Abstract
Serious disease outbreaks in cattle caused by Theileria orientalis have emerged in the Asia–Pacific region. Genetic variables of the major piroplasm surface protein (MPSP) expressed on the surface of the piroplasm inside T. orientalis-infected erythrocytes are considered to be associated with variation in the pathogenicity of T. orientalis. Our study describes the clinically relevant MPSP types associated with anemia in Theileria-infected cattle. These results revealed that MPSP expression plays an important role in hematological alterations in Theileria-infected cattle, and that MPSP type 1 is strongly associated with bovine anemia, which can be a potential target for the prevention of bovine theileriosis.
Keywords: Anemia, Major piroplasm surface protein, MPSP type 1, Theileria orientalis
Findings
Bovine theileriosis, caused by subspecies of the protozoan parasite Theileria, is one of the most economically important diseases of cattle throughout various regions of the world [1]. Theileria parva and T. annulata, commonly known as East Coast fever and tropical theileriosis, respectively, are highly pathogenic, whereas T. orientalis is believed to cause mild or asymptomatic disease [2]. However, recently, T. orientalis has emerged as an agent capable of causing outbreaks of clinical theileriosis resulting in losses to the Asia–Pacific cattle industry [3, 4].
Theileria orientalis proliferates inside erythrocytes as piroplasms [5]. During the intraerythrocytic stage, T. orientalis can cause hemolysis and subsequent anemia, which is the primary clinical finding [6, 7]. The major piroplasm surface protein (MPSP), conserved to the piroplasms of T. orientalis is expressed in the intraerythrocytic stage of the parasite [8]. The sequence variations in the MPSP gene have been used to define the genetic diversity of T. orientalis [9]. Currently, 11 genotypes of T. orientalis (types 1–8 and N1–N3) have been identified based on the MPSP gene sequence [9].
Despite few studies on pathogenicity of MPSP types [10], most studies are still focused on epidemiological investigations that identify and characterize the genotypes of T. orientalis [8, 9, 11, 12]. There is limited information on disease outbreaks related to the genotypes of T. orientalis [3, 10, 13] and the clinical relevance of the various MPSP types has not been clearly elucidated.
In a recent study, various MPSP types (types 1, 2, 3, and 7) were identified in the Republic of Korea [14]. Based on the MPSP genotypes identified from our previous report [14], we investigated the genotypes of T. orientalis associated with clinical disease by evaluating changes in red blood cell (RBC) profiles according to different MPSP types in Theileria-infected cattle. The objective of this study was to determine the main T. orientalis MPSP genotypes associated to disease severity and development of anemia in cattle.
A total of 143 Holstein cattle from three geographical regions of the Republic of Korea (Hoengseong, Namwon, and Jeju island) were randomly selected between April and September 2015. Blood samples for hematological analysis and Theileria polymerase chain reaction (PCR) analysis were collected from the jugular vein into ethylenediaminetetraacetic acid (EDTA) tubes. A RBC profile, including RBC count, hemoglobin (Hb) level, hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) was created using an automatic blood analyzer (Hemavet 960, Erba Diagnostics Inc., Miami, FL, USA). Cattle were divided into three categories based on RBC, Hb, and HCT values according to [8]. The categories were: severely anemic (RBC <3.0 M/μL, Hb <5.0 g/dL or HCT <15%); mildly anemic (RBC 3.0–5.0 M/μL, Hb 5.0–8.0 g/dL or HCT 15–24%), and without anemia (RBC >5.0 M/μL, Hb >8.0 g/dL or HCT >24%).
The T. orientalis types obtained have been reported previously [14]. Briefly, to identify T. orientalis infection, genomic DNA was extracted from whole blood using the DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, CA, USA). PCR was performed on all samples using a primer set targeting the 18S ribosomal RNA (rRNA) gene of Theileria [15]. In samples positive for the Theileria-18S rRNA gene, PCR was performed on the MPSP gene of T. orientalis [14]. To identify T. orientalis genotypes, DNA sequencing and phylogenetic analysis were performed on samples positive for the MPSP gene [14]. A total of four MPSP types (types 1, 2, 3 and 7) were identified, and deposited accession numbers were described in previous publication [14].
We used a SPSS 23.0 software package (SPSS, Chicago, IL, USA) to analyze data. The Shapiro–Wilk test was utilized for normality analysis. To compare alterations in the RBC profile according to 18S or MPSP gene expression, either an independent t test or Mann–Whitney U test was performed, depending on the results of a normality test. To compare anemic parameters among different MPSP genotypes, one-way analysis of variance (ANOVA) or Kruskal–Wallis was used accompanied by least significant difference (LSD) or Mann–Whitney test depending on the normal distribution of data. Data are expressed as mean ± standard deviation (SD) and a P value of <0.05 was considered significant.
To determine whether antigenic expression in erythrocytes infected with T. orientalis was a critical factor for progression of clinical disease, RBC profiles were compared among cattle positive for Theileria-18S or the T. orientalis-MPSP gene to cattle negative for Theileria-18S (non-infected with Theileria) or T. orientalis-MPSP gene (infected with Theileria but without MPSP expression) (Table 1). Cattle positive for the Theileria-18S gene had decreased RBC and HCT and increased MCV, MCH, and MCHC when compared to cattle negative for the Theileria-18S gene (P < 0.05). Of cattle positive for the Theileria-18S gene, RBC, Hb, and HCT of MPSP-positive cattle were significantly lower than those of MPSP-negative cattle (P < 0.005) or non-infected cattle (P < 0.01). In addition, the MCV and MCH of MPSP-positive cattle were significantly higher than found in MPSP-negative cattle (P < 0.005) or non-infected cattle (P < 0.05). In contrast, there were no differences in the RBC profile of MPSP-negative cattle when compared to non-infected cattle. Hematological alterations in Theileria-18S-positive cattle were dependent on the expression of the MPSP gene. This result revealed that detection of MPSP gene is a useful biomarker to predict development of clinical disease in Theileria-infected cattle.
Table 1.
Alterations in the red blood cell profile of cattle according to antigenicity of Theileria orientalis
| RBC profile | n | RBC (M/µL) | Hb (g/dL) | HCT (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) |
|---|---|---|---|---|---|---|---|
| Reference values | 5.0–10.0 | 8.0–15.0 | 24.0–46.0 | 40.0–60.0 | 11.1–17.0 | 28.2–36.0 | |
| 18S negative | 53 | 9.6 ± 1.6 | 10.0 ± 1.5 | 32.4 ± 5.5 | 34.4 ± 3.9 | 10.6 ± 1.8 | 31.1 ± 4.6 |
| 18S positive | 90 | 8.2 ± 2.1** | 9.9 ± 3.3 | 30.2 ± 6.5* | 37.6 ± 5.7** | 12.3 ± 2.6** | 32.5 ± 4.7* |
| MPSP negative | 34 | 9.8 ± 0.8 | 10.6 ± 0.5 | 32.5 ± 2.0 | 33.1 ± 2.1 | 10.5 ± 0.7 | 31.8 ± 1.5 |
| MPSP positive | 56 | 7.3 ± 2.1**a | 9.6 ± 4.1**a | 28.9 ± 7.8**a | 39.5 ± 5.7**a | 13.1 ± 2.7**a | 32.8 ± 5.5* |
* P < 0.05 and ** P < 0.01 vs. 18S negative, a P < 0.005 vs. major piroplasm surface protein (MPSP) negative
To investigate clinically relevant MPSP types, MPSP type-specific hematological changes and development of anemia were investigated in T. orientalis-infected cattle (Fig. 1). Of the four MPSP types identified, the RBC, Hb, and HCT values of type 1-infected cattle were significantly lower than those of MPSP-negative cattle (P < 0.001) or type 2-infected cattle (P < 0.05) (Fig. 1a). Type 2-infected cattle showed a significant decrease in RBC (P < 0.001), but no difference in Hb or HCT, when compared to MPSP-negative cattle.
Fig. 1.
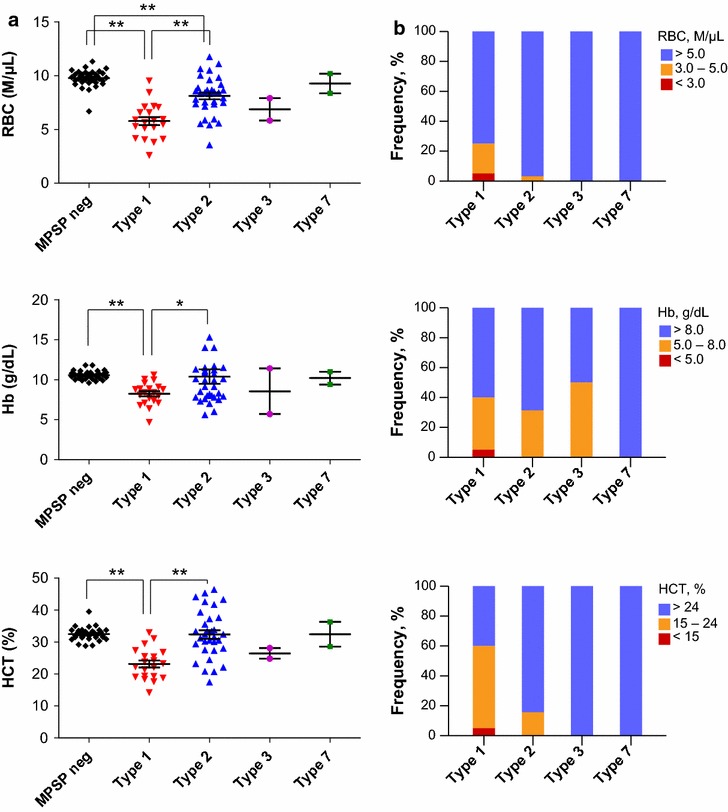
Development of anemia according to major piroplasm surface protein (MPSP) genotypes in Theileria-infected cattle. Comparison of red blood cell count (RBC), hemoglobin (Hb), and hematocrit (HCT) values among MPSP types (a) and the presence of anemia in each MPSP type (b). Colored bars indicated the level of anemia. Blue no anemia; orange mild anemia and red severe anemia. Number of animals: MPSP-negative (n = 34); types 1 (n = 20), 2 (n = 32), 3 (n = 2) and 7 (n = 2). *P < 0.05 and **P < 0.001
Type 1-infected cattle showed a greater proportion of severely and mildly anemic cattle when compared to cattle infected with other types (Fig. 1b). When the RBC count was used as the diagnostic criterium, severe or mild anemia was observed in 25% (n = 5) of the 20 type 1-infected cattle. However, only 3.1% (n = 1) of the 32 type 2-infected cattle had severe or mild anemia. Among type 1-infected cattle, 40% were anemic using the Hb criteria and 60% using the HCT criteria. Among type 2-infected cattle, 31.1 and 15.6% were anemic using the Hb and HCT criteria, respectively. There was a high incidence of anemia among type 1-infected cattle suggesting that infection with T. orientalis type 1 can be strongly associated with clinical disease.
The aim of this study was to explore if there was an association between the T. orientalis MPSP genotype and the development of anemia in T. orientalis-infected cattle. This is first report to show that T. orientalis type 1 is closely associated with anemia leading to clinical theileriosis. Although anemia associated with T. orientalis type 2-infection is less common than anemia associated with type 1-infection, this is also the first report of anemia associated with type 2-infection in the Republic of Korea.
This study has several limitations. First, due to the limited number of samples we were unable to determine and describe the pathogenicity of T. orientalis types 3 and 7 in detail. Based on other reports, types 3 and 5 are considered to be low pathogenic in cattle [3], while type 7 has been related to outbreaks of clinical disease in India [16]. A large-scale study with an increased number of samples is needed to explore this further. Second, pathogenic determinants for T. orientalis can include parasite load in addition to parasite genotype. High parasite loads correlate with clinical theileriosis. Type 2 infection with high parasite loads is negatively correlated with packed cell volume [6, 17]. Although we could not clearly elucidate whether clinical disease was a result of genotype, total parasite burden or a combination of genotype and burden, it was clear that type 1 infection was strongly linked to anemia in cattle. Further studies to determine critical factors responsible for clinical theileriosis in T. orientalis infection should include measurement of the parasite load in the blood.
Type 2 has been thought to be the key causal variant in bovine theileriosis [10]. Clinical cases of theileriosis were associated with type 2 alone or in combination with type 1 in Australia [10], while infection with type 1 alone was often associated with subclinical disease [17]. In contrast to previous reports, our study showed that type 1 infection alone can be a major causal factor for the outbreak of clinical theileriosis. Although several researches suggested that type 1 is a direct cause of disease [18, 19], the pathogenicity of type 1 is still less clear than that of type 2. Therefore, continuous monitoring of type 1-infected cattle is needed to assess the progression to clinical disease.
In conclusion, type 1 can be responsible for direct cause of disease outbreaks in cattle. Understanding the types of T. orientalis that result in theileriosis can aid in the development of effective strategies to prevent pathogenic orientalis theileriosis.
Authors’ contributions
JP, DHY, KSC, HCK, BKP, and JSC designed the study and provided consultation. SK conducted the analyses and drafted the manuscript. All the authors contributed to the interpretation of the data. JBC participated in the collection of materials and animal samples. KSC, HCK, and BKP contributed to the detection of pathogens. DHY performed the hematological analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset is available upon reasonable request.
Ethics approval
All procedures were carried out according to ethical guidelines for the use of animal samples, as approved by the Chonbuk National University (institutional animal care and use committee [IACUC] Decision No. CBU 2014-00026).
Funding
This work was supported by the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010092), funded by the Rural Development Administration of the Republic of Korea.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- Hb
hemoglobin
- HCT
hematocrit
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean cell volume
- MPSP
major piroplasm surface protein
- PCR
polymerase chain reaction
- RBC
red blood cell
- rRNA
ribosomal RNA
- T. orientalis
Theileria orientalis
Footnotes
Suhee Kim and Do-Hyeon Yu contributed equally to this work
Contributor Information
Suhee Kim, Email: vetksh@gmail.com.
Do-Hyeon Yu, Email: dyu@chonnam.ac.kr.
Jeong-Byoung Chae, Email: jbchae117@gmail.com.
Kyoung-Seong Choi, Email: kschoi3@knu.ac.kr.
Hyeon-Cheol Kim, Email: advs@kangwon.ac.kr.
Bae-Keun Park, Email: bkpark@cnu.ac.kr.
Joon-Seok Chae, Email: jschae@snu.ac.kr.
Jinho Park, Email: jpark@jbnu.ac.kr.
References
- 1.Gebrekidan H, Gasser RB, Baneth G, Yasur-Landau D, Nachum-Biala Y, Hailu A, Jabbar A. Molecular characterization of Theileria orientalis from cattle in Ethiopia. Ticks Tick Borne Dis. 2016;7:742–747. doi: 10.1016/j.ttbdis.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Aktas M, Altay K, Dumanli N. A molecular survey of bovine Theileria parasites among apparently healthy cattle and with a note on the distribution of ticks in eastern Turkey. Vet Parasitol. 2006;138:179–185. doi: 10.1016/j.vetpar.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasites Vectors. 2011;4:22. doi: 10.1186/1756-3305-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor A, Ball M, Freeman P, Jenkins C, Bogema DR. Prevalence of Theileria orientalis types in beef cattle herds on the North Coast of New South Wales. Aust Vet J. 2016;94:117–120. doi: 10.1111/avj.12415. [DOI] [PubMed] [Google Scholar]
- 5.George N, Bhandari V, Reddy DP, Sharma P. Emergence of new genotype and diversity of Theileria orientalis parasites from bovines in India. Infect Genet Evol. 2015;36:27–34. doi: 10.1016/j.meegid.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins C, Bogema DR. Factors associated with seroconversion to the major piroplasm surface protein of the bovine haemoparasite Theileria orientalis. Parasites Vectors. 2016;9:106. doi: 10.1186/s13071-016-1395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins C, Micallef M, Alex SM, Collins D, Djordjevic SP, Bogema DR. Temporal dynamics and subpopulation analysis of Theileria orientalis genotypes in cattle. Infect Genet Evol. 2015;32:199–207. doi: 10.1016/j.meegid.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Ota N, Mizuno D, Kuboki N, Igarashi I, Nakamura Y, Yamashina H, et al. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J Vet Med Sci. 2009;71:937–944. doi: 10.1292/jvms.71.937. [DOI] [PubMed] [Google Scholar]
- 9.Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. Evolution and genetic diversity of Theileria. Infect Genet Evol. 2014;27:250–263. doi: 10.1016/j.meegid.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Eamens GJ, Gonsalves JR, Jenkins C, Collins D, Bailey G. Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet Parasitol. 2013;191:209–217. doi: 10.1016/j.vetpar.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Jirapattharasate C, Moumouni PFA, Cao S, Iguchi A, Liu M, Wang G, et al. Molecular detection and genetic diversity of bovine Babesia spp., Theileria orientalis, and Anaplasma marginale in beef cattle in Thailand. Parasitol Res. 2017;116:751–762. doi: 10.1007/s00436-016-5345-2. [DOI] [PubMed] [Google Scholar]
- 12.Perera PK, Gasser RB, Anderson GA, Jeffers M, Bell CM, Jabbar A. Epidemiological survey following oriental theileriosis outbreaks in Victoria, Australia, on selected cattle farms. Vet Parasitol. 2013;197:509–521. doi: 10.1016/j.vetpar.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Pulford DJ, McFadden A, Hamilton JS, Donald J. Investigation of the index case herd and identification of the genotypes of Theileria orientalis associated with outbreaks of bovine anaemia in New Zealand in 2012. N Z Vet J. 2016;64:21–28. doi: 10.1080/00480169.2015.1090355. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Han YJ, Han DG, Chae JB, Chae JS, Yu DH, et al. Genetic characterization of Theileria orientalis from cattle in the Republic of Korea. Parasitol Res. 2017;116:449–454. doi: 10.1007/s00436-016-5316-7. [DOI] [PubMed] [Google Scholar]
- 15.Choi KS, Yu DH, Chae JS, Park BK, Yoo JG, Park J. Seasonal changes in hemograms and Theileria orientalis infection rates among Holstein cattle pastured in the mountains in the Republic of Korea. Prev Vet Med. 2016;127:77–83. doi: 10.1016/j.prevetmed.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Aparna M, Ravindran R, Vimalkumar MB, Lakshmanan B, Rameshkumar P, Kumar KG, et al. Molecular characterization of Theileria orientalis causing fatal infection in crossbred adult bovines of South India. Parasitol Int. 2011;60:524–529. doi: 10.1016/j.parint.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bogema DR, Deutscher AT, Fell S, Collins D, Eamens GJ, Jenkins C. Development and validation of a quantitative PCR assay using multiplexed hydrolysis probes for detection and quantification of Theileria orientalis isolates and differentiation of clinically relevant subtypes. J Clin Microbiol. 2015;53:941–950. doi: 10.1128/JCM.03387-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam MK, Jabbar A, Campbell BE, Cantacessi C, Gasser RB. Bovine theileriosis—an emerging problem in south-eastern Australia? Infect Genet Evol. 2011;11:2095–2097. doi: 10.1016/j.meegid.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 19.McFadden AM, Rawdon TG, Meyer J, Makin J, Morley CM, Clough RR, Tham K, Mullner P, Geysen D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naive cattle. N Z Vet J. 2011;59:79–85. doi: 10.1080/00480169.2011.552857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available upon reasonable request.


