patients with LADA, but also, in the latter duration was longer than in type 2 diabetes (23.7 ± 12.4, 13.2 ± 9.7 and 8.7 ± 7.9 years, respectively, P < 0.001). While the prevalence of diabetic retinopathy, as expected given the longer disease duration, was higher in type 1 diabetes than in LADA and type 2 diabetes, the frequency of microalbuminuria was similar. About half the patients with LADA were treated with metformin, and the majority were receiving insulin. Patients with type 2 diabetes, as expected, exhibited a more adverse lipid profile and anthropometric measures than the other two cohorts, though fewer were on statin treatment.
Table 1.
Characteristics of study subjects Table 1
| Variables | Type 1 diabetes (n = 116) | LADA (n = 71) | Type 2 diabetes (n = 191) | P value |
|---|---|---|---|---|
| Age (years) | 56.5 ± 10.8 | 58.3 ± 11.6 | 58.3 ± 10.5 | 0.321 |
| Male n (%) | 52 (44.8) | 37 (52.1) | 105 (55) | 0.224 |
| BMI (kg/m2) | 26.5 ± 3.9 | 27.4 ± 5 | 31.3 ± 5.3 | <0.001a |
| Waist (cm) | 91.6 ± 13.3 | 96.3 ± 14 | 104.8 ± 11.7 | <0.001 |
| Diabetes duration (years) | 23.7 ± 12.4 | 13.2 ± 9.7 | 8.7 ± 7.9 | <0.001 |
| Smoking (current/past/never) n (%) | 21 (18.1)/33 (28.4)/62 (53.4) | 17 (23.9)/22 (31)/32 (45.4) | 41 (21.5)/66 (34.6)/83 (43.5) | 0.647 |
| Anti-hypertensive treatment n (%) | 54 (46.6) | 37 (52.1) | 98 (51.3) | 0.667 |
| Statin treatment n (%) | 73 (62.9) | 42 (59.2) | 71 (37. 2) | <0.001a |
| Antiplatelet agents n (%) | 50 (43.1) | 40 (56.3) | 59 (30.9) | 0.002b |
| Metformin treatment n (%) | 0 | 31 (43.7) | 138 (72.3) | <0.001 |
| Insulin treatment n (%) | 100 | 63 (88.7) | 37 (19.4) | <0.001 |
| Fasting glycemia (mg/dl) | 170.9 ± 79.1 | 143.8 ± 53.4 | 151.7 ± 52.6 | 0.006c |
| HbA1c (%) | 7.7 ± 1 | 7.7 ± 1.1 | 7.4 ± 1.3 | 0.07 |
| HbA1c (mmol/mol) | 60.6 ± 12.5 | 61 ± 11.4 | 57.3 ± 18.6 | 0.07 |
| Total cholesterol (mg/dl) | 184.9 ± 29.8 | 178.9 ± 37.8 | 185.6 ± 35.3 | 0.357 |
| LDL-cholesterol (mg/dl) | 102.6 ± 25.4 | 102 ± 24.8 | 110.6 ± 29.4 | 0.015a |
| HDL-cholesterol (mg/dl) | 65.9 ± 14.6 | 61.5 ± 18.2 | 48.4 ± 12.1 | <0.001a |
| Triglycerides (mg/dl) | 79.9 ± 36.4 | 100.5 ± 72.9 | 141.3 ± 78.7 | <0.001a |
| Diabetic retinopathy n (%) | 52 (44.8) | 13 (18.3) | 36 (18.8) | <0.001c |
| Microalbuminuria > 30 mg/g creatinine | 10 (8.6) | 7 (9.9) | 12 (6.3) | 0.563 |
| Urinary albumin:creatinine ratio (mg/g) | 12.2 ± 29.8 | 20.1 ± 69.6 | 14.5 ± 32.9 | 0.451 |
| Mean IMT | 0.750 ± 0.109 | 0.775 ± 0.147 | 0.793 ± 0.135 | 0.047c |
Values are expressed as the mean ± SD or percentages
LADA latent autoimmune diabetes in adults, BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein, HbA1c glycosylated haemoglobin
a Type 2 diabetes different from LADA and type 1 diabetes
b Type 2 diabetes different from LADA
c Type 1 diabetes different from LADA and type 2 diabetes
For carotid ultrasound findings: mean carotid IMT was lower in patients with type 1 diabetes compared to both LADA and type 2 diabetes (Table 1). As shown in Fig. 1 subclinical carotid atherosclerosis (presence of atherosclerotic plaques) was more frequent in patients with LADA than in patients with type 1 diabetes and type 2 diabetes [73.2% vs. 57.1% (P = 0.026) and 56.9% (P = 0.018), respectively]. Multiple plaques were also more frequent in patients with LADA than in type 1 diabetes and type 2 diabetes [45.1% vs. 33.6% (P = 0.077), and 27.2% (P = 0.019), respectively] (Fig. 1).
Fig. 1.
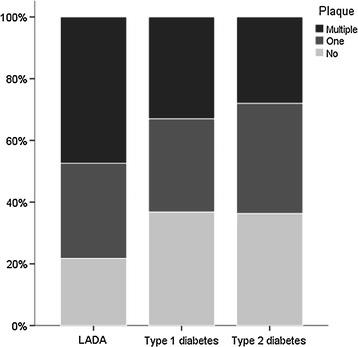
Carotid atherosclerosis in patients with LADA, type 1 and type 2 diabetes. The percentage of patients with carotid plaques was significantly higher in the LADA group (73.2%) than in the group of patients with type 1 diabetes (57.1%, P = 0.026) and type 2 diabetes (56.9%, P = 0.018). The difference was mainly due to the percentage of patients with multiple plaques, which was higher in LADA (45.1%), than in type 1 diabetes (33.6%), P = 0.077 and type 2 diabetes (27.2%), P = 0.019. LADA latent autoimmune diabetes in adults
Carotid plaques were also related to older age, longer diabetes duration, hypertension, retinopathy and dyslipidemia (Table 2).
Table 2.
Univariate analysis for the presence of carotid atherosclerotic plaques
| Odds ratio (95% confidence interval) | P value | |
|---|---|---|
| Diabetes | ||
| LADA | 1 | |
| Type 1 diabetes | 0.482 (0.254–0.916) | 0.026 |
| Type 2 diabetes | 0.486 (0.267–0.884) | 0.018 |
| Diabetic retinopathy | 1.954 (1.195–3.196) | 0.008 |
| Male sex | 1.275 (0.844–1.926) | 0.248 |
| Dyslipidemia | 1.808 (1.192–2.747) | 0.005 |
| Hypertension | 2.639 (1.721–4.032) | <0.001 |
| Smoker/former smoker | 1.464 (0.868–2.469) | 0.152 |
| Diabetes duration (tertiles) | ||
| ≤5 years | 1 | |
| 6–11 years | 1.360 (0.779–2.375) | 0.279 |
| ≥12 years | 1.875 (1.140–3.084) | 0.013 |
| Age (tertiles) | ||
| ≤52 years | 1 | |
| 53–63 years | 2.267 (1.371–3.748) | <0.001 |
| ≥64 years | 3.707 (2.177–6.313) | <0.001 |
LADA latent autoimmune diabetes in adults
A multivariate logistic regression model including age, diabetes duration, gender, type of diabetes, retinopathy, hypertension, smoking and dyslipidemia (Table 3) revealed that age, but not gender, type of diabetes, and the presence of hypertension and smoking habit were associated with carotid atherosclerosis. The adjusted inverse OR for the presence of carotid plaques in patients with LADA as compared with those with type 1 and type 2 diabetes was 2.44 [(1.65–3.6); P = 0.0225] and 2.06 [(1.47–2.9); P = 0.0334], respectively.
Table 3.
Adjusted Odds ratio for the presence of carotid atherosclerotic plaques
| Adjusted odds ratio (95% confidence interval) | P value | |
|---|---|---|
| Diabetes | ||
| LADA | 1 | |
| Type 1 diabetes | 0.410 (0.278–0.606) | 0.0225 |
| Type 2 diabetes | 0.485 (0.345–0.682) | 0.0334 |
| Diabetic retinopathy | 1.258 (0.910–1.741) | 0.4790 |
| Female sex | 0.764 (0.603–0.967) | 0.2530 |
| Dyslipidemia | 1.252 (0.980–1.599) | 0.3587 |
| Hypertension | 1.773 (1.389–2.264) | 0.0189 |
| Smoker/former smoker | 2.471 (1.824–3.349) | 0.0029 |
Odds ratio was adjusted by age and diabetes duration using a generalized additive model
LADA latent autoimmune diabetes in adults
To explore the impact of disease duration on carotid plaque frequency in patients with LADA, we limited the analysis to patients with LADA and type 2 diabetes, as few patients with type 1 diabetes had a short diabetes duration. We divided patients with LADA and type 2 diabetes into age-adjusted tertiles of disease duration: ≤5, 6–11, ≥12 years. Table 4 shows the results of this analysis; within 5 years of diabetes duration the prevalence of carotid plaques was lower, though not significantly so, in patients with LADA than in those with type 2 diabetes [26.7% vs. 54.8%, inverse OR 0.38 (0.11–1.39); P = 0.137]. However, with increasing diabetes duration the frequency of carotid atherosclerotic plaques increased in patients with LADA, but not in patients with type 2 diabetes [81.8% vs. 50%, inverse OR 5.72 (1.5–21.8); P = 0.009].
Table 4.
Age-adjusted frequency of carotid atherosclerotic plaques in patients with LADA and type 2 diabetes, divided by tertiles of disease duration
| Diabetes duration | N | Carotid plaque (%) | Inverse OR (95% confidence interval) | P value |
|---|---|---|---|---|
| ≤5 years | ||||
| Type 2 diabetes | 84 | 54.8 | 0.137 | |
| LADA | 15 | 26.7 | 0.38 (0.11–1.39) | |
| 6–11 years | ||||
| Type 2 diabetes | 60 | 50 | 0.009 | |
| LADA | 22 | 81.8 | 5.72 (1.5–21.8) | |
| ≥12 years | ||||
| Type 2 diabetes | 47 | 70.2 | 0.038 | |
| LADA | 34 | 88.2 | 4.19 (1.06–16.6) | |
LADA latent autoimmune diabetes in adults
Discussion
In this study, we unexpectedly found an increased frequency of subclinical carotid atherosclerosis in patients with LADA, as compared to subjects with classic type 1 diabetes and type 2 diabetes. That excess frequency of carotid disease was also evident when assessing multiple plaques in LADA compared with the other cohorts. We did not find the same results for cIMT as for carotid plaque, potentially due to the high variability and low intra-individual reproducibility of the former measurement [33]. Moreover, the pathophysiology underlying the development of carotid plaque and cIMT is distinct and the evidence regarding the value of cIMT in cardiovascular risk prediction is contradictory and of debatable value [34–36]. Most recent clinical guidelines on cardiovascular risk do not recommend carotid IMT for individual risk prediction [18, 37]. In contrast, the presence of carotid plaques has been shown to be a good predictor of future cardiac events [15, 18].
The high frequency of carotid plaques, despite the increased use of statins in LADA cases, remained after adjusting for the main cardiovascular risk factors, including diabetes duration. The current cross-sectional study does not allow for the identification of potential causes of our unexpected finding of higher atherosclerotic burden in LADA, even though patients with type 2 diabetes tend to exhibit, as in other studies, a worse cardiovascular risk profile including higher blood pressure, more obesity and an adverse lipid profile. In both the Freemantle Study [38] and the Collaborative Atorvastatin Study [7], patients designated LADA had comparable cardiovascular disease as the patients with type 2 diabetes; while in the Botnia study [6] after 13 years of diabetes 56% of LADA patients had ischemic heart disease and 5% had had cerebrovascular events. To date, only one other study has assessed the frequency of carotid atherosclerosis in patients with LADA and type 2 diabetes [8], and none have assessed that frequency in the three cohorts we assessed. That Chinese study was retrospective and not designed to answer the question we posed here, but they did find a comparable frequency of carotid atherosclerosis compared with type 2 diabetes patients. Taken together, these studies indicate that patients with adult-onset diabetes, irrespective of the cause of the diabetes, are at risk of carotid and coronary atherosclerosis. It is difficult to understand why this should be, given that autoimmune diabetes cases are less likely to have the metabolic syndrome and related features [7]. Two possibilities suggest themselves. First, the time of onset of autoimmune diabetes antedates the disease by months, even years, and it remains possible that LADA patients have had low-grade disease many years before clinical presentation, as can be the case with type 2 diabetes. However, the duration of LADA pre-disease is unknown both in this study and in general, while the risk of atherosclerosis in these cases was increased with increasing diabetes duration post-diagnosis. Second, patients with LADA are not well managed and are consistently shown to have higher HbA1c levels than comparable cohorts of patients with type 2 diabetes [4, 39–41]. Higher HbA1c has been found to be associated with higher coronary and carotid atherosclerotic burden in non-diabetic patients [42]. Additionally, deranged glycemic control has been independently related to coronary heart disease in both LADA patients [6], and diabetes patients in general [40, 43]. Higher HbA1c concentrations have also been related to cardiovascular disease in the non-diabetic population in prospective studies, including the Norfolk cohort of European Prospective Investigation into Cancer and Nutrition study [44] and The Copenhagen City Heart Study [45].
In subjects with diabetes, other potential different pathophysiological mechanisms may be involved in the atherosclerotic process. First, advanced glycation end products (AGEs) which are increased in diabetes are pro-oxidants that induce oxidative stress and are thought to play a major role in atherosclerosis [46, 47]. Although diet is the principal source of AGEs, their concentrations rise with increasing blood glucose. An increased level of advanced AGEs has been associated with cardiovascular disease in type 1 diabetes [48] and type 2 diabetes [49]; no such data is available in LADA. Secondly, patients with systemic autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, have an increased risk of atherosclerosis, with both cellular and humoral components of the immune system involved in its pathogenesis and some shared genetic risk [50], although the precise mechanisms underlying the high cardiovascular risk in these patients is unclear [51]. It is well known that chronic inflammation plays an important role in atherogenesis [52, 53], and inflammatory markers associated with macrovascular disease are elevated both in patients with type 1 diabetes and type 2 diabetes [54, 55]. Moreover, poor glycemic control and hypoglycaemic episodes are related to pro-inflammatory status [56, 57], and may be additional contributing factors in atherosclerosis in patients with autoimmune diabetes [58]. Unfortunately, in the current study we did not determine any markers of chronic inflammation in the LADA patients, though we have previously demonstrated such changes in LADA patients [59].
Nevertheless, this study has some weaknesses. First of all, the size of the populations studied is relatively small, and some important information is missing, notably the familial history of premature cardiovascular disease. While we found no differences in HbA1c between the three diabetes groups, this is a cross-sectional study, and the relationship between glycemic control and atherosclerosis can only be answered with a prospective design. The situation is made more complex because most of the patients with LADA in this study had already been referred to specialized care, usually because of poor glycemic control. The specialized diabetes care of all the patients with type 1 diabetes and most of the patients with LADA may explain the increased use of statins and antiplatelet agents in these groups compared to that of patients with type 2 diabetes, since the latter are usually under general practitioner’s care. Since LADA patients were recruited from a specialty-based cohort our results may show a bias; however, this group of LADA subjects is likely representative of LADA patients in general in Spain.
Nevertheless, despite its limitations, our study has the strength of comparing for the first time these three groups of diabetes, which come from a homogeneous population. While we use the term LADA throughout the text for clarity, we would prefer that LADA is called adult-onset autoimmune diabetes since there is no firm evidence that LADA can be categorically distinguished from type 1 (autoimmune) diabetes.
Conclusions
Our results indicate that the frequency of preclinical carotid atherosclerosis in patients with adult-onset initially non-insulin requiring autoimmune diabetes, also called LADA, is comparable, even greater, than in adults of similar age with classic type 1 diabetes and type 2 diabetes. Our data should be confirmed in both cross-sectional and prospective studies in other populations. However, the results draw attention to the importance of macrovascular disease in patients with diabetes, irrespective of the cause of the disease, and emphasize the potential value of rigorous treatment of macrovascular risk factors even in patients with autoimmune diabetes.
Authors’ contributions
MH, RDL and DM wrote the manuscript. AB, TM, EF, and ER performed all ultrasound examinations. EO, JR and JV performed the statistical analysis. CL, FV, JS, AB, NA, MG and AL contributed to all other data collection. DM and MH conceived the study and participated in its design and coordination. All authors contributed to the discussion and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work has been carried out within the framework of the Doctorate in Medicine of the Autonomous University of Barcelona.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval
The Ethics Committee of University Hospital Arnau de Vilanova and University Hospital Germans Trias i Pujol approved the study.
Funding
This project was funded by Grants Nos. PI12/00183 and PI15/00625, both included in Plan Nacional de I + D + I, and co-financed by Instituto de Salud Carlos III, Subdireccion General de Evaluacion, Ministry of Economy and Competitiveness, and Fondo Europeo de Desarrollo Regional (FEDER). CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM) is an initiative from Instituto de Salud Carlos III, Spain.
Informed consent
All the participants signed an informed consent form.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- LADA
latent autoimmune diabetes in adults
- IMT
intima-media-adventitia thickness
Contributor Information
Marta Hernández, Email: martahernandezg@gmail.com.
Carolina López, Email: karolopezc@gmail.com.
Jordi Real, Email: jreal.lleida.ics@gencat.cat.
Joan Valls, Email: jvalls@irblleida.cat.
Emilio Ortega-Martinez de Victoria, Email: eortega1@clinic.ub.es.
Federico Vázquez, Email: fvazquez@igtp.cat.
Esther Rubinat, Email: rubinatesther@gmail.com.
Minerva Granado-Casas, Email: mgranado@igtp.cat.
Nuria Alonso, Email: nalonso32416@yahoo.es.
Teresa Molí, Email: tere.nefrona@gmail.com.
Angels Betriu, Email: angels.betriu.bars@gmail.com.
Albert Lecube, Email: alecube@gmail.com.
Elvira Fernández, Email: efernandez@irblleida.cat.
Richard David Leslie, Email: r.d.g.leslie@qmul.ac.uk.
Dídac Mauricio, Phone: + 34 934978860, Email: didacmauricio@gmail.com.
References
- 1.Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017 doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 2.Laugesen E, Østergaard JA, Leslie RD. Danish Diabetes Academy Workshop and Workshop Speakers. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015 doi: 10.1111/dme.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawa MI, Thivolet C, Mauricio D, Alemanno I, Cipponeri E, Collier D, et al. Metabolic syndrome and autoimmune diabetes: action LADA 3. Diabetes Care. 2009 doi: 10.2337/dc08-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollo A, Hernandez M, Marsal JR, Esquerda A, Rius F, Blanco-Vaca F, et al. Latent autoimmune diabetes in adults is perched between type 1 and type 2: evidence from adults in one region of Spain. Diabetes Metab Res Rev. 2013 doi: 10.1002/dmrr.2411. [DOI] [PubMed] [Google Scholar]
- 5.Hawa MI, Kolb H, Schloot N, Beyan H, Paschou SA, Buzzetti R, et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care. 2013 doi: 10.2337/dc12-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isomaa B, Almgren P, Henricsson M, Taskinen MR, Tuomi T, Groop L, et al. Chronic complications in patients with slowly progressing autoimmune type 1 diabetes (LADA) Diabetes Care. 1999;22:1347. doi: 10.2337/diacare.22.8.1347. [DOI] [PubMed] [Google Scholar]
- 7.Hawa MI, Buchan AP, Ola T, Wun CC, DeMicco DA, Bao W, et al. LADA and CARDS: a prospective study of clinical outcome in established adult-onset autoimmune diabetes. Diabetes Care. 2014 doi: 10.2337/dc13-2383. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Hou X, Zhang L, Hu C, Zhou J, Pang C, et al. Associations between clinical characteristics and chronic complications in latent autoimmune diabetes in adults and type 2 diabetes. Diabetes Metab Res Rev. 2015 doi: 10.1002/dmrr.2626. [DOI] [PubMed] [Google Scholar]
- 9.Martinell M, Dorkhan M, Stålhammar J, Storm P, Groop L, Gustavsson C. Prevalence and risk factors for diabetic retinopathy at diagnosis (DRAD) in patients recently diagnosed with type 2 diabetes (T2D) or latent autoimmune diabetes in the adult (LADA) J Diabetes Complicat. 2016 doi: 10.1016/j.jdiacomp.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004 doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 11.Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008 doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease. The ARIC (Atherosclerosis Risk in Communities) Study. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011 doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigili de Kreutzenberg S, Fadini GP, Guzzinati S, Mazzucato M, Volpi A, Coracina A, et al. Carotid plaque calcification predicts future cardiovascular events in type 2 diabetes. Diabetes Care. 2015 doi: 10.2337/dc15-0327. [DOI] [PubMed] [Google Scholar]
- 15.Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Polak JF, Szklo M, O’Leary DH. Carotid intima-media thickness score, positive coronary artery calcium score, and incident coronary heart disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2017 doi: 10.1161/JAHA.116.004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013 [Google Scholar]
- 18.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016 doi: 10.1016/j.atherosclerosis.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017 doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 20.den Ruijter HM, Peters SA, Groenewegen KA, Anderson TJ, Britton AR, Dekker JM, et al. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative. Diabetologia. 2013 doi: 10.1007/s00125-013-2898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rassi CH, Churchill TW, Tavares CA, Fahel MG, Rassi FP, Uchida AH, et al. Use of imaging and clinical data to screen for cardiovascular disease in asymptomatic diabetics. Cardiovasc Diabetol. 2016 doi: 10.1186/s12933-016-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vouillarmet J, Helfre M, Maucort-Boulch D, Riche B, Thivolet C, Grange C. Carotid atherosclerosis progression and cerebrovascular events in patients with diabetes. J Diabetes Complicat. 2016 doi: 10.1016/j.jdiacomp.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association Cardiovascular disease and risk management. Diabetes Care. 2017 [Google Scholar]
- 24.Leslie RD, Kolb H, Schloot NC, Buzzetti R, Mauricio D, De Leiva A, et al. Diabetes classification: grey zones, sound and smoke: action LADA 1. Diabetes Metab Res Rev. 2008 doi: 10.1002/dmrr.877. [DOI] [PubMed] [Google Scholar]
- 25.Palomer X, Mauricio D, Rodríguez-Espinosa J, Zapico E, Mayoral C, González-Sastre F, et al. Evaluation of two nonisotopic immunoassays for determination of glutamic acid decarboxylase and tyrosine phosphatase autoantibodies in serum. Clin Chem. 2004 doi: 10.1373/clinchem.2004.031799. [DOI] [PubMed] [Google Scholar]
- 26.Betriu A, Martinez-Alonso M, Arcidiacono MV, Cannata-Andia J, Pascual J, Valdivielso JM, et al. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: the NEFRONA study. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu038. [DOI] [PubMed] [Google Scholar]
- 27.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007. doi:10.1159/000097034. [DOI] [PubMed]
- 28.Störk S, van den Beld AW, von Schacky C, Angermann CE, Lamberts SW, Grobbee DE, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation. 2004 doi: 10.1161/01.CIR.0000134966.10793.C9. [DOI] [PubMed] [Google Scholar]
- 29.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, et al. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–2877. doi: 10.1161/01.CIR.0000018650.58984.75. [DOI] [PubMed] [Google Scholar]
- 30.Figueiras A, Cadarso-Suárez C. Application of nonparametric models for calculating odds ratios and their confidence intervals for continuous exposures. Am J Epidemiol. 2001;154(3):264–275. doi: 10.1093/aje/154.3.264. [DOI] [PubMed] [Google Scholar]
- 31.Hastie T, Tibshirani R. Generalized additive models. Encycl Stat Sci. 2006 doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 32.R Core Team. R: A language and environment for statistical computing 2013. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 16 May 2016.
- 33.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014 doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 35.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012 doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 36.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012 doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 37.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myhill P, Davis WA, Bruce DG, Mackay IR, Zimmet P, Davis TM. Chronic complications and mortality in community-based patients with latent autoimmune diabetes in adults: the Fremantle Diabetes Study. Diabet Med. 2008 doi: 10.1111/j.1464-5491.2008.02562.x. [DOI] [PubMed] [Google Scholar]
- 39.Balme M, McAllister I, Davis WA, Davis TM. Retinopathy in latent autoimmune diabetes of adults: the Fremantle Diabetes Study. Diabet Med. 2002 doi: 10.1046/j.1464-5491.2002.00739.x. [DOI] [PubMed] [Google Scholar]
- 40.Olsson L, Grill V, Midthjell K, Ahlbom A, Andersson T, Carlsson S. Mortality in adult-onset autoimmune diabetes is associated with poor glycemic control: results from the HUNT Study. Diabetes Care. 2013 doi: 10.2337/dc13-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen CD, Bennet L, Nyström L, Lindblad U, Lindholm E, Groop L, et al. Worse glycaemic control in LADA patients than in those with type 2 diabetes, despite a longer time on insulin therapy. Diabetologia. 2013 doi: 10.1007/s00125-012-2759-y. [DOI] [PubMed] [Google Scholar]
- 42.Scicali R, Giral P, Gallo A, Di Pino A, Rabuazzo AM, Purrello F. HbA1c increase is associated with higher coronary and peripheral atherosclerotic burden in non diabetic patients. Atherosclerosis. 2016 doi: 10.1016/j.atherosclerosis.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015 doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001;322:15. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eskesen K, Jensen MT, Galatius S, Vestergaard H, Hildebrandt P, Marott JL, et al. Glycated haemoglobin and the risk of cardiovascular disease, diabetes and all-cause mortality in the Copenhagen City Heart Study. J Intern Med. 2013 doi: 10.1111/j.1365-2796.2012.02594.x. [DOI] [PubMed] [Google Scholar]
- 46.Vlassara H, Striker GE. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol Metab Clin N Am. 2013 doi: 10.1016/j.ecl.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 48.Monnier VM, Sun W, Gao X, Sell DR, Cleary PA, Lachin JM, Research Group et al. Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc Diabetol. 2015 doi: 10.1186/s12933-015-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007 doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 50.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006 doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014 doi: 10.1186/1741-7015-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 53.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012 doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Aguilera E, Serra-Planas E, Granada ML, Pellitero S, Reverter JL, Alonso N, et al. Relationship of YKL-40 and adiponectin and subclinical atherosclerosis in asymptomatic patients with type 1 diabetes mellitus from a European Mediterranean population. Cardiovasc Diabetol. 2015 doi: 10.1186/s12933-015-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heier M, Margeirsdottir HD, Brunborg C, Hanssen KF, Dahl-Jørgensen K, Seljeflot I. Inflammation in childhood type 1 diabetes; influence of glycemic control. Atherosclerosis. 2015 doi: 10.1016/j.atherosclerosis.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Kiec-Wilk B, Matejko B, Razny U, Stankiewicz M, Skupien J, Klupa T, et al. Hypoglycemic episodes are associated with inflammatory status in patients with type 1 diabetes mellitus. Atherosclerosis. 2016 doi: 10.1016/j.atherosclerosis.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Giménez M, Gilabert R, Monteagudo J, Alonso A, Casamitjana R, Paré C, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care. 2011 doi: 10.2337/dc10-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schloot NC, Pham MN, Hawa MI, Pozzilli P, Scherbaum WA, Schott M, et al. Inverse relationship between organ-specific autoantibodies and systemic immune mediators in type 1 diabetes and type 2 diabetes: action LADA 11. Diabetes Care. 2016 doi: 10.2337/dc16-0293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


