Abstract
Reperfusion therapy decreases myocardium damage during an acute coronary event and consequently mortality. However, there are unmet needs in the treatment of acute myocardial infarction, consequently mortality and heart failure continue to occur in about 10% and 20% of cases, respectively. Different strategies could improve reperfusion. These strategies, like generation of warning sign recognition and being initially assisted and transferred by an emergency service, could reduce the time to reperfusion. If the first electrocardiogram is performed en route, it can be transmitted and interpreted in a timely manner by a specialist at the receiving center, bypassing community hospitals without percutaneous coronary intervention capabilities. To administer thrombolytic therapy during transport to the catheterization laboratory could reduce time to reperfusion in cases with expected prolonged transport time to a percutaneous coronary intervention center or to a center without primary percutaneous coronary intervention capabilities with additional expected delay, known as pharmaco-invasive strategy. Myocardial reperfusion is known to produce damage and cell death, which defines the reperfusion injury. Lack of resolution of ST segment is used as a marker of reperfusion failure. In patients without ST segment resolution, mortality triples. It is important to note that, until recently, reperfusion injury and no-reflow were interpreted as a single entity and we should differentiate them as different entities; whereas no-reflow is the failure to obtain tissue flow, reperfusion injury is actually the damage produced by achieving flow. Therefore, treatment of no-reflow is obtained by tissue flow, whereas in reperfusion injury the treatment objective is protection of susceptible myocardium from reperfusion injury. Numerous trials for the treatment of reperfusion injury have been unsuccessful. Newer hypotheses such as “ controlled reperfusion”, in which the interventional cardiologist assumes not only the treatment of the culprit vessel but also the way to reperfuse the myocardium at risk, could reduce reperfusion injury.
Keywords: Myocardial Infarction, Reperfusion, Reperfusion Injury, Controlled reperfusion
Introduction
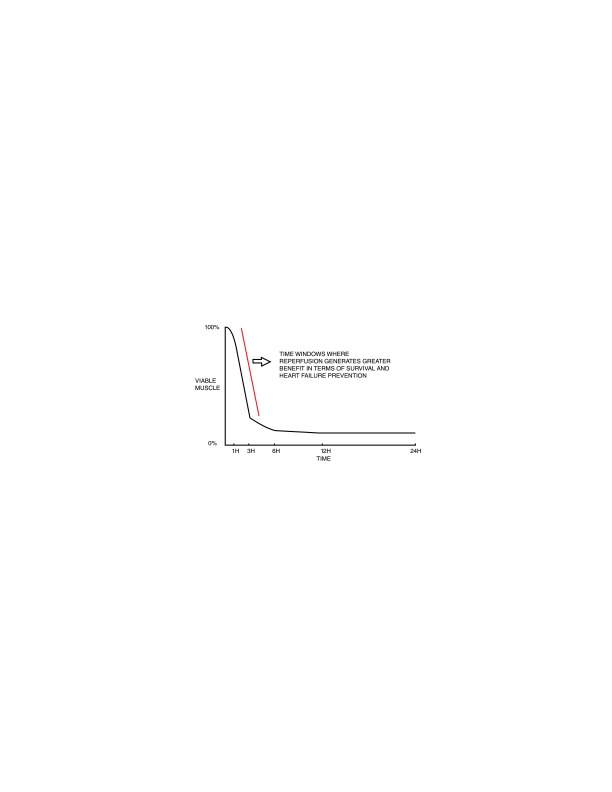
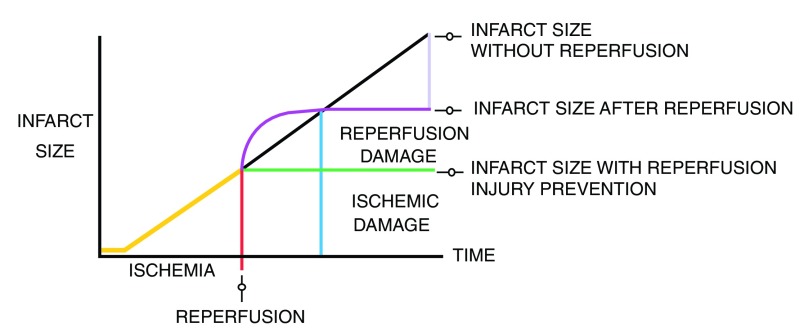
Atherosclerotic cardiovascular disease is the leading cause of death around the world 1. Acute myocardial infarction (AMI) is the event that causes most deaths or new cases of heart failure (HF) 2– 5. Early reperfusion therapy decreases the amount of myocardium damaged during an acute event and consequently mortality 6, 7. Primary percutaneous coronary intervention (PPCI) has become the optimal reperfusion strategy when performed in a timely manner 8– 10. However, there are unmet needs in the treatment of AMI, limiting the benefits that could be obtained with PPCI, since mortality and HF continue to occur in about 10% and 20% of cases each year, respectively 2– 5. In the current state of AMI treatment, two different stages can be recognized in which decrease of reperfusion benefits and in which the wavefront of necrosis could potentially be aborted. The first stage is the time from the onset of symptoms to reperfusion ( Figure 1). The second stage occurs during reperfusion ( Figure 2).
Figure 1. Relationship between time, extent of myocardial salvage, and mortality reduction.
Figure 2. Ischemic injury and reperfusion injury contributions to final myocardial infarction size.
Efforts to optimize the benefit of PPCI are aimed at decreasing the time from onset of symptoms to reperfusion, reducing myocardial damage during the delay, and preventing reperfusion injury.
Time reperfusion
The greatest benefit of reperfusion is obtained within the first 2 to 3 hours of ischemia 11, 12. The guidelines for the treatment of AMI indicate that the time from first contact with the health team for acquisition and interpretation of electrocardiogram (ECG) must be less than 10 minutes 13, 14. PPCI is chosen for reperfusion if it is done in a timely manner by a trained team within 120 minutes of the first medical contact (FMC) 12, 15– 17. If the FMC occurs in a PPCI center, the accepted delay to reperfusion is 90 minutes 17, 18 but preferably would be less than 60 minutes. Since most patients present to centers without PPCI capabilities, door-in to door-out time in the non-PPCI center has to be less than 30 minutes for patients transferred to a PPCI center 12, 19, 20.
If the FMC occurs in an institution without primary angioplasty (or in emergency medical services) and the expected delay for transfer for primary angioplasty has an estimated time of longer than 120 minutes, reperfusion with thrombolytic is recommended for patients without contraindications 17, 21, 22. In this case, the recommended time from arrival of the patient to starting the application of thrombolytic is less than 30 minutes 17, 23, 24.
But in the real world, the time from onset of symptoms to FMC varies widely, and usually patients wait 1.5 to 2 hours to seek medical attention, and only 66% of patients receive reperfusion within the recommendations of scientific guidelines 25. The variables related to delay from onset of symptoms to the FMC are the following: female gender; older age and those younger than 40 years; previous cardiovascular disease, particularly coronary heart disease; renal failure; and walk-in hospital presentation and geographical location 26– 28. The average time from onset of symptoms to FMC has not decreased in the last 10 years 28, 29. An additional delay is generated when the initial ECG is performed by a general practitioner who takes an average of 23.9 minutes 30. There is a close correlation between system delay and short- and long-term mortality; 1-hour delay in the system involves mortality of 15% at 3.4 years, and a delay of 3 hours increases mortality to 28.1% in the same period 31. Factors related to system delay are transfers from remote regions, presentation in a center not trained in reperfusion therapy, transfers between centers, delay for the administration of thrombolytics, and delayed activation of the catheterization laboratory.
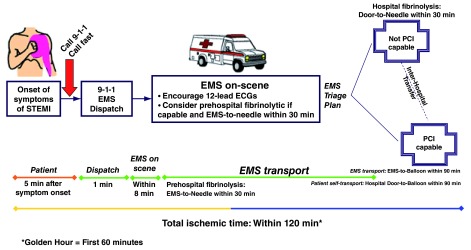
Strategies that could reduce the time to reperfusion are the following: education of the general population, generation of warning sign recognition 32 and being initially assisted and transferred by an emergency service; as in the case of cardiac arrest, they may benefit from receiving timely CPR 33. If the first ECG is performed during transport, it can be transmitted and interpreted by a specialist at the receiving center. This could allow the system to be activated while the patient is en route to the hospital 34. This might also allow thrombolytic therapy to be administered as a pharmaco-invasive strategy in those patients with a long transport time to the catheterization laboratory. The pharmaco-therapy with aspirin, clopidogrel, unfractionated heparin, and tenecteplase and subsequent interventionism demonstrated outcomes equivalent to those of primary angioplasty but with twice the major bleeding, so it has to be selected only in those patients with expected long delays for PPCI 35 and half the dose in the elderly population ( Figure 3).
Figure 3. Strategies to optimize time to reperfusion.
ECG, electrocardiogram; EMS, emergency medical services; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Reperfusion injury
Reperfusion therapy for AMI saves viable myocardium, but paradoxically the re-establishment of coronary blood flow also induces myocyte damage and death, limiting the full benefit of reperfusion in terms of reduction of infarct size and preservation of ventricular function 36, 37. Reperfusion itself can cause more damage and cell death; this process defines the phenomenon of reperfusion injury 36, 38 that potentially is prevented by applying additional therapies 39. Some evidence suggests that reperfusion injury may be responsible for up to 50% of the final myocardial damage during AMI 36 ( Figure 4).
Figure 4. Potential benefits of reperfusion injury treatment.
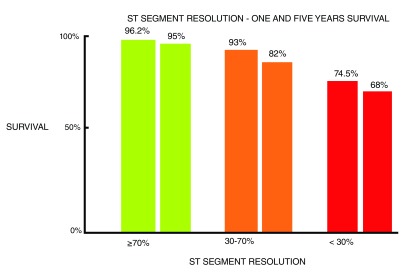
The time from the symptom onset, diabetes, thrombolysis in myocardial infarction flow 0 in the baseline angiography, culprit lesion located at the proximal anterior descending artery, and presentation with HF are related to a higher chance of reperfusion injury 40, 41. Elevated white blood cells, increased platelet activation (size and reactivity), high thromboxane A2 and ET1 levels, hyperglycemia with or without diabetes, and C-reactive protein before reperfusion are predictors of this phenomenon 42– 44. It is possible that some degree of reperfusion injury is always present, but those patients with a short time from symptom onset or with previous angina seem less susceptible 45, 46. There is a useful rule of thumb to estimate its magnitude: the greater and more intense the ischemia, the greater the reperfusion injury 41, 47– 49. In everyday practice, the lack of ST segment resolution after achieving epicardial coronary flow is used as a marker of reperfusion failure. ST segment elevation does not decrease, mortality of AMI triples regardless of the achievement of adequate epicardial flow 50, 51 ( Figure 5).
Figure 5. Relationship between lack of ST segment resolution and mortality.
Diagnosis and differential diagnosis of reperfusion injury. The presence of reperfusion is a condition for reperfusion injury to exist. Clinical, electrocardiographic, and angiographic elements must be present. Clinical symptoms include increasing pain, anxiety, vegetative symptoms, and impaired hemodynamic status 52, 53. Electrocardiographic changes include ST segment elevation, onset of sinus tachycardia (by adrenergic discharge), malignant ventricular arrhythmias, extreme bradycardia, and electromechanical dissociation 52– 54. Angiographic elements include epicardial artery with signs of reperfusion and adequate antegrade flow and contrast extravasation in the microvasculature evidenced by persistent myocardial blush 55– 57.
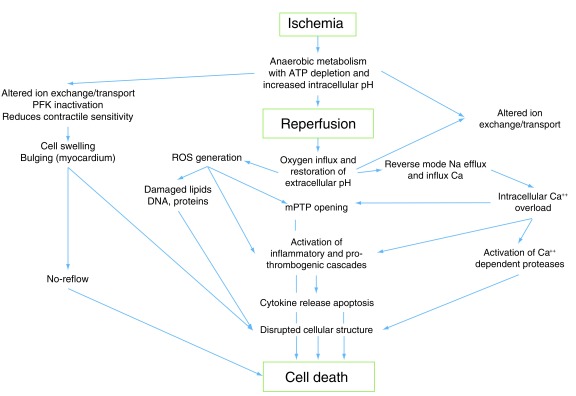
Cell damage may be caused by different pathways during reperfusion ( Figure 6). The main event occurring during reperfusion and trigger of reperfusion injury is the abrupt increase of oxygen content in a medium with low pH (acidosis tissue caused by ischemia). In this scenario, the O 2 reacts with hydrogen protons to reactive oxygen species (ROS), causing damage to DNA, protein, and lipid membranes, producing myocardial cell death 58, 59. In addition, ROS have pro-inflammatory effects, causing apoptosis and cell necroptosis 60. At the mitochondrial level, ROS open mitochondrial permeability transition pores, making them susceptible to irreversible damage 60. The damage produced by ROS at the level of the endoplasmic reticulum alters calcium dynamics, which in the context of acidotic reperfusion generates calcium influx into the sarcolemma, producing sustained hypercontraction and contraction band necrosis 59– 61. In addition, the influx of calcium-dependent proteases degrades structural components of the cell.
Figure 6. Physiopathologic events contributing to ischemic and reperfusion injury.
PTP: membrane protein transition pore, ROS: reactive oxigen species, PFK: Phosphofructokinase.
Reperfusion injury affects not only myocytes but also the microvasculature, where ROS produce direct damage of endothelial cells, causing increased permeability of the capillary wall and edema. ROS are chemotactic for neutrophils, activate complement, and trigger pro-thrombotic events 60– 63 ( Table 1 and Table 2). Finally, microvascular occlusion by perivascular edema, accumulation of neutrophils, and local thrombosis occur.
Table 1. Reperfusion injury: physiopathogenic elements.
| Oxidative/nitrosative stress |
| Calcium overload |
| Endoplasmic reticulum stress |
| Mitochondrial dysfunction |
| Activation of apoptotic and autophagic pathways |
| Protein kinases |
| Epigenetic changes |
| Inflammation |
| Protein cleavage products and other degradation products |
Table 2. Reactions that produce free radicals.
| Superoxide production | O 2 + e − : O 2 − |
| Hydrogen peroxide production | 2H + O 2 : H 2O 2 |
| Haber-Weiss reaction | O 2 − + H 2O 2 : O 2 + 2OH |
| Fenton reaction | Fe 2+ + H 2O 2 : OH + OH − Fe 3+ |
| Peroxynitrite production | O 2 − + NO : ONOO |
| Peroxynitrous acid production | ONOO − + H + : ONOOH |
| Breakdown of peroxynitrous acid | ONOOH : OH + NO 2 |
| NO 2 and CO 3 production | ONOO − + CO 2 : NO 2 + CO 3 |
Reperfusion injury occurs by the influx of O 2-saturated blood to a myocardial tissue that is made vulnerable by metabolic changes and a local internal environment that are produced during ongoing ischemia. Reperfusion injury is a rapid and irreversible phenomenon; therefore, the therapeutic strategy should focus on reducing the vulnerability of the myocardium or modify the blood that arrives to the susceptible muscle. Any therapy administered after reperfusion will be ineffective or of limited clinical benefit.
Different approaches were tested to reduce or prevent reperfusion injury and many of them failed ( Table 3) 64. Occasionally, conflicting results were found in selective therapies 64. Therefore, it is difficult to establish standardized treatment guidelines. Current scientific guidelines do not include reperfusion injury as a therapeutic target. It is important to note that, until recently, reperfusion injury and no-reflow were interpreted as a single entity ( Table 4) and we should differentiate them as different entities; whereas no-reflow is the failure to obtain tissue flow, reperfusion injury is actually the damage produced by achieving flow. Therefore, the way to treat no-reflow is to obtain tissue flow, whereas in reperfusion injury the treatment objective is to protect the susceptible myocardium from reperfusion injury. Another problem for the evaluation of clinical trials is that it is difficult to detect successful treatment for no-reflow and distinguish it from success in treating reperfusion injury if ultimately the common goal is to preserve the myocardium and there is no diagnosis of any of the phenomena before therapy is applied.
Table 3. Simplified evaluation scheme treatment of reperfusion injury.
| Therapeutic
target |
Treatment | Route of
administration |
Result |
|---|---|---|---|
| Indeterminate | Hypothermia | IV | − |
| Hypothermia | Peritoneal | − | |
| MMTP | Delcasetrib | IV before reperfusion | − |
| TR040303 | IV before reperfusion | − | |
| Bendavia | IV before reperfusion | − | |
| Ciclosporin A | IV before reperfusion | + − | |
| Nitric oxide
signaling |
Nitrite sodium | IV | − |
| Nitrite sodium | Intracoronary | − | |
| Nitric oxide | Inhaled | − | |
| Pro-survival
kinase |
Copertide | IV | + |
| Exenatide | IV | + | |
| Indeterminate | Metoprolol | IV | + |
| Indeterminate | Post-conditioning | IC balloon inflations | + + − − |
| Indeterminate | RIC | Limb ischemia | + + + +
+ + + − |
+, one trial with positive results; −, one trial with negative results; IC, intracoronary; IV, intravascular; MMTP, mitochondrial permeability transition pore; RIC, remote ischemic conditioning.
Table 4. Differential diagnosis with no-reflow.
| Reperfusion injury | No-reflow | |
|---|---|---|
| Clinic | Sudden clinical deterioration | No changes to the state prior to
reperfusion |
| Electrocardiography | ST segment elevation | ST unchanged with respect to
electrocardiogram prior to reperfusion |
| Angiography | Persistent myocardial blush | No blush or slow blush |
| Thrombolysis in myocardial infarction
(TIMI) 2–3 at epicardial artery |
TIMI 2–3 at epicardial artery |
Given the pathophysiological difference of both entities, it may be considered that there is no reperfusion injury if no-reflow occurs. If a treatment is useful for no-reflow, this does not imply that it is useful for reperfusion injury. For example, perhaps thromboaspiration, glycoprotein IIb IIIa inhibitors, and vasodilators such as adenosine are effective for treatment of no-reflow but this does not mean that they avoid damage caused by ROS and pro-inflammatory cytokines. Likely, in a given patient, any therapeutic option for reperfusion injury is effective if the no-reflow phenomenon is solved first, the patient is being treated for an event that will not happen. Therefore the efficacy of treatment for each phenomena should be assessed separately in clinical trials. We also have to consider the treatment of both entities as predominantly preventive; therefore, clinicians need to start treatment before the phenomenon occurs and compare their effectiveness with controls.
It is reasonable to choose, as the definition of success for trials evaluating therapies in no-reflow, the presence of myocardial blush, whereas reperfusion injury therapies should define success by ST correction in the presence of positive myocardial blush ( Table 5).
Table 5. Theoretical model to evaluate success for no-reflow and reperfusion injury treatment.
| Treatment | Myocardial Blush | ST | No-reflow/Reperfusion injury |
|---|---|---|---|
| + | ↓ | Success/Success | |
| + | ↑ | Success/Failure | |
| − | ↑ | Failure/? | |
| a | ? | ↑ | ?/Failure |
| a | ? | ↓ | ?/Success |
aThe existence of the latter two possibilities in this table is explained if one can evaluate a treatment for reperfusion injury by administering it by a microcatheter, balloon over the wire, or other similar device that can administer treatment before acting on the epicardial occlusion.
Pro-inflammatory and cytotoxic phenomena (not only local but systemic), which are triggered during ischemia and reperfusion, may continue to produce myocardial damage. These mechanisms could explain why some patients with successful reperfusion continue to lose myocardium (R wave of ECG) in the following reperfusion hours.
Perspectives
The development of reperfusion therapies for AMI meaningfully reduced mortality. There are possibilities to optimize their use. Health teams should continue fighting to shorten the system delay and identify the best strategy according to the context in which they operate. To this end, initiatives such as Stent for Life are expanding around the world. There are working groups that conduct research in basic science, translational research, and clinical research against reperfusion injury, such as the Hatter Cardiology Institute, which (led by Derek Yellon) is making progress in myocardial protection using remote ischemic conditioning. We are working on primary controlled reperfusion and starting a clinical assay using intracoronary dextran plus vein blood through the balloon catheter before opening the artery. See Dextran Use for Primary Angioplasty Protection in Acute Myocardial Infarction. DUPAP Trial at ClinicalTrials.gov.
We hypothesized that developing treatment protocols for “ continuous myocardial protection” with different drugs, such as cyclosporine or other modulators of inflammation, administered from the time of diagnosis to the patient convalescence at the critical unit, could preserve myocardium during the delay of the system and during the early evolution of the event. To develop procedures of “ controlled reperfusion” where interventional cardiologists assume treatment not only for the culprit vessel infarction but also for myocardium could reduce reperfusion injury. The newer concept of “ controlled reperfusion” means deciding how to reperfuse (for example, post-conditioning with successive balloon inflations) and which adjunct compound to use during reperfusion (for example, administering to the ischemic myocardium, through dedicated catheters, prior to the opening of the artery, modified blood or enriched with drugs), preparing the myocardium for a more complete and definitive recovery. These two concepts—“ continuous myocardial protection” and “ controlled reperfusion”—open a wide field of research and development with potential benefits that could decrease myocardial damage and mortality.
Abbreviations
AMI, acute myocardial infarction; ECG, electrocardiogram; FMC, first medical contact; HF, heart failure; PPCI, primary percutaneous coronary intervention; ROS, reactive oxygen species.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Antonio Abbate, VCU Pauley Heart Center, Virginia Commonwealth University, Virginia, USA
Takashi Akasaka, Department of Cardiovascular Medicine, Wakayama Medical University, Kimiidera, Wakayama, Japan
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Global Projections of mortality and causes of death, 2015 and 2030 MORTALITY – WHO. On line publication.2016. [Google Scholar]
- 2. Steg PG, Dabbous OH, Feldman LJ, et al. : Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109(4):494–9. 10.1161/01.CIR.0000109691.16944.DA [DOI] [PubMed] [Google Scholar]
- 3. Velazquez EJ, Francis GS, Armstrong PW, et al. : An international perspective on heart failure and left ventricular systolic dysfunction complicating myocardial infarction: the VALIANT registry. Eur Heart J. 2004;25(21):1911–9. 10.1016/j.ehj.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 4. Hellermann JP, Jacobsen SJ, Redfield MM, et al. : Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7(1):119–25. 10.1016/j.ejheart.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 5. Jhund PS, McMurray JJ: Heart failure after acute myocardial infarction: a lost battle in the war on heart failure? Circulation. 2008;118(20):2019–21. 10.1161/CIRCULATIONAHA.108.813493 [DOI] [PubMed] [Google Scholar]
- 6. Gibson CM: NRMI and current treatment patterns for ST-elevation myocardial infarction. Am Heart J. 2004;148(5 Suppl):S29–33. 10.1016/j.ahj.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 7. Keeley EC, Hillis LD: Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med. 2007;356(1):47–54. 10.1056/NEJMct063503 [DOI] [PubMed] [Google Scholar]
- 8. Grines CL, Cox DA, Stone GW, et al. : Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1999;341(26):1949–56. 10.1056/NEJM199912233412601 [DOI] [PubMed] [Google Scholar]
- 9. Stone GW, Brodie BR, Griffin JJ, et al. : Prospective, multicenter study of the safety and feasibility of primary stenting in acute myocardial infarction: in-hospital and 30-day results of the PAMI stent pilot trial. Primary Angioplasty in Myocardial Infarction Stent Pilot Trial Investigators. J Am Coll Cardiol. 1998;31(1):23–30. 10.1016/S0735-1097(97)00439-7 [DOI] [PubMed] [Google Scholar]
- 10. Keeley EC, Boura JA, Grines CL: Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. 10.1016/S0140-6736(03)12113-7 [DOI] [PubMed] [Google Scholar]
- 11. Gershlick AH, Banning AP, Myat A, et al. : Reperfusion therapy for STEMI: is there still a role for thrombolysis in the era of primary percutaneous coronary intervention? Lancet. 2013;382(9892):624–32. 10.1016/S0140-6736(13)61454-3 [DOI] [PubMed] [Google Scholar]
- 12. Kolh P, Windecker S: ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J. 2014;35(46):3235–6. 10.1093/eurheartj/ehu422 [DOI] [PubMed] [Google Scholar]
- 13. Rokos IC, French WJ, Koenig WJ, et al. : Integration of pre-hospital electrocardiograms and ST-elevation myocardial infarction receiving center (SRC) networks: impact on Door-to-Balloon times across 10 independent regions. JACC Cardiovasc Interv. 2009;2(4):339–46. 10.1016/j.jcin.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 14. Diercks DB, Kontos MC, Chen AY, et al. : Utilization and impact of pre-hospital electrocardiograms for patients with acute ST-segment elevation myocardial infarction: data from the NCDR (National Cardiovascular Data Registry) ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry. J Am Coll Cardiol. 2009;53(2):161–6. 10.1016/j.jacc.2008.09.030 [DOI] [PubMed] [Google Scholar]
- 15. Andersen HR, Nielsen TT, Rasmussen K, et al. : A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349(8):733–42. 10.1056/NEJMoa025142 [DOI] [PubMed] [Google Scholar]
- 16. Nielsen PH, Terkelsen CJ, Nielsen TT, et al. : System delay and timing of intervention in acute myocardial infarction (from the Danish Acute Myocardial Infarction-2 [DANAMI-2] trial). Am J Cardiol. 2011;108(6):776–81. 10.1016/j.amjcard.2011.05.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. O'Gara PT, Kushner FG, Ascheim DD, et al. : 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 18. Sorensen JT, Terkelsen CJ, Norgaard BL, et al. : Urban and rural implementation of pre-hospital diagnosis and direct referral for primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2011;32(4):430–6. 10.1093/eurheartj/ehq437 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Herrin J, Miller LE, Turkmani DF, et al. : National performance on door-in to door-out time among patients transferred for primary percutaneous coronary intervention. Arch Intern Med. 2011;171(21):1879–86. 10.1001/archinternmed.2011.481 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Wang TY, Nallamothu BK, Krumholz HM, et al. : Association of door-in to door-out time with reperfusion delays and outcomes among patients transferred for primary percutaneous coronary intervention. JAMA. 2011;305(24):2540–7. 10.1001/jama.2011.862 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Nallamothu BK, Bates ER: Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol. 2003;92(7):824–6. 10.1016/S0002-9149(03)00891-9 [DOI] [PubMed] [Google Scholar]
- 22. Pinto DS, Kirtane AJ, Nallamothu BK, et al. : Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation. 2006;114(19):2019–25. 10.1161/CIRCULATIONAHA.106.638353 [DOI] [PubMed] [Google Scholar]
- 23. Boersma E, Maas AC, Deckers JW, et al. : Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348(9030):771–5. 10.1016/S0140-6736(96)02514-7 [DOI] [PubMed] [Google Scholar]
- 24. Newby LK, Rutsch WR, Califf RM, et al. : Time from symptom onset to treatment and outcomes after thrombolytic therapy. GUSTO-1 Investigators. J Am Coll Cardiol. 1996;27(7):1646–55. 10.1016/0735-1097(96)00053-8 [DOI] [PubMed] [Google Scholar]
- 25. Miedema MD, Newell MC, Duval S, et al. : Causes of delay and associated mortality in patients transferred with ST-segment-elevation myocardial infarction. Circulation. 2011;124(15):1636–44. 10.1161/CIRCULATIONAHA.111.033118 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Goff DC, Jr, Feldman HA, McGovern PG, et al. : Prehospital delay in patients hospitalized with heart attack symptoms in the United States: the REACT trial. Rapid Early Action for Coronary Treatment (REACT) Study Group. Am Heart J. 1999;138(6 Pt 1):1046–57. 10.1016/S0002-8703(99)70069-4 [DOI] [PubMed] [Google Scholar]
- 27. Goldberg RJ, Steg PG, Sadiq I, et al. : Extent of, and factors associated with, delay to hospital presentation in patients with acute coronary disease (the GRACE registry). Am J Cardiol. 2002;89(7):791–6. 10.1016/S0002-9149(02)02186-0 [DOI] [PubMed] [Google Scholar]
- 28. Spencer FA, Montalescot G, Fox KA, et al. : Delay to reperfusion in patients with acute myocardial infarction presenting to acute care hospitals: an international perspective. Eur Heart J. 2010;31(11):1328–36. 10.1093/eurheartj/ehq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg RJ, Spencer FA, Fox KA, et al. : Prehospital Delay in Patients With Acute Coronary Syndromes (from the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol. 2009;103(5):598–603. 10.1016/j.amjcard.2008.10.038 [DOI] [PubMed] [Google Scholar]
- 30. Tra J, van der Wulp I, de Bruijne MC, et al. : Exploring the treatment delay in the care of patients with ST-elevation myocardial infarction undergoing acute percutaneous coronary intervention: a cross-sectional study. BMC Health Serv Res. 2015;15:340. 10.1186/s12913-015-0993-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Terkelsen CJ, Sorensen JT, Maeng M, et al. : System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304(7):763–71. 10.1001/jama.2010.1139 [DOI] [PubMed] [Google Scholar]
- 32. McDermott MM, Mandapat AL, Moates A, et al. : Knowledge and attitudes regarding cardiovascular disease risk and prevention in patients with coronary or peripheral arterial disease. Arch Intern Med. 2003;163(18):2157–62. 10.1001/archinte.163.18.2157 [DOI] [PubMed] [Google Scholar]
- 33. Becker L, Larsen MP, Eisenberg MS: Incidence of cardiac arrest during self-transport for chest pain. Ann Emerg Med. 1996;28(6):612–6. 10.1016/S0196-0644(96)70082-3 [DOI] [PubMed] [Google Scholar]
- 34. Barbagelata A, Perna ER, Clemmensen P, et al. : Time to reperfusion in acute myocardial infarction. It is time to reduce it! J Electrocardiol. 2007;40(3):257–64. 10.1016/j.jelectrocard.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 35. Rashid MK, Guron N, Bernick J, et al. : Safety and Efficacy of a Pharmacoinvasive Strategy in ST-Segment Elevation Myocardial Infarction: A Patient Population Study Comparing a Pharmacoinvasive Strategy With a Primary Percutaneous Coronary Intervention Strategy Within a Regional System. JACC Cardiovasc Interv. 2016;9(19):2014–20. 10.1016/j.jcin.2016.07.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Yellon DM, Hausenloy DJ: Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35. 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 37. Hausenloy DJ, Yellon DM: Targeting Myocardial Reperfusion Injury--The Search Continues. N Engl J Med. 2015;373(11):1073–5. 10.1056/NEJMe1509718 [DOI] [PubMed] [Google Scholar]
- 38. Braunwald E, Kloner RA: Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76(5):1713–9. 10.1172/JCI112160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piper HM, García-Dorado D, Ovize M: A fresh look at reperfusion injury. Cardiovasc Res. 1998;38(2):291–300. 10.1016/S0008-6363(98)00033-9 [DOI] [PubMed] [Google Scholar]
- 40. Collet JP, Montalescot G: The acute reperfusion management of STEMI in patients with impaired glucose tolerance and type 2 diabetes. Diab Vasc Dis Res. 2005;2(3):136–43. 10.3132/dvdr.2005.021 [DOI] [PubMed] [Google Scholar]
- 41. Iwakura K, Ito H, Kawano S, et al. : Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J Am Coll Cardiol. 2001;38(2):472–7. 10.1016/S0735-1097(01)01405-X [DOI] [PubMed] [Google Scholar]
- 42. Campo G, Valgimigli M, Gemmati D, et al. : Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY Study. J Am Coll Cardiol. 2006;48(11):2178–85. 10.1016/j.jacc.2005.12.085 [DOI] [PubMed] [Google Scholar]
- 43. Niccoli G, Giubilato S, Russo E, et al. : Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur Heart J. 2008;29(15):1843–50. 10.1093/eurheartj/ehn325 [DOI] [PubMed] [Google Scholar]
- 44. Niccoli G, Lanza GA, Shaw S, et al. : Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 2006;27(15):1793–8. 10.1093/eurheartj/ehl119 [DOI] [PubMed] [Google Scholar]
- 45. Karila-Cohen D, Czitrom D, Brochet E, et al. : Decreased no-reflow in patients with anterior myocardial infarction and pre-infarction angina. Eur Heart J. 1999;20(23):1724–30. 10.1053/euhj.1999.1714 [DOI] [PubMed] [Google Scholar]
- 46. Komamura K, Kitakaze M, Nishida K, et al. : Progressive decreases in coronary vein flow during reperfusion in acute myocardial infarction: clinical documentation of the no reflow phenomenon after successful thrombolysis. J Am Coll Cardiol. 1994;24(2):370–7. 10.1016/0735-1097(94)90290-9 [DOI] [PubMed] [Google Scholar]
- 47. Nallamothu BK, Bradley EH, Krumholz HM: Time to treatment in primary percutaneous coronary intervention. N Engl J Med. 2007;357(16):1631–8. 10.1056/NEJMra065985 [DOI] [PubMed] [Google Scholar]
- 48. Turschner O, D'hooge J, Dommke C, et al. : The sequential changes in myocardial thickness and thickening which occur during acute transmural infarction, infarct reperfusion and the resultant expression of reperfusion injury. Eur Heart J. 2004;25(9):794–803. 10.1016/j.ehj.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 49. Uyarel H, Cam N, Okmen E, et al. : Level of Selvester QRS score is predictive of ST-segment resolution and 30-day outcomes in patients with acute myocardial infarction undergoing primary coronary intervention. Am Heart J. 2006;151(6):1239.e1–7. 10.1016/j.ahj.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 50. de Lemos JA, Braunwald E: ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol. 2001;38(5):1283–94. 10.1016/S0735-1097(01)01550-9 [DOI] [PubMed] [Google Scholar]
- 51. Buller CE, Fu Y, Mahaffey KW, et al. : ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction: insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Circulation. 2008;118(13):1335–46. 10.1161/CIRCULATIONAHA.108.767772 [DOI] [PubMed] [Google Scholar]
- 52. Aiello EA, Jabr RI, Cole WC: Arrhythmia and delayed recovery of cardiac action potential during reperfusion after ischemia. Role of oxygen radical-induced no-reflow phenomenon. Circ Res. 1995;77(1):153–62. 10.1161/01.RES.77.1.153 [DOI] [PubMed] [Google Scholar]
- 53. Ito H, Maruyama A, Iwakura K, et al. : Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93(2):223–8. 10.1161/01.CIR.93.2.223 [DOI] [PubMed] [Google Scholar]
- 54. Rezkalla SH, Kloner RA: No-reflow phenomenon. Circulation. 2002;105(5):656–62. 10.1161/hc0502.102867 [DOI] [PubMed] [Google Scholar]
- 55. Perez de Prado A, Fernández-Vázquez F, Cuellas-Ramón JC, et al. : Coronary clearance frame count: a new index of microvascular perfusion. J Thromb Thrombolysis. 2005;19(2):97–100. 10.1007/s11239-005-1379-5 [DOI] [PubMed] [Google Scholar]
- 56. Henriques JP, Zijlstra F, van 't Hof AW, et al. : Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107(16):2115–9. 10.1161/01.CIR.0000065221.06430.ED [DOI] [PubMed] [Google Scholar]
- 57. Appelbaum E, Kirtane AJ, Clark A, et al. : Association of TIMI myocardial perfusion grade and ST-segment resolution with cardiovascular magnetic resonance measures of microvascular obstruction and infarct size following ST-segment elevation myocardial infarction. J Thromb Thrombolysis. 2009;27(2):123–9. 10.1007/s11239-008-0197-y [DOI] [PubMed] [Google Scholar]
- 58. Zweier JL, Talukder MA: The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70(2):181–90. 10.1016/j.cardiores.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 59. Barber AM, Maizel JV, Jr: SequenceEditingAligner: a multiple sequence editor and aligner. Genet Anal Tech Appl. 1990;7(2):39–45. 10.1016/0735-0651(90)90011-4 [DOI] [PubMed] [Google Scholar]
- 60. Kalogeris T, Baines CP, Krenz M, et al. : Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Hoffman JW, Jr, Gilbert TB, Poston RS, et al. : Myocardial reperfusion injury: etiology, mechanisms, and therapies. J Extra Corpor Technol. 2004;36(4):391–411. [PubMed] [Google Scholar]
- 62. Verma S, Fedak PW, Weisel RD, et al. : Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105(20):2332–6. 10.1161/01.CIR.0000016602.96363.36 [DOI] [PubMed] [Google Scholar]
- 63. Reffelmann T, Hale SL, Dow JS, et al. : No-reflow phenomenon persists long-term after ischemia/reperfusion in the rat and predicts infarct expansion. Circulation. 2003;108(23):2911–7. 10.1161/01.CIR.0000101917.80668.E1 [DOI] [PubMed] [Google Scholar]
- 64. Bulluck H, Yellon DM, Hausenloy DJ: Reducing myocardial infarct size: challenges and future opportunities. Heart. 2016;102(5):341–8. 10.1136/heartjnl-2015-307855 [DOI] [PMC free article] [PubMed] [Google Scholar]