Abstract
Objective
Our objective was to develop and validate a prognostic score for predicting mortality at time of extracorporeal membrane oxygenation (ECMO) initiation for children with respiratory failure. Pre-ECMO mortality prediction is important for determining center-specific risk-adjusted outcomes and counseling families.
Design
Multivariable logistic regression of a large international cohort of pediatric ECMO patients.
Setting
Multi institutional data.
Patients
Prognostic score development: 4352 children aged >7 days to <18 years, with an initial ECMO run for respiratory failure reported to the Extracorporeal Life Support Organization’s data registry during 2001–2013 were used for derivation (70%) and validation (30%). Bidirectional stepwise logistic regression was used to identify factors associated with mortality. Retained variables were assigned a score based on the odds of mortality with higher scores indicating greater mortality. External validation was accomplished using 2007 patients from the Pediatric Health Information System dataset.
Interventions
None
Measurements and Main Results
The Pediatric Pulmonary Rescue with Extracorporeal Membrane Oxygenation Prediction (P-PREP) score included mode of ECMO; pre-ECMO mechanical ventilation > 14 days; pre-ECMO severity of hypoxia; primary pulmonary diagnostic categories including, asthma, aspiration, respiratory syncytial virus, sepsis-induced respiratory failure, pertussis and ‘other’; and pre-ECMO comorbid conditions of cardiac arrest, cancer, renal and liver dysfunction. The area under the receiver operating characteristic curve for internal and external validation data-sets were 0.69 (95% CI, 0.67–0.71) and 0.66 (95% CI, 0.63–0.69).
Conclusions
P-PREP is a validated tool for predicting in-hospital mortality among children with respiratory failure receiving ECMO support.
Keywords: predictive score model, extracorporeal membrane oxygenation, respiratory failure, pediatric, decision support, validated
INTRODUCTION
The stated indications for extracorporeal membrane oxygenation (ECMO) candidacy include a “reversible condition with a high predicted mortality rate if conventional management is continued”. (1) However, lack of randomized control trials and prognostic prediction models complicates the ability to predict reversibility of the condition or mortality if ECMO is used.
Despite innovations in ECMO support, in-hospital mortality over the last decade for children > 30 days who receive ECMO for respiratory failure has remained stable at ~50%. (2, 3) Mortality is influenced by an increasing prevalence of comorbid conditions, in addition to the primary pulmonary diagnoses. (2, 3)
Recently, adult scoring systems have been developed using a variety of pre-ECMO factors, to help guide prognosis related to respiratory failure supported by ECMO. (4, 5) Pediatric scoring systems are not yet reported.
Our aim was to develop and validate a prognostic scoring tool to predict in-hospital mortality for children with respiratory failure who received ECMO by using pre-ECMO data available in the extracorporeal life support organization (ELSO) registry. The ELSO registry uses a standardized form to voluntarily collect ECMO patient data from over 449 international centers. The Pediatric-Pulmonary Rescue with ECMO Prediction (P-PREP) score we developed is beneficial for purposes of risk-adjusting for severity of illness when determining center-specific outcomes and family counseling.
METHODS
With approval from the ECMO Registry Committee of ELSO, we analyzed the de-identified data for all children aged > 7 days to < 18 years who required ECMO primarily for respiratory failure from 2001 through 2013 (http://www.elso.org accessed May 2014). Our primary outcome was in-hospital mortality after ECMO. We excluded neonates from our study defined as < 14 days for data from 2001–2007. We excluded infants < 7 days for data collected from 2008–2013 given a recent report demonstrating that pediatric and neonatal outcomes do not differ after 7 days of life. (6) Only those with an International Classification of Diseases-9 (ICD-9) diagnostic code that indicated a primary pulmonary diagnosis were included. Analysis was restricted to the first ECMO run and included demographic data, year of ECMO run, mode of ECMO, pre-ECMO variables, ICD-9 diagnostic codes, procedure and complication codes, and hospital outcome. ECMO mode was categorized as veno-arterial (VA) for any mode using an arterial cannula, and veno-venous (VV) for any mode without an arterial cannula. Pre-ECMO variables included pre-ECMO cardiac arrest, pre-ECMO therapies (high frequency ventilation, use of inhaled nitric oxide, ventilator settings, neuromuscular blockers, and fraction of inspired oxygen), and blood gases.
Prediction Variables
Predictors of mortality were limited to variables in the ELSO dataset and were selected by clinical reasoning and published reports. (2, 7–10) Severity of hypoxia was categorized using the partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio just prior to ECMO initiation (mild = 201–300mmHg, moderate = 101–200 mmHg, and severe = ≤100 mmHg). (11) Initial blood pH prior to ECMO was categorized using the middle quartiles compared to the lowest and highest quartile and categorized. ‘Low quartile was < 7.11, ‘middle quartiles’ was 7.11–7.34, and ‘high quartile was > 7.34. The primary pulmonary diagnostic categories were independently assigned by the authors (SB, LZ, DB) according to the ICD-9 codes with disagreements resolved by consensus (Supplemental Table 1). When more than one relevant ICD-9 code existed for the primary pulmonary diagnosis, we used the first relevant code recorded in the ELSO registry. Sepsis-induced respiratory failure was restricted to children whose primary diagnostic code was sepsis without additional pulmonary infections.
Because timing of injuries (with the exception of cardiac arrest), is not recorded in the ELSO registry we assumed that all comorbid conditions occurred prior to ECMO, including renal and liver dysfunction. Bleeding and thrombotic events were excluded given the known propensity for these conditions to occur after ECMO initiation. (12) Hematopoetic stem cell transplant patients were excluded due to small sample size (n=10, 9 died), known high mortality odds, and concerns for over-fitting the multivariable model. (2, 10)
Statistical analysis
Tool development and analysis utilized SAS 9.4. The modeling dataset consisting of 4352 ECMO runs was randomly divided into a development set (70%) and a validation set (30%), stratified by year. We used a complete-case analysis approach and only considered variables missing <10% of measurements. The development set was used for all variable selection and model building, and the validation set was retained to validate the final model. Candidate predictors of mortality were assessed with univariate logistic regression. Variables associated with mortality (p < 0.1) were included in the multivariate model building. The multivariate logistic regression model was built using a bidirectional stepwise selection process in which variables were added or removed from the model at each step based on the p-value from the residual chi-squared score statistic of ≤ 0.05. No variables were forced into the model.
The P-PREP tool was created using the estimated log-odds of mortality for patients supported with ECMO for respiratory failure. The log of the odds ratio for each variable was scaled and rounded to provide a simple, integer value.
External Validation
External validation was performed using the Pediatric Health Information System (PHIS), which includes data from more than 40 children’s hospitals. We queried for ECMO encounters using ICD-9 and charge codes from 2004 through the second quarter of 2014 (Supplemental Table 2). Patient inclusion required coding for both ECMO management and cannulation at age > 7days and an ICD-9 code for respiratory failure. (13) We excluded all cases with ICD-9 or charge codes for cardiac surgery, cardiac transplant, cystic fibrosis or mechanical support with a ventricular assist device. PHIS diagnosis and procedure classification used the same codes and rules as the ELSO data classifications. External validation was not performed using ECMO mode and severity of hypoxia because these variables are not collected in PHIS. Duration of mechanical ventilation is also not collected so we used the admission date as a proxy for ventilation start date given that all patients were admitted for respiratory failure.
RESULTS
A total of 4621 ECMO runs for pediatric respiratory failure were reported to ELSO of which 4498 (97.3%) represented first runs between 2001–2013. We analyzed 4352 (96.7% of first runs) patients after excluding 146 for missing data. The following variables (% missing) were excluded from the model due to missing > 10% of measurements: peak end expiratory pressure (53%), mean airway pressure (29%), peak inspiratory pressure (18%), ventilator rate (14%). The rates of missing measurements for variables included in the model were: fraction of inspired oxygen (7%), ventilation duration (6%), PaCO2 and PaO2 (5%), and sex (1%). Less than 1% of all other variables were missing. The distribution of ELSO patients across the development and validation data sets are shown in supplemental Table 3.
Characteristics of ECMO survivors and non-survivors among ELSO registry patients are shown in Table 1. Overall mortality was 43% and did not differ by sex. Later year of ECMO was associated with decreased mortality (p = 0.02) and 43% of all runs occurred from 2010–2013. The frequency of ECMO mode did not change over time and mortality with VV ECMO was lower compared to VA ECMO (p < 0.01). Median overall duration of ECMO support was 7.9 days (interquartile range 4.2–14.3 days). Median duration of mechanical ventilation prior to ECMO was 6.0 days, and overall 8% were ventilated > 14 days which was significantly associated with increased mortality (p < 0.001). Mortality risk increased with increasing severity of hypoxia (p = <0.001), with severe hypoxia representing 88% of patients. Lower pH increased mortality (p < 0.001). The most common primary pulmonary diagnostic category was ‘other’ (67%) followed by sepsis-induced respiratory failure (13%). Comparing across pulmonary diagnosis, death was more frequent in those with sepsis-induced respiratory failure and pertussis. The rates of co-existing comorbid conditions with each primary pulmonary diagnostic category are shown in supplemental Table 4.
Table 1.
Clinical Characteristics of Extracorporeal Life Support Registry Subjects
| Vital Status | ||||
|---|---|---|---|---|
| Alive (N=2495) |
Dead (N=1857) |
Overall (N=4352) |
P-value | |
| Patient Characteristics | n (%) | n (%) | n (%) | |
| Male | 1300 (53%) | 969 (53%) | 2269 (53%) | 0.926 |
| Age (years) | 0.003 | |||
| <1 | 1139 (46%) | 886 (48%) | 2025 (47%) | |
| 1–2 | 452 (18%) | 271 (15%) | 723 (17%) | |
| 3–5 | 242 (10%) | 165 (9%) | 407 (9%) | |
| 6–9 | 185 (7%) | 119 (6%) | 304 (7%) | |
| >9 | 477 (19%) | 416 (22%) | 893 (21%) | |
| Year of ECMO | 0.020 | |||
| 2001–2005 | 623 (25%) | 483 (26%) | 1106 (25%) | |
| 2006–2009 | 744 (30%) | 611 (33%) | 1355 (31%) | |
| 2010–2013 | 1128 (45%) | 763 (41%) | 1891 (43%) | |
| Veno-venous ECMO | 1239 (50%) | 626 (34%) | 1865 (43%) | <0.001 |
| Pre-ECMO therapies | ||||
| Mechanical ventilation > 14 days | 157 (7%) | 184 (11%) | 341 (8%) | <0.001 |
| High frequency ventilation | 1289 (52%) | 983 (53%) | 2272 (52%) | 0.408 |
| Inhaled nitric oxide | 1317 (53%) | 1007 (54%) | 2324 (53%) | 0.357 |
| Neuromuscular blockers | 1648 (66%) | 1197 (64%) | 2845 (65%) | 0.288 |
| PaO2/FiO2 | <0.001 | |||
| >300 mmHg | 59 (2%) | 19 (1%) | 78 (2%) | |
| 201–300 mmHg | 63 (3%) | 27 (1%) | 90 (2%) | |
| 101–200 mmHg | 227 (9%) | 128 (7%) | 355 (8%) | |
| ≤ 100 mmHg | 2146 (86%) | 1683 (91%) | 3829 (88%) | |
| pH | <0.001 | |||
| Median [Q1,Q3] | 7.25 [7.12,7.36] | 7.21 [7.10,7.32] | 7.23 [7.11, 7.34] | |
| < 7.11 | 647 (26%) | 572 (31%) | 1219 (28%) | |
| 7.11–7.34 | 1172 (47%) | 937 (50%) | 2109 (48%0 | |
| >7.34 | 676 (27%) | 348 (19%) | 1024 (24%) | |
| PaO2 (mmHg) | 52.0 [40.0, 68.0] | 50.0 [39.0, 64.0] | 51.0 [39.0, 66.0] | 0.030 |
| PaCO2 (mmHg) | 61.0 [47.0, 83.2] | 63.0 [48.0, 82.0] | 62.0 [47.0, 83.0] | 0.255 |
| Primary Pulmonary Diagnosis | ||||
| ‘Other’ | 1645 (66%) | 1260 (68%) | 2905 (67%) | |
| Asthma | 134 (5%) | 30 (2%) | 164 (4%) | <0.001 |
| Aspiration | 100 (4%) | 37 (2%) | 137 (3%) | <0.001 |
| Respiratory syncytial virus | 329 (13%) | 152 (8%) | 481 (11%) | <0.001 |
| Sepsis induced ARDS | 276 (11%) | 311 (17%) | 587 (13%) | <0.001 |
| Pertussis | 47 (2%) | 93 (5%) | 140 (3%) | <0.001 |
| Comorbid conditions | ||||
| Structural heart disease | 232 (9%) | 193 (10%) | 425 (10%) | 0.235 |
| Chronic lung disease | 85 (3%) | 76 (4%) | 161 (4%) | 0.256 |
| Immunodeficiency | 34 (1%) | 42 (2%) | 76 (2%) | 0.027 |
| Myocarditis | 22 (1%) | 29 (2%) | 51 (1%) | 0.046 |
| Pre-ECMO cardiac arrest | 316 (13%) | 335 (18%) | 651 (15%) | <0.001 |
| Cancer | 60 (2%) | 101 (5%) | 161 (4%0 | <0.001 |
| Acute renal failure | 301 (12%) | 522 (28%) | 823 (19%) | <0.001 |
| Acute liver necrosis | 8 (0%) | 68 (4%) | 76 (2%) | <0.001 |
Statistics presented are n (%) or Median [Q1, Q3]. 6% of ventilation duration, 5% of PaCO2 and PaO2, and 1% of sex is missing in the data; less than 1% of other variables are missing.
P-values are based on Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. P-values for Fisher’s exact test are approximated by Markov chain Monte Carlo for tables larger than 2 by 2.
Veno-arterial was used for any mode using an arterial cannula
Restricted to patients without additional pulmonary infections
ARDS = acute respiratory distress syndrome according to Berlin definitions; ECMO = extracorporeal membrane oxygenation
Risk factors associated with mortality at time of ECMO initiation by univariate analysis were age > 10 years, year of ECMO, veno-arterial ECMO, mechanical ventilation > 14 days, neuromuscular blockade, lower PaO2/FiO2 ratio, lower pH, sepsis-induced ARDS, pertussis, immunodeficiency, myocarditis, cardiac arrest, cancer, acute renal failure, and acute liver necrosis (see Table 2). Asthma, aspiration, and RSV were protective against mortality.
Table 2.
Pre-ECMO Candidate Variables Associated with Hospital Mortality by Univariate Analysis
| Pre-ECMO Variables | Odds ratio (90% CI) |
P-value |
|---|---|---|
| Male vs. female | 01.00 (0.88–1.13) | 0.962 |
| Age ≥10 years vs. <10 years | 1.249 (1.08–1.45) | 0.014 |
| Year of ECMO | 0.98 (0.96–1.00) | 0.036 |
| Veno-venous vs. Veno-arterial ECMOa | 0.52 (0.46–0.58) | <.0001 |
| Pre-ECMO therapies | ||
| Mechanical ventilation > 14 days | 1.73 (1.37–2.17) | <.0001 |
| High frequency ventilation | 1.05 (0.93–1.18) | 0.5435 |
| Inhaled nitric oxide | 1.03 (0.91–1.16) | 0.678 |
| Neuromuscular blockers | 0.88 (0.77–0.99) | 0.0830 |
| Pa02/FiO2 ratio | ||
| 201–300 vs. >300 mmHg | 1.53 (0.80–2.91) | 0.001 |
| 101–200 vs. >300 mmHg | 1.95 (1.15–3.31) | * |
| ≤ 100 vs. > 300 mmHg | 2.53 (1.55–4.13) | * |
| pH | ||
| pH >7.34 vs. 7.11–7.34 | 0.71 (0.60–0.82) | <.0001 |
| pH <7.11 vs. 7.11–7.34 | 1.17 (1.01–1.34) | * |
| Primary pulmonary diagnoses | ||
| Aspiration | 0.50 (0.34–0.74) | 0.0021 |
| Respiratory syncytial virus | 0.60 (0.49–0.74) | <.0001 |
| Sepsis induced ARDSb | 1.67 (1.40–1.99) | <.0001 |
| Pertussis | 2.34 (1.66–3.28) | <.0001 |
| Comorbid conditions | ||
| Structural heart disease | 1.05 (0.86–1.29) | 0.681 |
| Chronic lung disease | 1.20 (0.86–1.66) | 0.368 |
| Immunodeficiency | 1.62 (1.03–2.55) | 0.080 |
| Myocarditis | 1.89 (1.11–3.20) | 0.047 |
| Pre-ECMO cardiac arrest | 1.63 (1.38–1.92) | <.0001 |
| Cancer | 2.20 (1.61–3.00) | <.0001 |
| Acute renal failure | 3.24 (2.77–3.79) | <.0001 |
| Acute liver necrosis | 12.9 (5.91–28.00) | <.0001 |
Each row of this table represents a separate univariate model. Records with either the vital status or predictor missing were dropped from the analysis. P-values are based on a likelihood ratio.
6% of ventilation duration, 5% of PaCO2 and PaO2, and 1% of sex is missing in the data; less than 1% of other variables are missing.
Veno-arterial was used for any mode using an arterial cannula
Restricted to patients without additional pulmonary infections
ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation;
After final multiple logistic regression analysis, the P-PREP score retained mode of ECMO, mechanical ventilation > 14 days, PaO2/FiO2 ratio, pH, primary pulmonary diagnosis group, pre-ECMO cardiac arrest, cancer, acute renal failure, and acute liver necrosis and year of ECMO (Table 3). Year of ECMO use was not assigned a score because the tool is intended to assist with clinical decisions at time of ECMO initiation. A full description of the P-PREP score is shown in Table 3 and an online calculator is available at http://www.picuscientist.org/pprep. The prediction equation is: exp (Score/8.358–1.2769)/(1+ exp(Score/8.358–1.2769)). Individual predicted in-hospital ECMO mortality risk is calculated by applying the P-PREP score to Figure 1 which displays the 95% confidence interval (CI) for mortality of the development data set used to derive the score. Higher scores are associated with greater predicted mortality. P-PREP scores between −1 and +20 represented 95% of all patients and 45% of patients scored between +3 to +8. All patients with score of ≤ −10 survived (n=15) and all patients with scores ≥42 died (n=2). Figure 2 displays the distribution of primary pulmonary diagnoses and comorbid conditions across the P-PREP scores.
Table 3.
The P-PREP Score at ECMO Initiation for Pediatric Respiratory Failure
| Prognostic Variables | Adjusted odds ratio (95% CI) | Score |
|---|---|---|
| Veno-arterial ECMOa | Reference | 0 |
| Veno-venous ECMO | 0.62 (0.52, 0.73) | −4 |
| Mechanical ventilation ≤14 days | Reference | 0 |
| Mechanical ventilation > 14 days | 1.84 (1.38, 2.45) | 5 |
| PaO2/FiO2 ratio | ||
| > 300 mmHg | Reference | 0 |
| 201–300 | 1.64 (0.70, 3.83) | 4 |
| 101–200 | 1.78 (0.89, 3.59) | 5 |
| ≤ 100 | 2.31 (1.21, 4.40) | 7 |
| pH | ||
| < 7.11 vs. 7.11–7.34 | 1.16 (0.96, 1.41) | 1 |
| > 7.34 vs. 7.11.–7.34 | 0.84 (0.69, 1.03 | −1 |
| Primary pulmonary diagnoses (select only one) | ||
| All other | ------------------- | 0 |
| Asthma | 0.39 (0.24, 0.66) | −8 |
| Aspiration | 0.55 (0.34, 0.89) | −5 |
| Respiratory syncytial virus | 0.62 (0.47, 0.82) | −4 |
| Sepsis induced ARDSb | 1.39 (1.10, 1.75) | 3 |
| Pertussis | 1.88 (1.22, 2.90) | 5 |
| Comorbid conditions | ||
| Pre-ECMO cardiac arrest | 1.44 (1.16, 1.80) | 3 |
| Cancer | 2.07 (1.37, 3.13) | 6 |
| Acute renal failure | 2.66 (2.17, 3.26) | 8 |
| Acute liver necrosis | 8.61 (2.97, 24.90) | 18 |
| Year of ECMO | 0.97 (0.95, 0.99) | – |
| Total Score | −13 to 53 |
An online calculator is available at www.picuscientist.org/pprep
The prediction equation is: exp(Score/8.358–1.2769)/(1+ exp(Score/8.358–1.2769)).
Veno-arterial is used for any mode using an arterial cannula
Restricted to patients without additional pulmonary infections
ARDS = acute respiratory distress syndrome according to Berlin definitions; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; P-PREP = pediatric pulmonary rescue with extracorporeal membrane oxygenation prediction
Figure 1.
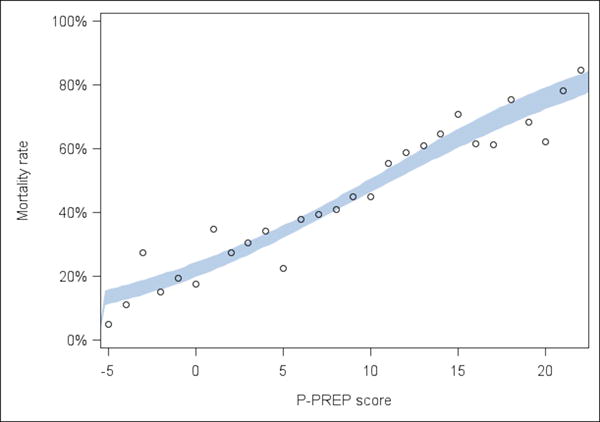
Observed death rate in the development set used to create the Pediatric-Pulmonary Rescue with Extracorporeal Membrane Oxygenation Prediction tool. Each dot represents the observed death rate in the development dataset. The shaded band is a 95% pointwise confidence band obtained from logistic regression on the score in the derivation dataset. Scores <−5 and > +21 are not represented because of small numbers of patients (n<18) in the development set.
P-PREP = Pediatric Pulmonary Rescue with Extracorporeal Membrane Oxygenation Prediction
Figure 2.
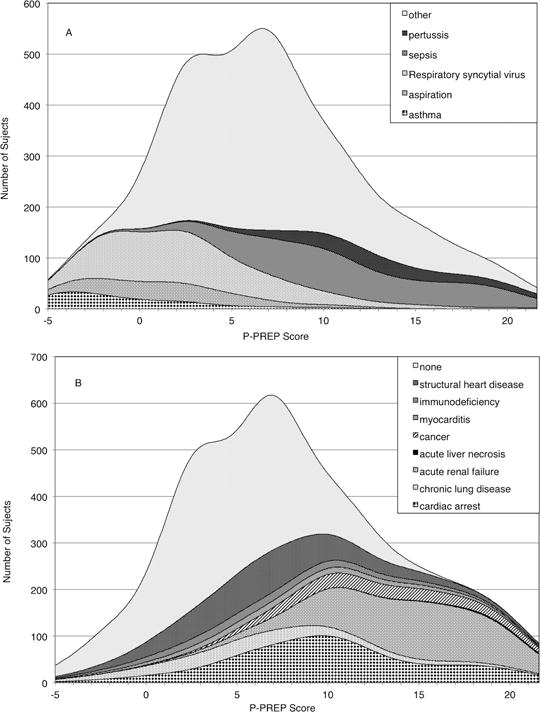
Stacked kernel density plots of the P-PREP score plotting for (A) Primary Pulmonary Diagnoses and (B) Comorbid Conditions.
P-PREP = Pediatric Pulmonary Rescue with Extracorporeal Membrane Oxygenation Prediction
Model performance
The areas under the receiver-operating characteristic curves (AUROC) in the development and internal validation sets were reasonable at 0.69 (95% CI: 0.67 – 0.71) and 0.66 (95% CI: 0.63 – 0.69) respectively. Hosmer-Lemeshow p values of 0.34 and 0.44 in the development and internal validation sets indicated good model calibration. Despite decreased mortality from 44% to 40% during 2001–2009 (n = 2461) to 2009–2013 (n=1891), the P-PREP score exhibited similar performance across both eras (2001–2009; c = 0.68 [95% CI, 0.64 – 0.72] and in 2010–2013; c = 0.66 [95% CI, 0.61 – 0.71]).
The model was externally validated using a PHIS dataset consisting of 2007 patients with respiratory failure treated with ECMO and included a similar distribution of primary pulmonary diagnoses compared to data obtained from the ELSO registry with the exception of a larger proportion of primary sepsis-induced respiratory failure (26% versus 11%) and acute renal failure (38% vs. 19%) (supplemental Table 1). External validation was reasonable with an AUROC of 0.69 (95% CI: 0.67–0.71).
DISCUSSION
We report on a prognostic tool to predict in-hospital mortality when ECMO is used for respiratory failure in pediatric patients. The tool was developed and validated using 4352 patients in the ELSO registry over a 12-year period and externally validated using the PHIS administrative database. Our tool provides a real time prognostication of ECMO mortality to compare against the risks of the current therapies, including the risk of transporting to an ECMO center (14, 15).
Mortality among pediatric patients with acute respiratory failure is significantly higher when there are two or more extra pulmonary organ system failures compared to one or none. (2, 14–20) Thus, the decision to transition to ECMO is difficult when comorbid conditions exist because the risk of death with conventional therapies may appear similar to the risk of death if ECMO is used. Often the transition to ECMO occurs only after a protracted treatment course or a sentinel event. We found that 8% of ELSO patients received mechanical ventilation for >14 days and >15% had a cardiac arrest.
Pre-ECMO assessment of mortality allows for risk stratification, which is increasingly important as we attempt to deal with the technical, ethical, and administrative issues inherent to high-risk, high-cost rescue therapies such as ECMO. An assessment of risk of mortality can also help risk-adjust for severity of illness when determining center-specific outcomes, patient selection in clinical trials, and guiding conversations related to ECMO training and maintenance requirements.
Family counseling relative to clinical outcomes is currently limited by lack of trials and prognostic tools. The P-PREP score incorporates a variety of pre-ECMO clinical characteristics known to influence mortality into a single prognostic score that is unique to each patient. As therapies are deployed and the clinical condition evolves, the score can be re-calculated to re-calibrate the family and caregivers to the current risk of in-hospital mortality for pediatric respiratory failure, should ECMO be used.
Prior Prediction Models
The most recent adult predictive tool; the RESP score, also used the ELSO registry and was developed using 2355 adults over 13 years with acute respiratory failure. (12) Acute non-pulmonary-associated infections and central nervous system dysfunction were strongly associated with mortality and heavily influenced the RESP score. The RESP tool was validated using the PRESERVE data set consisting of 140 patients from three French intensive care units diagnosed exclusively with ARDS, and achieved an AUROC of 0.74 (95% CI: 0.72–0.76). (4, 12) Only duration of mechanical ventilation and pre-ECMO cardiac arrest were common to both the RESP and P-PREP tools.
Patients with a P-PREP score between 5 and 15, and RESP patients in category III, represent ~50% of both cohorts and have an estimated risk of mortality between 40–60% which closely approximates overall mortality, thereby reducing discrimination in both tools.
The RESP study utilized enrollment and validation methods that enhanced its discrimination compared to the P-PREP tool. The RESP study excluded over 30% of patients for missing data compared with exclusion of only 5% of patients in our study which resulted in a larger proportion of our patients falling into the primary pulmonary diagnostic category “other” (67% in our cohort = “other” compared to ~30% in the RESP cohort). Analysis of the P-PREP tool after exclusion of the category “other” increases the AUROC to 0.74 (95% CI: 0.69–0.79) nearly matching the performance of the RESP score. The performance of the RESP tool is further enhanced by a validation cohort which is small (represented only ~ 5% of the development cohort) and discrete (contained only patients with ARDS compared to the development cohort which included multiple types of ‘acute respiratory failure’).
Limitations
Limitations include those inherent to the use of a retrospective registry-based analysis. Specifically, the influence of ECMO center characteristics, physiologic data, specific timing of injuries, interventions, morbidities and functional status of survivors is not incorporated into the tool. Center infrastructure characteristics are likely to affect patient outcomes particularly for the highest risk patients who often require timely initiation of advanced treatments such as dialysis and bronchoscopy. (6, 13, 21–23) Although timing of injuries is not reported to ELSO, Gupta found that renal function commonly improved after ECMO initiation suggesting most acute kidney injury occurs prior to ECMO. (24)
Misclassification bias may have been introduced because many ICD-9 related conditions lack explicit definitions. We analyzed ‘acute renal failure’ first using ICD-9 codes and second using creatinine > 1.5 mg/dL (which is also reported in the ELSO registry), and found no change in the tool’s performance. Some children had multiple primary pulmonary diagnostic codes making it difficult to be certain that the first code in sequence truly represented the primary etiology for respiratory failure. Our tool prognosticates for mode of ECMO at time of cannulation, however our model assumed VA for any patient with an arterial cannula at any point in time. ICD-9 coding also lacks illness severity scaling and activity which may be particularly important for patients with immune dysfunction which was not included in our model.
Our external validation was limited to variables in the PHIS database thereby excluding mode of ECMO and severity of hypoxia from analysis. Furthermore, duration of mechanical ventilation may be overestimated since patients could have been intubated at any time since admission. Given that all PHIS hospitals except two have submitted data to ELSO within the last 5 years it is likely that there is patient overlap between the two registries. Also, the eras of comparison between ELSO (2001–2013) and PHIS (2004–2014) did not entirely over-lap.
Finally, we lack a comparison database of non-ECMO patients with similar illnesses and thus our findings are applicable only to pediatric patients with respiratory failure for whom ECMO is chosen as a therapy and are not applicable to patients who do not receive ECMO.
CONCLUSIONS
The P-PREP tool is a validated tool to predict in-hospital mortality for children who receive ECMO for respiratory failure. It demonstrates that comorbid conditions strongly influence overall mortality regardless of primary pulmonary diagnosis. We anticipate it will be most useful for risk stratification and family counseling.
Supplementary Material
Acknowledgments
The authors thank the Extracorporeal Life Support Organization for providing data to conduct this research. We also thank Renee Enriques and Mihai Virtosu for building the online calculator.
Grants and Support: No relevant disclosures.
Copyright form disclosures:
Dr. Bailly received support for article research from the National Institutes of Health (NIH) and received funding from the Primary Children’s Hospital Early Career Development Award, the NIH loan repayment grant, and Orca Health (consumer mobile outpatient decision support). Dr. Thiagarajan disclosed other support (Co-Chair ECMO registry of ELSO). His institution received funding from Bristol Myer Squibb - Events Adjudication Committee.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Annich GM, Lynch WR, MacLaren G, Wilson JM, Bartlett RH. Extracorporeal Cardiopulmonary Support in Critical Care. Fourth. Ann Arbor: Extracorporeal Life Support Organization; 2012. [Google Scholar]
- 2.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 3.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59:202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt C-E, Trouillet J-L, Brechot N, Nieszkowska A, Dupont H, Ouattara A, Leprince P, Chastre J, Combes A. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A, Ranieri VM, Gattinoni L, Landoni G, Holzgraefe B, Beutel G, Zangrillo A. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275–281. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KM, McMullan DM, Bratton SL, Rycus P, Kinsella JP, Brogan TV. Is age at initiation of extracorporeal life support associated with mortality and intraventricular hemorrhage in neonates with respiratory failure? J Perinatol. 2014;34:386–391. doi: 10.1038/jp.2013.156. [DOI] [PubMed] [Google Scholar]
- 7.Nance ML, Nadkarni VM, Hedrick HL, Cullen JA, Wiebe DJ. Effect of preextracorporeal membrane oxygenation ventilation days and age on extracorporeal membrane oxygenation survival in critically ill children. J Pediatr Surg. 2009;44:1606–1610. doi: 10.1016/j.jpedsurg.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Minneci PC, Kilbaugh TJ, Chandler HK, Behar BJ, Localio AR, Deans KJ. Factors associated with mortality in pediatric patients requiring extracorporeal life support for severe pneumonia. Pediatr Crit Care Med. 2013;14:e26–33. doi: 10.1097/PCC.0b013e31826e7254. [DOI] [PubMed] [Google Scholar]
- 9.Pathan N, Ridout DA, Smith E, Goldman AP, Brown KL. Predictors of outcome for children requiring respiratory extra-corporeal life support: implications for inclusion and exclusion criteria. Intensive Care Med. 2008;34:2256–2263. doi: 10.1007/s00134-008-1232-3. [DOI] [PubMed] [Google Scholar]
- 10.Gow KW, Heiss KF, Wulkan ML, Katzenstein HM, Rosenberg ES, Heard ML, Rycus PT, Fortenberry JD. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Crit Care Med. 2009;37:1308–1316. doi: 10.1097/CCM.0b013e31819cf01a. [DOI] [PubMed] [Google Scholar]
- 11.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, Pellegrino V, Combes A, Pilcher D. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 13.Freeman CL, Bennett TD, Casper TC, Larsen GY, Hubbard A, Wilkes J, Bratton SL. Pediatric and neonatal extracorporeal membrane oxygenation: does center volume impact mortality? Crit Care Med. 2014;42:512–519. doi: 10.1097/01.ccm.0000435674.83682.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flori H, Glidden D, Rutherford G, Matthay M. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman J, Akhtar S, Caldwell E, Rubenfeld G. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124:87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 16.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 17.Roupie E, Lepage E, Wysocki M, Fagon JY, Chastre J, Dreyfuss D, Mentec H, Carlet J, Brun-Buisson C, Lemaire F, Brochard L. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation. Société de Réanimation de Langue Française. Intensive Care Med. 1999;25:920–929. doi: 10.1007/s001340050983. [DOI] [PubMed] [Google Scholar]
- 18.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F. Epidemiology and outcome of acute lung injury in European intensive care units Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 19.Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, Pozo M, Gómez A, Baredes N, Jannello G, Osatnik J. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Keenan HT, Bratton SL, Martin LD, Crawford SW, Weiss NS. Outcome of children who require mechanical ventilatory support after bone marrow transplantation. Crit Care Med. 2000;28:830–835. doi: 10.1097/00003246-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 21.Karamlou T, Vafaeezadeh M, Parrish AM, Cohen GA, Welke KF, Permut L, McMullan DM. Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg. 2013;145:470–475. doi: 10.1016/j.jtcvs.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Jen HC, Shew SB. Hospital readmissions and survival after nonneonatal pediatric ECMO. Pediatrics. 2010;125:1217–1223. doi: 10.1542/peds.2009-0696. [DOI] [PubMed] [Google Scholar]
- 23.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, Annich GM. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta P, Carlson J, Wells D, Selakovich P, Robertson MJ, Gossett JM, Fontenot EE, Steiner MB. Relationship Between Renal Function and Extracorporeal Membrane Oxygenation Use: A Single-Center Experience. Artif Organs. 2015;39:369–374. doi: 10.1111/aor.12379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


