Abstract
Objective
Obstructive sleep apnea (OSA) is a prevalent condition, especially in obese children, and is associated with increased risk for metabolic syndrome. Angiopoietins have been identified as potential biomarkers of endothelial dysfunction, and MetS. In adults, angiopoietin-2 (Ang-2) and its soluble receptor (sTie-2) are associated with diabetes, hypertension and obesity, and could be increased in children with OSA, obesity, particularly those with evidence of cardiometabolic alterations.
Methods
126 children (7.4±2.0 years) were consecutively recruited, underwent overnight polysomnography, endothelial function, and BMI z score assessments and a fasting blood draw the morning after the sleep study. In addition to lipid profile, glucose and insulin levels and calculation of HOMA-IR, Ang-2 and sTie-2 concentrations were determined.
Results
Obese and OSA children had significantly elevated plasma Ang-2 and sTie-2 levels compared to corresponding non-obese and obese controls. Furthermore, endothelial function (Tmax) and HOMA-IR were linearly and independently associated with Ang-2 and sTie-2 levels. In a small subset of children (n=14), treatment of OSA by adenotonsillectomy resulted in reductions of Ang-2 and sTie-2 (p<0.01).
Conclusions
Ang-2 and sTie-2 plasma levels are increased in pediatric OSA and obesity, particularly when endothelial dysfunction or insulin resistance are detectable, and appear to decrease upon OSA treatment.
Keywords: Obstructive sleep apnea, Obesity, Inflammation, Insulin Resistance, Lipid Profile, Endothelial Dysfunction, Angiopoietins
Introduction
The prevalence of obesity in children has clearly increased all over the world, and such trends are further reflected by the increased emergence of cardiometabolic diseases in the pediatric age range (1–4). Among the obesity-related morbidities, insulin resistance and endothelial dysfunction are early manifestations and carry adverse prognosis if left untreated. Conversely, the presence of elevated BMI z scores in the overweight-obese range does not necessarily indicate the presence of cardiometabolic dysfunction, and as such, identification of candidate biomarkers would be desirable for early detection and intervention (5,6).
Another highly prevalent pediatric condition affecting around 3–4% of children that is associated with increased risk for cardiometabolic dysfunction is obstructive sleep apnea (OSA) (7). This disease is characterized by recurring episodes of increased upper airway resistance during sleep, intermittent oxygen desaturations and hypercapnia, as well as sleep fragmentation (8, 9). The mechanisms underlying the association between cardiometabolic dysfunction and OSA remain unclear, even though both systemic low grade inflammation and oxidative stress pathways appear to be involved (10).
Angiopoietins are an important group of endothelial growth factors that modulate angiogenesis and vascular remodeling. Although the various angiopoietins bind to the tyrosine kinase receptor Tie-2, they exhibit contextually divergent biological functions. As such, when angiopoietin-1 (Ang-1) binds to Tie-2, it will enhance endothelial cell survival, and promote angiogenesis (11–13). In contrast, Angiopoietin-2 (Ang-2) operates as a competitor inhibitor of Ang-1-Tie-2 binding. Ang-2, an approximately 70 kDa glycoprotein, is expressed in vascular endothelial and smooth muscle cells (13), as well as in several other cell types, where it promotes leukocyte adhesion to the vascular endothelium and extravasation (11–13). Not surprisingly, Ang-2 and soluble Tie-2 (sTie-2) are elevated in adult patients with metabolic syndrome (14), as well as in a proportion of obese adults (15,16), with Ang-2 apparently being primarily correlated with vascular dysfunction and atherosclerosis (17, 18), and sTie-2 being associated with obesity and dyslipidemia (19).
We hypothesized that the presence of OSA or obesity in children would be associated with increased plasma levels of Ang-2 and sTie-2, particularly among those children presenting evidence of endothelial dysfunction or insulin resistance.
Materials and Methods
The research protocol was approved by the University of Chicago (protocol 09–115-B) human research ethics committee. Informed consent was obtained from the parents, and age appropriate assent was also obtained from the children. Children (4–11 years of age) were recruited from the Sleep and ENT clinics at Comer Children’s Hospital at the University of Chicago, as well as by advertisement in the community. Children found to be hypertensive or using anti-hypertensive drug therapies were excluded (n=6). Furthermore, children with overt diabetes (fasting serum glucose ≥120 mg/dL; n=9), with a craniofacial, neuromuscular or defined genetic syndrome, and children on chronic anti-inflammatory therapy (n=12), or with any known acute or chronic illness were also excluded. In addition, children placed on sympathomimetic agents such as psychostimulants were not tested (n=18).
Overnight Polysomnographic Studies
All children underwent standard nocturnal polysomnography (NPSG) evaluation as previously described (20), with assessment of 8 standard EEG channels, bilateral EOG, EMG, 2-lead ECG, oronasal airflow measurement using thermistor, nasal pressure transducer, and end tidal CO2, chest and abdominal movement by respiratory inductance plethysmography, and pulse oximetry including pulse waveform using a commercially available data acquisition system (Polysmith; Nihon Kohden America Inc, CA, USA). The NPSG studies were scored as per the 2012 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events (21). The proportion of time spent in each stage of sleep was calculated as a percentage of total sleep time (TST). A respiratory event was scored as an obstructive apnea if it was associated with a >90% fall in signal amplitude for >90% of the entire event compared to the baseline amplitude, the event lasted for at least 2 breaths and there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort in the initial part of the event, followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, the event lasted for at least 2 missed breaths, and was associated with an arousal/ awakening or a ≥3% desaturation. A hypopnea was scored if the event was associated with a ≥50% fall in amplitude of the nasal pressure transducer, lasted at least for 2 breaths, and was associated with an arousal/ awakening or ≥3% desaturation. The obstructive apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of TST. Arousals were classified as either spontaneous or respiratory, and corresponding indices (SAI and RAI, respectively), were computed.
The diagnosis of OSA was defined by the presence of an AHI≥2/hour of total sleep time. Control children were non-snoring children who had AHI<2/hour of total sleep time.
Endothelial Function Measurements
Endothelial function was assessed in a fasted state in the morning, using the hyperemic test after cuff-induced occlusion of wrist arteries, as previously described (22, 23). In brief, a laser Doppler sensor (Periflux 5000 System, Perimed, Järfälla, Sweden) was placed over the volar aspect of the hand at the 1st finger distal metacarpal surface and the hand was secured and immobilized (24). Once cutaneous blood flow readings became stable, a cuff placed at the forearm and connected to a computer-controlled was inflated to supra-systolic pressures and blood flow signal declined to undetectable levels. The cuff was rapidly deflated and the laser Doppler measured hyperemic responses. The time to maximal regional blood flow after occlusion release (Tmax) is representative of the post-occlusion hyperemic response, an index of NO-dependent endothelial function (25). A Tmax>45 sec was considered as indicative of abnormal endothelial function (26), and represents three standard deviations above the mean. The intra-observer and inter-observer variability of the test has been previously examined and is 8.9 and 13.8% respectively.
According to our recruitment strategies, 4 distinctly different groups of children were identified: Controls (CO): Non-obese and either non-snoring or snoring children with normal polysomnographic tests; Obese children (OB), i.e., BMI z score≥1.65 with either normal polysomnographic tests or evidence of OSA; Non-obese snoring children with abnormal polysomnographic findings confirming the presence of OSA.
Plasma Assays
Blood samples were drawn into either EDTA containing tubes (purple top) or tubes without any additive. Samples were centrifuged within 30 min at 3,000 g for 10 min and plasma or serum were separated and either analyzed immediately or kept at −80°C. High sensitivity CRP (hsCRP) was measured within 2 to 3 hours after collection using the Flex reagent Cartridge (Date Behring, Newark, DE), which is based on a particle-enhanced turbidimetric immunoassay technique. Serum levels of lipids, including total cholesterol, high-density lipoprotein (HDL) cholesterol, calculated low-density lipoprotein (LDL) cholesterol, and triglycerides, were also assessed with a Flex reagent cartridge (Date Behring, Newark, DE). Plasma insulin levels were measured using a commercially available radioimmunoassay kit (Coat-A-Count Insulin, Cambridge Diagnostic Products, Inc, Fort Lauderdale, FL). Plasma glucose levels were measured using a commercial kit based on the hexokinase-glucose-6-phosphate dehydrogenase method (Flex Reagent Cartridges, Dade Behring, Newark, DE). Insulin resistance was then assessed using the homeostasis model assessment (HOMA-IR) equation (fasting insulin × fasting glucose ÷ 405) (27). In addition, plasma samples were frozen at −80°C till assay.
Ang-2 and sTie-2 Plasma Assays
Circulating levels of plasma Ang-2 and sTie-2 were assayed using commercially available Quantikine human ELISA kits (R&D systems). The intra-and inter-assay coefficients of variation were 6.5% and 9.1%, respectively, for Ang-2 and 4.4% and 6.5%, respectively, for Tie-2. The assays were performed according to the manufacturer’s instructions.
Statistical Analysis
All analyses were conducted using either SPSS software (version 19.0; SPPS Inc., Chicago, Ill.) or STATA, and data are presented as mean ± SD. Children were subdivided into 4 groups, based on the presence or absence of obesity (i.e., BMI z score >1.65) and OSA. A priori assumptions on the presence of differences in Ang-2 and sTie2 levels between children with and without OSA were formulated such as to allow for 80% power and a 2-sided confidence level at 0.01, and indicated the need for 86–112 subjects in the cohort. Significant differences between groups were analyzed using 2-way ANOVA. If the data were not normally distributed, they were logarithmically transformed (i.e., AHI, hsCRP, respiratory arousal index). Pearson correlation analyses and linear regression analyses were conducted to examine potential associations between BMI, sleep variables, lipid profiles, log hsCRP, Tmax, and HOMA-IR and plasma concentrations of Ang-2 or sTie-2. To explore potential causal pathways in our data, we developed 3 logistic regression models with incremental complexity. First, we generated a simple model that was adjusted only for age. Then, a second model was adjusted for BMI z score, and the third model was adjusted BMI z score and sleep variables. Finally, a model was constructed by adjusting for demographic, anthropometric, sleep measures and plasma levels of metabolic and inflammatory markers simultaneously, i.e., a fully adjusted model. All variables associated with Ang-2 or sTie-2 (p<0.05) in one model were included in the next modeling steps, except when the information contained in two or more variables was so similar (co-linear), in which case only one variable was included in the next modeling step. We also calculated the attributable Ang-1 or sTie-2 change fraction, which corresponds to the proportion of Ang-1 or sTie-2 change that could be explained assuming causality of the associations and elimination of the various confounding factors by using the aflogit command in STATA (28) on the logistic regression framework, since such approach enables taking into account potential confounders. All p-values reported are 2-tailed with statistical significance set at <0.05.
Results
A total of 124 children fulfilling eligibility entry criteria completed all phases of the assessments and also provided a fasting blood sample after the sleep study. However, 34 additional children refused to participate in the study (14 parents declined to participate altogether and 20 parents were not willing to participate in the blood draw portion of the study). The demographic and polysomnographic characteristics of these 34 children were similar to those of the cohort, which are shown in Table 1.
Table 1.
Demographic, polysomnographic, and endothelial function findings among obese and non-obese children with and without OSA.
| Non-Obese with OSA (n=38) |
Non-Obese without OSA (n=30) |
Obese with OSA (n=40) |
Obese without OSA (n=16) |
|
|---|---|---|---|---|
| Age (years) | 7.2±2.4 | 7.3±1.5 | 7.2±1.9 | 7.3±2.7 |
| Gender (male, %) | 50.0 | 53.3 | 57.5 | 50.0 |
| Ethnicity (African American, %) | 71.1 | 70.0 | 72.5 | 62.5 |
| BMI-z score | 0.34±0.36§ | 0.28±0.81¥ | 2.57±0.58§ | 2.32±0.62¥ |
|
Tmax (sec) N (% ED) |
42.2±10.1 13 (34.2%) |
28.2±5.7 0 (0%) |
49.5±8.8 5 (65.0%) |
43.8±9.1 5 (31.2%) |
| Total sleep duration (min) | 484.1±53.1 | 471.2±56.3 | 487.2±51.8 | 469.2±55.3 |
| Stage 1 (%) | 7.0±3.3** | 4.2±3.1** | 8.2±5.1 | 4.8±3.7 |
| Stage 2 (%) | 38.3±7.8 | 38.4±10.9 | 40.3±8.9 | 38.4±13.5 |
| Stage 3 (%) | 36.7±12.8** | 47.1±11.4** | 39.6±13.5 | 40.0±14.2 |
| REM sleep (%) | 18.3±8.1** | 25.0±7.5** | 17.8±8.6¶ | 24.2±12.2¶ |
| Sleep latency (min) | 20.2±15.6**,§ | 30.7±13.6**,¥ | 15.2±13.0¶,§ | 24.6±17.2¶,¥ |
| REM latency (min) | 119.7±49.6**,§ | 139.5±57.7**,¥ | 103.8±48.7¶,§ | 138.7±66.6¶,¥ |
| Total Arousal Index (events /hour TST) | 23.4±12.4** | 8.9±8.3** | 27.4±15.2¶ | 12.5±9.6¶ |
| Respiratory Arousal Index (events /hour TST) | 7.2±3.2**,§ | 0.2±0.2**,¥ | 8.9±4.7¶,§ | 0.6±0.3¶,¥ |
| Obstructive Apnea Hypopnea Index (events/ hour TST) | 18.9±8.1** | 0.4±0.1** | 20.9±9.4¶ | 0.6±0.3¶ |
|
SpO2 Nadir (%) ODI3% |
81.7±7.8** 17.4±8.0** |
95.1±0.7** 0.2±0.1** |
76.9±8.2¶ 20.3±8.8¶ |
93.1±1.8¶ 0.5±0.2¶ |
All data are expressed as mean±SD. Non-obese+OSA: n=38; non-obese, no-OSA: n=30; obese, no OSA: n=16; obese +OSA: n=40
- non-obese OSA vs. obese OSA–p<0.01
- non-obese non- OSA vs. obese non-OSA – p<0.01
- OSA non-obese vs. non-obese no-OSA–p<0.01
- OSA obese vs. obese no-OSA – p<0.01
In general, there were no significant differences in age, gender, or ethnicity across the 4 sub-groups. However, obese children exhibited higher BMI z scores, as well as higher HOMA-IR, serum lipids, and hs-CRP, and reduced HDL cholesterol levels (Table 2). In addition, the proportion of obese children with evidence of ED (Tmax>45 sec) was significantly higher than in non-obese children (54.4% vs. 13.2%, p<0.001). Similarly, children with OSA had significantly higher HOMA-IR, LDL cholesterol, and hs-CRP concentrations, and lower HDL cholesterol levels (Table 2). Furthermore, ED was more likely to occur in the presence of OSA (OSA vs. no-OSA: 46.0% vs. 10.9%, p<0.001).
Table 2.
Lipid profile, HOMA-IR, hs-CRP, Ang-2 and sTie-2 plasma levels in obese and non-obese children with and without OSA.
| Non-Obese with OSA (n=38) |
Non-Obese without OSA (n=30) |
Obese with OSA (n=40) |
Obese without OSA (n=16) |
|
|---|---|---|---|---|
| Total Cholesterol (mg/dl)† | 148.8±21.7**,§ | 143.8±18.3**,¥ | 159.3±27.2¶,§ | 153.9±31.6¶,¥ |
| HDL cholesterol (mg/dl)† | 50.1±9.4**,§ | 63.3±10.7**,¥ | 48.8±10.4¶,§ | 50.4±13.6¶,¥ |
| LDL cholesterol (mg/dl)† | 90.8±18.7,§ | 89.7±14.0**,¥ | 101.5±26.8¶,§ | 95.8±29.1¶,¥ |
| Triglycerides (mg/dl)† | 75.4±31.6§ | 75.3±27.9§ | 96.8±34.1¥ | 104.7±59.4¥ |
| HOMA-IR N, % with values >2.0 | 1.7±0.6**,§ 11 |
1.2±0.3**,¥ | 2.9±0.7¶,§ | 2.3±0.4¶,¥ |
| Log hsCRP† [mean actual levels] | 0.27±0.19**,§ [2.84±1.87 mg/dl] |
−0.05±0.07**,¥ [1.22±1.19 mg/dl] |
1.28±0.36¶,§ [3.59±1.97 mg/dl] |
0.44±0.32¶,¥ [2.12±1.87 mg/dl] |
| Ang-2 (ng/ml) | 1.28±0.33**,§ | 0.78±0.25**,¥ | 1.48±0.27§ | 1.37±0.28¥ |
| sTie-2 (ng/ml) | 11.2±3.3**,§ | 8.9±1.7**,¥ | 14.8±2.7§ | 15.2±2.1¥ |
HDL: high density lipid cholesterol; LDL: low density lipid cholesterol; hsCRP: high sensitivity C-reactive protein.
- non-obese OSA vs. obese OSA–p<0.01
- non-obese non- OSA vs. obese non-OSA – p<0.01
- OSA non-obese vs. non-obese no-OSA–p<0.01
- OSA obese vs. obese no-OSA – p<0.01
Primary sleep disturbance measures clinically used to characterize the severity of OSA were not significantly different in obese and non-obese children with OSA, although obese children exhibited a trend toward higher AHI and ODI3% and lower nadir SpO2 values. Similarly, there were no differences in sleep architecture measures among obese and non-obese children without OSA (Table 1).
Obese children without OSA had higher Ang-2 and sTie-2 levels than non-obese children without OSA (p<0.01; Table 2). Similarly, non-obese children with OSA also exhibited higher Ang-2 and sTie-2 plasma concentrations compared to non-obese controls (p<0.01; Table 2). However, obese children with OSA did not exhibit higher Ang-2 or sTie-2 levels compared to obese children without OSA (p>0.05; Table 2).
Individual HOMA-IR values were significantly associated with either BMI z score (r2: 0.18, p<0.01) or with AHI (r2: 0.16, p<0.01) but not with age as illustrated by Pearson bivariate correlation analyses (Figure 1). Similarly, Tmax values were also significantly associated with BMI z scores (r2: 0.13, p<0.01), and with AHI (r2: 0.30, p<0.001) but not with age (Figure 1). In addition, no evidence of significant associations emerged between age, BMI z score or AHI and total cholesterol, LDL and HDL cholesterol levels. However, a very strong association emerged between HOMA-IR and Tmax (r2: 0.44, p<0.0001).
Figure 1. Scatterplots of Tmax and HOMA-IR vs. age, BMI z score, and obstructive apnea hypopnea index (AHI) in children with and without obesity or OSA.
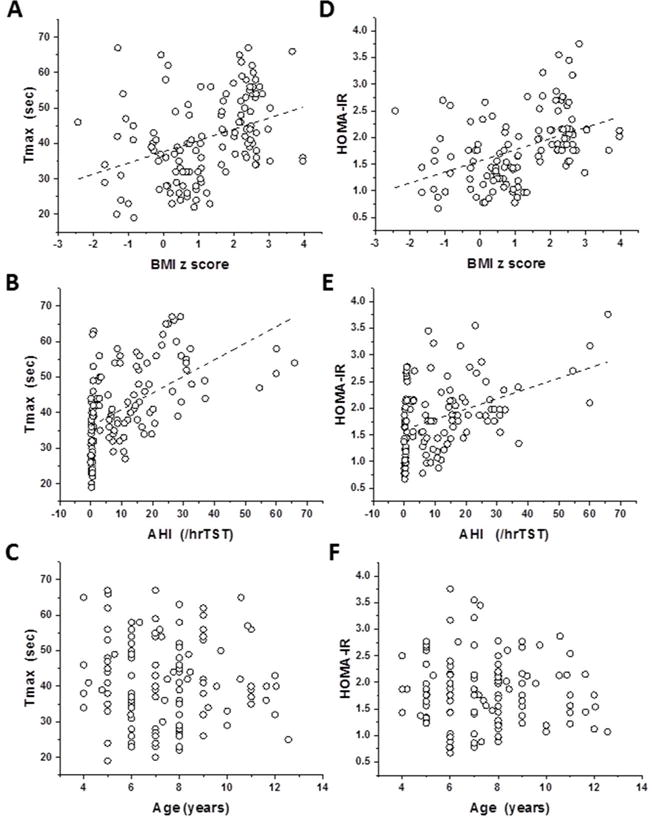
Panel A - r2=0.133, p<0.001
Panel B - r2=0.299, p<0.001
Panel C - p>0.05
Panel D - r2=0.190, p<0.001
Panel E - r2=0.169, p<0.001
Panel F - p>0.05
In order to estimate potential associations between Ang-2 or sTie-2 plasma levels, polysomnographic measures and metabolic (HOMA-IR) or endothelial function (Tmax) indices, we also performed Pearson correlation analyses. As shown in Figure 2, Ang-2 plasma levels were significantly associated with BMI z score (r2: 0.10, p<0.01), AHI (r2: 0.22, p<0.001), and particularly with Tmax (r2: 0.57, p<0.001) and HOMA-IR (r2: 0.29, p<0.001). Similarly, sTie-2 levels were significantly correlated with BMI z score (r2: 0.22, p<0.001), AHI (r2: 0.12, p<0.01), Tmax (r2: 0.34, p<0.001), and particularly with HOMA-IR (r2: 0.59, p<0.0001). Ang-2 levels were also inversely correlated with HDL (r2: 0.12, p<0.01 and r2: 0.08, p<0.01) but not with LDL or total cholesterol.
Figure 2. Scatterplots of Ang-2 (A panels 1–4) and sTie-2 plasma levels (B panels 1–4) vs. BMI z score, obstructive apnea hypopnea index (AHI), Tmax, and HOMA-IR in children with and without obesity or OSA.
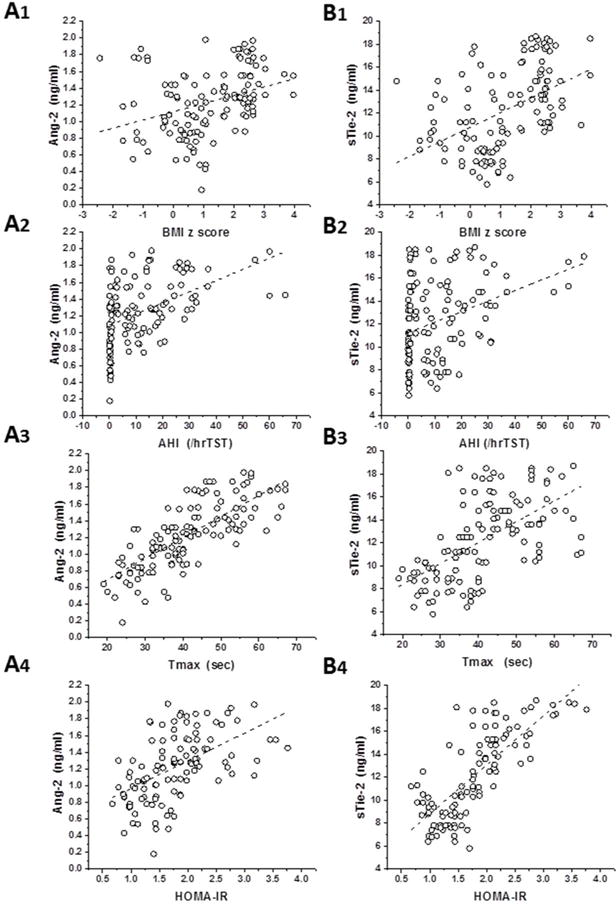
Panel A1 - r2=0.10, p<0.01
Panel A2 - r2=0.22, p<0.001
Panel A3 - r2=0.57, p<0.001
Panel A4, r2=0.29, p<0.001
Panel B1: r2=0.22, p<0.001
Panel B2: r2=0.12, p<0.01
Panel B3: r2=0.34, p<0.001
Panel B4: r2=0.59, p<0.001
To further explore independent predictors of Ang-2 and sTie-2 levels, we performed stepwise multiple regression analyses for OSA severity with age and BMI z score included as potential confounders. In the first model (adjusted only for age), AHI was independently associated with Ang-2 and sTie-2 levels (Table 3, standardized coefficients: 0.490 and 0.306, p<0.05). In the second model (adjusted for age and BMI z score) the severity of OSA accounted for 21.0% and 13.4% of the variance in Ang-2 and sTie-2 respectively. In addition, in the context of iterative variations on model 2, BMI z score contributed approximately 13.2% of the variance in Ang-2 and 24.3% of the variance in sTie-2 levels after adjusting for age and OSA severity (Table 3). However, when HOMA-IR was included in models, the proportion of variability in Ang-2 explained by AHI was reduced to 11.0, though AHI remained a statistically significant predictor (standardized coefficient: 0.382; p<0.001) despite a strong positive relationship between HOMA-IR and AHI (r2: 0.413, p<0.001). However, AHI was no longer a significant predictor of sTie-2 with HOMA-IR in the model (p = 0.242). Similarly, due to the shared variance between Tmax and AHI (r2: 0.298, p < 0.001) when Tmax was included in the model AHI was no longer a significant predictor of Ang-2 (p = 0.130) or sTie-2 (p = 0.681). In this comprehensive and adjusted model, and using a statistical approach that allows multiple risk factors to be taken into account (28), OSA severity accounted for 13% of the variance in Ang-2 levels (standardized coefficient: 0.184; p<0.01) and for 8% of the variance in sTie-2 levels (standardized coefficient: 0.184; p<0.05), with BMI z score accounting for 36% for Ang-2 and 38% of the variance for sTie-2 (standardized coefficient: 0.387; p<0.001 and 0.402; p<0.001, respectively). There were no apparent interactions in the fully adjusted model between OSA and BMI z score severity.
Table 3.
Multivariate stepwise regression analyses between sleep measures, HOMA-IR, Tmax, and Ang-2 or sTie-2 plasma levels.
| Variables | Ang-2 Plasma Levels | STie-2 Plasma Levels | ||
|---|---|---|---|---|
| Standardized Coefficients | P-value | Standardized Coefficients | P-value | |
| Age | −0.130 | 0.147 | −0.002 | 0.982 |
| BMI-z score | 0.373 | < 0.001 | 0.490 | < 0.001 |
| AHI† | 0.490 | < 0.001 | 0.306 | < 0.001 |
| HOMA-IR | 0.468 | < 0.001 | 0.690 | < 0.001 |
Data were log-transformed; Data for age, gender and race are not adjusted. Data for BMI z score are shown after adjusting for age only, and after adjusting for all sleep measures. All other data are shown after controlling for age and BMI z score.
AHI – obstructive apnea-hypopnea index; HOMA-IR – homeostatic model of insulin resistance
In a subset of 14 children with OSA (7 non-obese and 7 obese), the same protocol was repeated within 12–16 weeks after undergoing adenotonsillectomy. In this group, AHI decreased from 18.4±5.4 /hrTST to 3.7±4.7/hrTST (p<0.001) and no changes in BMI z scores were apparent. In parallel with the improvements in respiratory patterns during sleep, decreases in both Ang-2 (1.75±0.41 to 0.93±0.38 ng/ml; p<0.01) and sTie- 2 (14.6±3.7 to 9.3±2.7 ng/ml; p<0.01) were documented. In addition, hsCRP levels decreased after T&A (3.12±1.22 to 2.23±1.17 mg/dl ; p<0.01), but improvements in HOMA-IR were only borderline significant (2.1±0.9 to 1.7±0.8; p<0.05).
Discussion
This study shows that both obese children and children with OSA exhibit significantly higher Ang-2 and sTie-2 plasma levels when compared to healthy controls. However, a ceiling effect for both Ang-2 and sTie-2 levels emerges if both obesity and OSA are concurrently present. Significant linear bivariate associations were detected between Ang-2 plasma levels and BMI z score and the degree of respiratory disturbance during sleep as indicated by the AHI. However, particularly prominent associations between Ang-2 and sTie-2 emerged with Tmax and HOMA-IR, but neither total, LDL, HDL cholesterol or hsCRP levels exhibited any significant correlation. Finally, in a small subset of children with OSA who underwent adenotonsillectomy, follow-up assessments revealed significant decreases in AHI as well in Ang-2 and sTie-2 plasma concentrations after treatment. Taken together, these findings suggest that assessment of Ang-2 and sTie-2 plasma levels may provide reliable indicators for children at risk cardiometabolic dysfunction in the context of either obesity or OSA.
Several methodological considerations and study limitations deserve comment. First, blood collection procedures were carefully standardized to coincide with an overnight fast after the overnight sleep study. Furthermore, the overall sleep duration in the night before the blood sample draw was available from the polysomnogram, such that we could ascertain that differences in sleep duration were not present among the 4 subgroups and could have potentially accounted for the findings. Second, to reduce potential sources of variability all ELISA assays were performed concomitantly. Finally, non-snoring non-obese or obese children were recruited from the community rather than from clinical referral populations. As far as potential limitations, we should point out that the cohort included in the study as well as the group of children who were evaluated before and after treatment were relatively small, and therefore future validation studies in more extensive and potentially age and ethnicity diverse communities would be required to enable more accurate assessments of the predictive ability of Ang-2 and sTie-2 to serve as biomarkers of cardiometabolic dysfunction in the pediatric age range. Also, the roles of food intake and physical activity, or of single nucleotide polymorphisms in the variance of Ang-2 and sTie-2 were not evaluated (19, 29).
We are not aware of any published studies that examined Ang-2 or sTie-2 as potential indicators of cardiometabolic risk in children. In a recent population-based study conducted in northeast Germany in adults (14), both of these analytes were cross-sectionally associated with the metabolic syndrome. Globally similar findings were also reported in another large US-based cohort that evaluated the impact of diet-induced weight loss and exercise on angiogenic factors, whereby significant improvements in Ang-2 levels emerged after weight reduction (16), further attesting to the potential responsiveness of Ang-2 to treatment. The evidence linking Ang-2 and sTie-2 with cardiovascular and metabolic morbidity is further buttressed by the fact that Ang-2 levels were significantly higher in adult patients with type 2 diabetes who had evidence of microvascular angiopathy, and that Ang-2 levels were also strongly correlated with HOMA-IR (30). In another study in diabetic patients, Ang-2 levels were significantly and independently elevated among subjects with insulin therapy, diabetic polyneuropathy, and diabetic macroangiopathy, while sTie-2 was independently associated with HbA1c, insulin levels and HOMA-IR (31). Based on our current findings and aforementioned studies, it would appear that the potential role of assessing Ang-2 and sTie-2 as indicators of cardiometabolic risk can be expanded to at-risk children. Although the mechanisms underlying the putative link between OSA and plasma levels of Ang-2 and sTie-2 remain unclear at this stage, it is very likely that the systemic oxidative stress and inflammation that accompany the presence of OSA and obesity may alter the transcriptional and translational regulation of these important molecules whose roles in the microcirculation and metabolic function may reflect the interdependency between Ang- and sTie-2 and inflammatory mediators both systemically and at the tissue level (32–35).
We should also remark that in addition to changes in Ang-2 and sTie-2 levels with treatment, we also significant found significant improvements in hsCRP, a finding that has now been reported on multiple occasions and further proposed as a biomarker for residual OSA after T&A (36, 37). Furthermore, the anticipated effects of T&A on HOMA-IR are relatively small, a finding that is further recapitulated herein (38).
In summary, we have shown that obese children or those with OSA manifest elevated plasma levels of Ang-2 and sTie-2, particularly when such children exhibit evidence of insulin resistance or endothelial dysfunction. Furthermore, declines in Ang-2 and sTie-2 levels occur after treatment of OSA. Thus, assessment of these markers may permit future identification of obese or OSA children who may be at risk for cardiometabolic dysfunction.
What is known
Obstructive sleep apnea and obesity in children increase the risk for cardiometabolic morbidity
Angiopoietins such as Ang-2 and sTie-2 have been associated with atherosclerosis and insulin resistance
What this study adds
Children with obstructive sleep apnea and obesity have higher plasma levels of Ang-2 and sTie-2
Ang-2 and sTie-2 levels are independently associated with HOMA-IR and endothelial function as we as with BMI z score and sleep apnea severity
Treatment of sleep apnea reduces levels of Ang-2 and sTie-2 in children
Acknowledgments
DG provided the conceptual design of the project, analyzed data, and drafted the manuscript. AK and ZQ conducted ELISA assay experiments and analyzed data. DLS performed statistical analyses and modeling. MFP performed endothelial function experiments, reviewed data and provided critical input to data analysis. DK assisted with subject recruitment and data analysis. LKG provided conceptual initiative and design for the project, recruited subjects, performed endothelial function experiments, analyzed sleep data and metabolic data, and drafted components of the manuscript. LKG is also responsible for the financial support of the project. All authors have reviewed and approved the final version of the manuscript. Drs. Gozal are the guarantors of this work, had full access to all the data, and take full responsibility for the integrity of data and the accuracy of data analysis.
Funding: This project was supported by National Institutes of Health grant HL130984 (to LKG).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation. 2012;126:1770–1779. doi: 10.1161/CIRCULATIONAHA.111.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Shen W, Baxter-Jones A, Bell JD, Butte NF, Demerath EW, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatr Obes. 2012;7:e42–61. doi: 10.1111/j.2047-6310.2012.00073.x. [DOI] [PubMed] [Google Scholar]
- 3.Berends LM, Ozanne SE. Early determinants of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2012;26:569–580. doi: 10.1016/j.beem.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev. 2012;13:985–1000. doi: 10.1111/j.1467-789X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 5.Canas JA, Sweeten S, Balagopal PB. Biomarkers for cardiovascular risk in children. Curr Opin Cardiol. 2013;28:103–114. doi: 10.1097/HCO.0b013e32835dd0ce. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes Metab Syndr Obes. 2016;9:281–310. doi: 10.2147/DMSO.S95120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. American Academy of Pediatrics Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 9.Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev. 2006;7:247–259. doi: 10.1016/j.prrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Gileles-Hillel A, Kheirandish-Gozal L, Gozal D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat Rev Endocrinol. 2016;12(5):290–8. doi: 10.1038/nrendo.2016.22. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Ward NL, Dumont DJ. The angiopoietins and Tie2/Tek: adding to the complexity of cardiovascular development. Semin Cell Dev Biol. 2002;13:19–27. doi: 10.1006/scdb.2001.0288. [DOI] [PubMed] [Google Scholar]
- 13.Thorin-Trescases N, Thorin E. Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties. Expert Rev Mol Med. 2014;16:e17. doi: 10.1017/erm.2014.19. [DOI] [PubMed] [Google Scholar]
- 14.Lorbeer R, Baumeister SE, Dörr M, Nauck M, Grotevendt A, Schlesinger S, et al. Angiopoietin-2, its soluble receptor Tie-2, and metabolic syndrome components in a population-based sample. Obesity (Silver Spring) 2016 Oct;24(10):2038–41. doi: 10.1002/oby.21632. [DOI] [PubMed] [Google Scholar]
- 15.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005 Nov;29(11):1308–14. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 16.Kaess BM, Pedley A, Massaro JM, Larson MG, Corsini E, Hoffmann U, et al. Relation of vascular growth factors with CT-derived measures of body fat distribution: the Framingham Heart Study. J Clin Endocrinol Metab. 2012;97(3):987–94. doi: 10.1210/jc.2011-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David S, Kümpers P, Lukasz A, Kielstein JT, Haller H, Fliser D. Circulating angiopoietin-2 in essential hypertension: relation to atherosclerosis, vascular inflammation, and treatment with olmesartan/pravastatin. J Hypertens. 2009;27(8):1641–7. doi: 10.1097/HJH.0b013e32832be575. [DOI] [PubMed] [Google Scholar]
- 18.Trollope AF, Golledge J. Angiopoietins, abdominal aortic aneurysm and atherosclerosis. Atherosclerosis. 2011;214(2):237–43. doi: 10.1016/j.atherosclerosis.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieb W, Zachariah JP, Xanthakis V, Safa R, Chen MH, Sullivan LM, et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet. 2010;3(3):300–6. doi: 10.1161/CIRCGENETICS.109.914556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–14. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–91. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansell J, Henareh L, Agewall S, Norman M. Non-invasive assessment of endothelial function - relation between vasodilatory responses in skin microcirculation and brachial artery. Clinical physiology and functional imaging. 2004;24:317–322. doi: 10.1111/j.1475-097X.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- 25.Kheirandish-Gozal L, Khalyfa A, Gozal D, Bhattacharjee R, Wang Y. Endothelial dysfunction in children with obstructive sleep apnea is associated with epigenetic changes in the eNOS gene. Chest. 2013;143:971–977. doi: 10.1378/chest.12-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, Philby MF, Alonso-Álvarez ML, Mohammadi M, et al. Circulating plasma extracellular microvesicle miRNA cargo and endothelial dysfunction in OSA children. Am J Respir Crit Care Med. 2016;194:1116–1126. doi: 10.1164/rccm.201602-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.Brady AR. sbe21-Adjusted population attributable fractions from logistic regressions. STB Reprints. 1998;7:137–143. [Google Scholar]
- 29.Lieb W, Chen MH, Larson MG, et al. Genome-wide association study for endothelial growth factors. Circ Cardiovasc Genet. 2015;8:389–397. doi: 10.1161/CIRCGENETICS.114.000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Qian L, Yu ZQ. Serum angiopoietin-2 is associated with angiopathy in type 2 diabetes mellitus. J Diabet Comp. 2015;29:568–571. doi: 10.1016/j.jdiacomp.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Rasul S, Reiter MH, Ilhan A, Lampichler K, Wagner L, Kautzky-Willer A. Circulating angiopoietin-2 and soluble Tie-2 in type 2 diabetes mellitus: a cross-sectional study. Cardiovascular Diabetology. 2011;10:55. doi: 10.1186/1475-2840-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong JH, Kim K, Lim D, et al. Microvasculature remodeling in the mouse lower gut during inflammaging. Sci Rep. 2017;7:39848. doi: 10.1038/srep39848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Linge HM, Ochani K, Lin K, Miller EJ. Regulation of angiopoietin-2 secretion from human pulmonary microvascular endothelial cells. Exp Lung Res. 2016;42(7):335–345. doi: 10.1080/01902148.2016.1218977. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa-Vega N, Jordán B, Pérez-Luque EL, Parra-Laporte L, Garnelo S, Malacara JM. Effects of sleeve gastrectomy and rs9930506 FTO variants on angiopoietin/Tie-2 system in fat expansion and M1 macrophages recruitment in morbidly obese subjects. Endocrine. 54(3):700–713. doi: 10.1007/s12020-016-1070-y. 206. [DOI] [PubMed] [Google Scholar]
- 35.Giri H, Chandel S, Dwarakanath LS, Sreekumar S, Dixit M. Increased endothelial inflammation, sTie-2 and arginase activity in umbilical cords obtained from gestational diabetic mothers. PLoS One. 2013;8(12):e84546. doi: 10.1371/journal.pone.0084546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-Reactive Protein and obstructive sleep apnea syndrome in children. Frontiers in Bioscience. 2012;4:2410–22. doi: 10.2741/e553. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharjee R, Kheirandish-Gozal L, Kaditis AG, Verhulst S, Gozal D. C-reactive protein as a potential biomarker of residual obstructive sleep apnea following adenotonsillectomy in children. Sleep. 2016;39(2):283–91. doi: 10.5665/sleep.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koren D, Gozal D, Philby MF, Bhattacharjee R, Kheirandish-Gozal L. Impact of adenotonsillectomy on insulin resistance and lipoprotein profile in nonobese and obese children. CHEST. 2016;149(4):999–1010. doi: 10.1378/chest.15-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]


