Abstract
Purpose
Performing a sentinel lymph node biopsy (SLNB) is the standard of care for axillary nodal staging in patients with invasive breast cancer and clinically negative nodes. The procedure provides valuable staging information with few complications when performed by experienced surgeons. However, variation in proficiency exists for this procedure, and a great amount of experience is required to master the technique, especially when faced with challenging cases. The purpose of this paper is to provide a troubleshooting guide for commonly encountered technical difficulties in SLNB, and offer potential solutions so that surgeons can improve their own technical performance from the collective knowledge of experienced specialists in the field.
Methods
Information was obtained from a convenience sample of six experienced breast cancer specialists, each actively involved in training surgeons and residents/fellows in SLNB. Each surgeon responded to a structured interview in order to provide salient points of the SLNB procedure.
Results
Four of the key opinion surgical specialists provided their perspective using technetium-99m sulfur colloid, and two shared their experience using blue dye only. Distinct categories of commonly encountered problem scenarios were presented and agreed upon by the panel of surgeons. The responses to each of these scenarios were collected and organized into a troubleshooting guide.
Discussion
We present a compilation of “tips” organized as a troubleshooting guide to be used to guide surgeons of varying levels of experience when encountering technical difficulties with SLNB.
Introduction
Sentinel lymph node biopsy (SLNB) is the standard of care for axillary staging in clinically node negative breast cancer.1-6 Prior studies confirm a high degree of variation in the technical proficiency of SLNB.7-10 The importance of experience in the accuracy of sentinel node identification was illustrated in a recent multicenter trial.11 After five training cases, the success rates for individual surgeons identifying a sentinel lymph node (SLN) ranged from 79 to 98 percent. Although the false negative rate varied between 0 and 29 percent among the participating surgeons, proficiency improved with increasing number of cases. Furthermore, Cox et al showed that surgeons who performed more than six SLNB per month had lower failure rates than surgeons who performed fewer SLNB procedures.12 This data is relevant since the majority of breast cancer procedures are performed by surgeons whose practices may not be predominately or exclusively dedicated to breast cancer.13-15 Proper surgical technique in SLNB influences outcomes and minimizes the risk of understaging and undertreating patients.
The purpose of this paper is to present a troubleshooting guide of the most commonly encountered problems in SLNB and potential solutions created from the combined experience of breast surgeons active in training and evaluating SLNB performance. Our goal is that this troubleshooting guide will help improve the technical performance and success rates of SLNB, especially when presented with technically challenging cases.
Data Generation
A select panel of seven experienced surgeons with recognized expertise in SLNB was contacted for participation in this project. All were active breast cancer surgeons recognized for their expertise in the field and involved in training surgeons in SLNB. All but one of the seven surgeons approached agreed to participate. The participating surgeons reviewed, edited, and agreed upon the technical steps included in the description of the SLNB procedure.
Topics for inclusion in the troubleshooting guide were vetted individually among the group, and consensus was achieved on major areas to be discussed. These topics directly informed the problem scenarios to be addressed. Individual semi-structured interviews were then conducted with each surgeon in order to ascertain their responses and advice pertaining to these scenarios, including solutions to common pitfalls and technical conundrums with SLNB. From the semi-structured interviews, common themes emerged and were included as content in the formal troubleshooting guide. Additional suggestions and tips were included separately in figures detailing further approaches to addressing the problem scenarios.
SLNB Technique
Debate exists regarding the optimal technique for SLNB. Each surgeon needs to find the method that works best for their practice. The literature indicates that sentinel node identification rates can be optimized, and false negative rates can be minimized by using dual agents as opposed to a single agent, particularly for surgeons with limited experience and in cases where misidentification and false negative rates are known to be higher (e.g. neoadjuvant therapy, prior breast/axillary surgery, and high BMI).16, 17 Consideration can also be given to employing lymphoscintigraphy in higher risk cases. A checklist of key steps for SLNB is presented (Table 1).
Table 1.
Checklist of key steps for the sentinel lymph node biopsy procedure in breast cancer.
| □ | Consider SLNB for all invasive breast cancer and cases of DCIS undergoing mastectomy |
| □ | Utilize dual tracer (blue dye and radiocolloid) to optimize identification and reduce false negative rates, especially following neoadjuvant therapy, prior breast/axillary surgery or in patients with elevated BMI |
| □ | Consider IV prophylaxis if blue dye utilized |
| □ | Inject blue dye around tumor periphery, at the palpable edge of the biopsy cavity, or into the subareolar plexus |
| □ | Inject radiocolloid peritumorally, intradermally, or into the subareolar plexus |
| □ | Avoid injection into the tumor itself or into a seroma cavity |
| □ | Consider lower dose or subareolar injection for tumors located in the axillary tail |
| □ | Massage breast can be performed |
| □ | Remove any suspicious palpable nodes |
Abbreviations: SLNB=sentinel lymph node biopsy; DCIS=ductal carcinoma in situ; BMI=body mass index; IV=intravenous
Blue dye method
The surgeon injects typically 3 to 5 mL of blue dye (isosulfan blue or diluted methylene blue) around the tumor periphery, at the palpable edge of the biopsy cavity, or into the subareolar plexus. Subareolar injection may be preferable to avoid staining of the lumpectomy cavity. Intradermal injections of blue dye are avoided to prevent tattooing of the breast or dermal necrosis. The use of isosulfan blue dye is associated with anaphylactic reactions in 0.7 to 1.1 percent of cases.18-20 Patients should be screened for make-up allergies (contain blue dye) and prior tattooing, as both are associated with an increased risk of allergic reaction. Prophylaxis can be achieved by administering any one of the following: 100 mg of hydrocortisone, 20 mg of methylprednisolone, 4 mg of dexamethasone, 50 mg of diphenhydramine, or 20 mg of famotidine intravenously. Prophylaxis appears to decrease the severity but not the incidence of dye reactions.18 Neither of the two contributing surgeons who map with blue dye alone utilize prophylaxis given the low reported rate of anaphylaxis. In cases of severe reactions leading to cardiopulmonary collapse, the procedure should be aborted and resumed sometime after the patient is stabilized.
Methylene blue has been proposed as an alternative to isosulfan blue dye, but false negative rates have not been validated with studies including immediate completion ALND, as has been performed for isosulfan blue. Methylene blue is also associated with side effects including skin necrosis and induration, as well as reports of pulmonary edema and central nervous system reactions in patients who take serotonin-acting medications.21-23 Side effects can be potentially minimized by diluting the methylene blue (1:7 dilution; 1.25mg/mL – 0.5cc of methylene blue mixed with 3.5cc of normal saline).24 Despite these issues, methylene blue is widely used and has become the “de-facto” standard in the United States because of difficulties obtaining Lymphazurin, and the cost of generic 1% isosulfan blue dye (available from a single manufacturer [Mylan]). Of note, isosulfan blue can be made by a compounded pharmacy (utilized by one of the authors when Lymphazurin was in short supply).
When performing SLNB using blue dye only it is important not to inject the dye into the tumor itself because the lymphatics can be occluded by tumor. It is also important not to inject into a seroma cavity following an excisional biopsy, as the seroma itself does not contain lymphatic channels. Pericavitary injection is preferred to a subareolar technique when upper outer quadrant excisions have already been performed, as the scar can obstruct lymphatic drainage from the nipple-areolar complex to the axilla leading to a failure of mapping. Breast massage can be performed for approximately five minutes to dilate the breast lymphatics. The axillary fascia is entered through an axillary incision. Some surgeons prefer the incision at the inferior border of the axillary hair and extend medially to the edge of the pectoralis major muscle. A careful search is made for blue lymphatic channels leading to blue-stained lymph nodes. All blue lymph nodes and any lymph nodes at the end of a blue lymphatic channel are removed and designated as SLNs.25 The dye-filled tract is dissected to the first blue lymph node. If possible, the tract is followed proximally to the tail of the breast to ensure that the identified lymph node is the most proximal lymph node and thus, the sentinel node. Care must be taken to identify proximal blue nodes because the dye transit time is rapid and blue staining of distal, nonsentinel axillary lymph nodes is not uncommon.3 Failure to consider the node at the end of a blue lymphatic channel as a sentinel node whether or not the node itself appears blue, and failure to remove the most proximal blue lymph node(s) are the two most common technical errors. Suspicious palpable nodes should also be removed for evaluation, as a lymph node replaced with tumor is not likely to take up the localizing dye.
Radiocolloid method
Radioactive tracer may be injected peritumorally, intradermally, or into the subareolar plexus. There is ongoing debate about the best site for injection. Typically 0.5 mCi is injected the day of the surgery, or 2.5 mCi is injected the day before as the half-life for technetium-99m sulfur colloid is six hours. As with blue dye injection, technetium should not be injected directly into the tumor or into a seroma cavity. The “10 percent rule” is a guideline referring to removal of all SLNs with counts over 10 percent of the most radioactive node.26 Surgeons should confirm ex-vivo counts to limit falsely positive counts due to in vivo scatter. All nodes that qualify as sentinel nodes should be removed; not just the hottest nodes. A median of 2-3 SLNs is removed.27 Suspicious palpable nodes should also be removed for evaluation, as a lymph node replaced with tumor is not likely to absorb technetium.
Troubleshooting Guide for Sentinel Lymph Node Biopsy
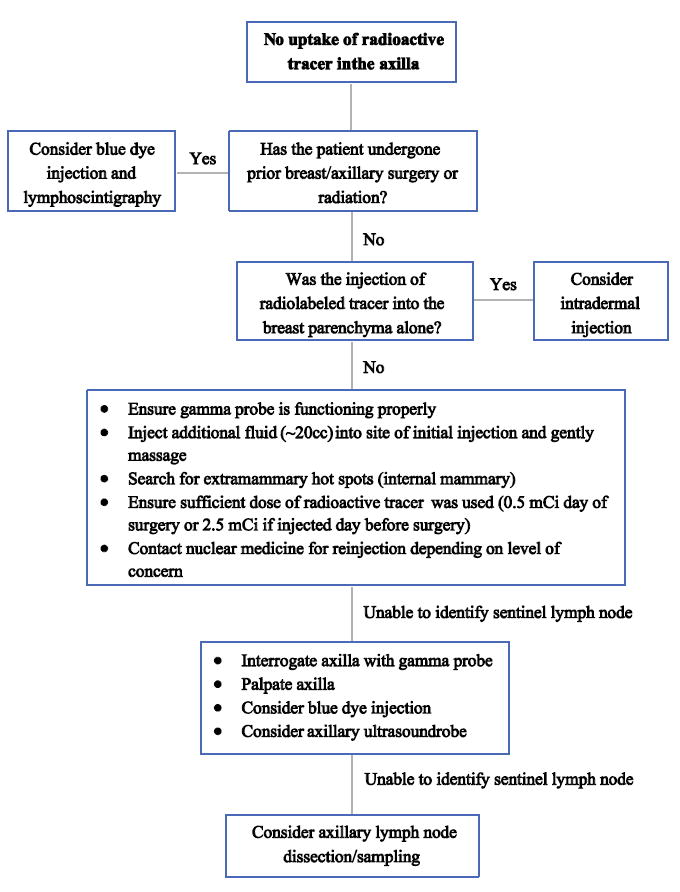
1. No uptake of radioactive tracer in the axilla
This problem is seen most often when the radiotracer is injected into the breast parenchyma alone. The use of a small dermal injection of tracer greatly enhances the activity that reaches the axillary nodes.28 Some surgeons use the dermal injection technique exclusively as it leads to smaller areas of radioactivity diffusion. However, it should be noted that extra-axillary sites of drainage are rarely identified if only intradermal injections are used.29
In cases where there is difficulty finding a pre-incision hot spot with a gamma probe, there are some potential remedies. First, be sure the gamma probe is functioning and set to the appropriate settings to maximize the sensitivity of the audio feedback. If there is still difficulty identifying the hot spot, the next step is to inject blue dye to increase the SLN identification rate. Often a SLN can still be identified after an incision has been made and the gamma probe is placed into the axilla. Therefore, proceed with an incision in the axilla and re-evaluate the nodes with the gamma probe. This is especially true in patients with higher BMI. Another technique involves injecting fluid into the site of the technetium injection, using 10 to 40 mL of sterile saline or local anesthetic. This increases the interstitial pressures, which forces more tracer into the lymphatic channels. It is recommended to perform gentle massage at the injection site after which the pre-incision hot spot is reassessed with the gamma probe. This process may be repeated as needed if a hot spot is still not identified.
Under circumstances of prior breast/axillary surgery or prior radiation therapy, lymphatic channels may be disrupted causing alternate drainage pathways to be formed. In these situations, a lymphoscintigraphy can be used preoperatively to identify the appropriate drainage basin. Using dual tracer with radiocolloid and blue dye can be considered as well. It is also important to palpate the axilla and resect any palpable abnormal nodes as SLNs. Intra-operative ultrasound may help identify nodes. If all else fails, the default option is to proceed to ALND or axillary sampling. However, first consider how important the nodal staging information is and the likelihood of nodal positivity. For instance, not identifying a SLN in a T1a low grade, ER+, Her2- tumor in an older woman may not require an ALND. Figure 1 depicts the algorithm for troubleshooting no uptake of radioactive tracer.
Figure 1.
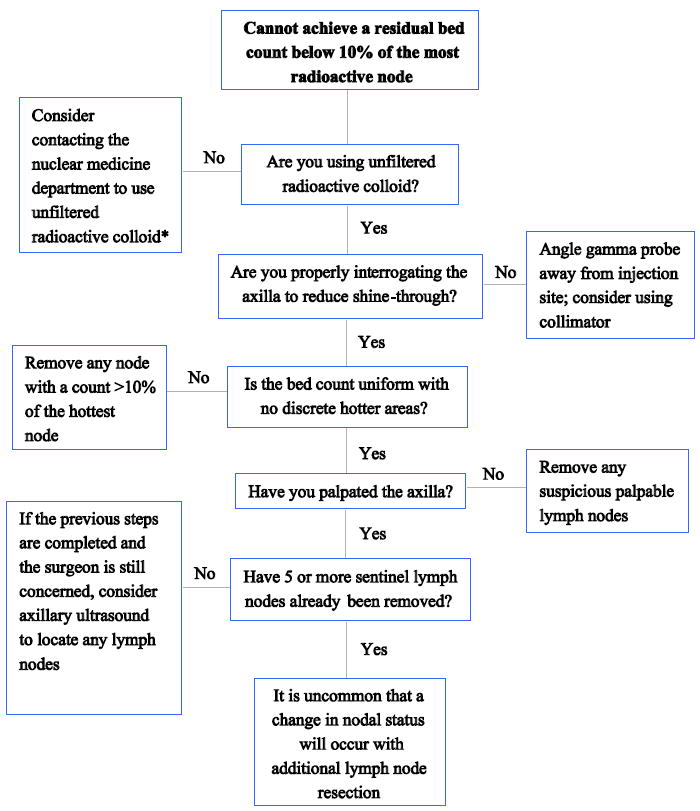
2. Cannot achieve a residual bed count below 10% of the most radioactive node
In the event a surgeon is confronted with a “high residual bed count” in the axilla, it is important to be sure that all of the “hottest” nodes have been removed. If the remaining bed counts are uniform, with no ‘discreet’ areas of greater radioactivity found and the bed count remains over 10%, then the surgeon need not remove any other nodes unless they are suspicious by palpation. Data indicates that once four or five SLNs have been resected, the value of additional SLNs is extremely low.30,31 Some have reported that taking three SLNs is sufficient,32 but this has been controversial.33 Additional details for addressing this situation are presented in Figure 2.
Figure 2.
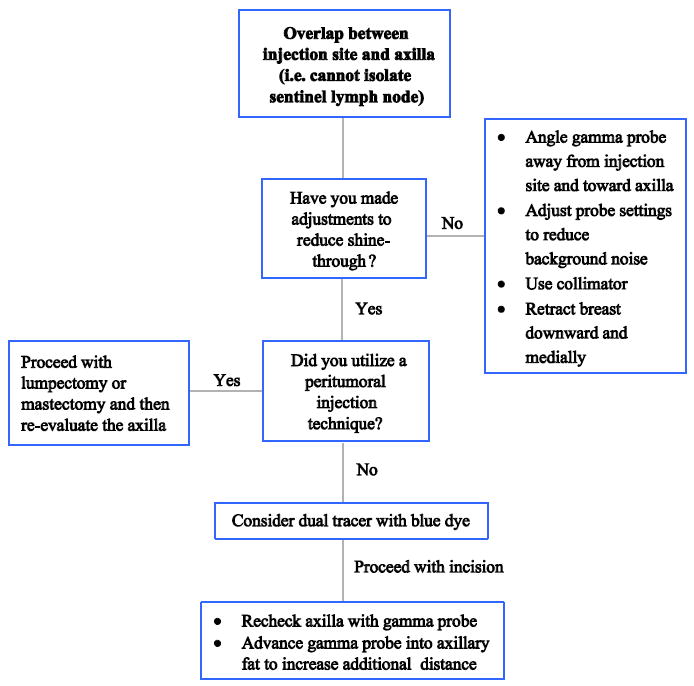
3. Overlap between injection site and axilla (i.e. cannot isolate sentinel node)
The problem of overlap of the injection site diffusion zone with the axillary nodes is often an issue with tumors located in the upper outer quadrant and axillary tail of the breast. Utilizing a subareolar injection technique as opposed to a peritumoral injection increases the distance between injection site and axilla, minimizing the potential for overlap. There is an abundance of data in support of this technique.34 A second potential solution to this problem is to limit the volume of injection as much as possible. For tumors located in the upper outer quadrant/axillary tail, this may be a good situation in which to use small volume intradermal injections alone, as it would be rare for lymphatic drainage in this area to go to extra-axillary sites. The use of small volumes limits the size of the diffusion zone, facilitating identification of the axillary hot spots. Both of these solutions require the surgeon to anticipate the problem prior to injection. Additional potential solutions are illustrated in Figure 3.
Figure 3.
4. Radioactive node identified in internal mammary site
Surgeons have debated the utility of dissecting nodes from the internal mammary (IM) chain given the relative lack of familiarity with the procedure and the associated potential risks (e.g. pneumothorax, bleeding). Current evidence indicates that the prognostic significance of sentinel nodes in the IM chain is similar to sentinel nodes in the axilla.35 Other reports demonstrate the incidence of isolated positive IM nodes (i.e. without concurrent positive axillary SLNs) to be low.36 Therefore, evidence suggests that the status of the axillary SLNs also reflect the status of the IM nodes in the vast majority of cases. Removal of the IM nodes may not change treatment, particularly if radiation oncologists treat IM nodes in patients with positive axillary nodes.37 Many of the authors do not routinely evaluate the IM chain with the gamma probe, unless the IM node(s) appears enlarged or abnormal on pre-operative imaging (e.g. ultrasound or MRI). The procedure for identifying and removing sentinel nodes in the IM chain has been previously described.38
5. General troubleshooting techniques for blue dye alone
The most common cause for a lack of blue dye uptake in the axilla is extensive tumor infiltration. Therefore, the surgeon should always palpate the axilla carefully and remove any palpable suspicious nodes. Incision placement is also critical since the surgeon cannot rely on a gamma probe signal to identify the location of the sentinel node. The incision should be made at the inferior border of the axillary hair and extend medially to the edge of the pectoralis major muscle instead of being centered within the axilla.
In patients with very large breasts or those over the age of 65 where failure to map is slightly more frequent, the surgeon can consider increasing the injection volume.39 A volume of 8 to 10cc of blue dye can be used depending on the breast size.
Finally, a common error with the blue dye technique when a SLN is easily identified immediately beneath the incision is failure to actively search for other SLNs. Failure to search for additional blue nodes contributes to a high false negative identification rate. Since the majority of sentinel nodes are in close proximity to one another, it is not necessary to open the entire axilla to search for additional nodes.
Discussion
SLNB has gained widespread acceptance as the primary means of axillary staging for patients with clinically node-negative invasive breast cancer. Many surgeons have obtained appropriate training and experience in the procedure, and have reached an ideal level of proficiency performing the technique. However, some variation in technical performance remains, and practical guidance can help success rates of SLNB, especially when first commencing with the procedure in practice or when encountering unusual or difficult circumstances.
Troubleshooting has long been used in industries such as engineering, computer science and mechanics. The application of this process to a surgical procedure is a relatively novel endeavor. The techniques outlined in this guide offer a concise and practical approach to addressing problems with SLNB; compiling ‘tips’ learned through years of collective experience. The information presented is intended to provide a logical, systematic approach to problem solving, thereby enhancing the success rate of SLNB.
Supplementary Material
Acknowledgments
We would like to thank Nancy Bianchi from the Dana Medical Library at the University of Vermont for her contribution to the literature review.
Funding Source: None
Footnotes
Disclosures: None
Contributor Information
Ted A. James, Department of Surgery, University of Vermont, Burlington, VT 05405, USA.
Alex R. Coffman, University of Vermont College of Medicine, Burlington, VT 05405, USA.
Anees B. Chagpar, Department of Surgery, Yale University, New Haven, CT 06510, USA.
Judy C. Boughey, Department of Surgery, Mayo Clinic, Rochester, MN 55905, USA.
V. Suzanne Klimberg, Department of Surgery, University of Arkansas, Little Rock, AR 72205, USA.
Monica Morrow, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Armando E. Giuliano, Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Seth P. Harlow, Department of Surgery, University of Vermont, Burlington, VT 05405, USA.
References
- 1.Singletary S. Current status of axillary node dissection. Contemp Surg. 2002;58:334–340. [Google Scholar]
- 2.Wong SL, Abell TD, Chao C, Edwards MJ, McMasters KM. Optimal use of sentinel lymph node biopsy versus axillary lymph node dissection in patients with breast carcinoma: a decision analysis. Cancer. 2002;95(3):478–487. doi: 10.1002/cncr.10696. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. doi: 10.1097/00000658-199409000-00015. discussion 398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2(6):335–339. doi: 10.1016/0960-7404(93)90064-6. discussion 340. [DOI] [PubMed] [Google Scholar]
- 5.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen AY, Halpern MT, Schrag NM, Stewart A, Leitch M, Ward E. Disparities and trends in sentinel lymph node biopsy among early-stage breast cancer patients (1998-2005) J Natl Cancer Inst. 2008;100(7):462–474. doi: 10.1093/jnci/djn057. [DOI] [PubMed] [Google Scholar]
- 7.Sanidas EE, de Bree E, Tsiftsis DD. How many cases are enough for accreditation in sentinel lymph node biopsy in breast cancer? Am J Surg. 2003;185(3):202–210. doi: 10.1016/s0002-9610(02)01367-3. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JM, Orr RK, Moline SR. Institutional learning curve for sentinel node biopsy at a community teaching hospital. Am Surg. 2001;67(11):1030–1033. [PubMed] [Google Scholar]
- 9.Moonka R, Hunter JA, Cray WK, Duncan M, Wechter DG. A comparison of rates of lymph node metastases between patients undergoing sentinel and axillary lymphadenectomy. Am J Surg. 2002;183(5):558–561. doi: 10.1016/s0002-9610(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 10.Kiluk JV, Ly QP, Meade T, et al. Axillary recurrence rate following negative sentinel node biopsy for invasive breast cancer: long-term follow-up. Ann Surg Oncol. 2011;18(Suppl 3):S339–S342. doi: 10.1245/s10434-009-0704-1. [DOI] [PubMed] [Google Scholar]
- 11.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer--a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 12.Cox CE, Salud CJ, Cantor A, et al. Learning curves for breast cancer sentinel lymph node mapping based on surgical volume analysis. J Am Coll Surg. 2001;193(6):593–600. doi: 10.1016/s1072-7515(01)01086-9. [DOI] [PubMed] [Google Scholar]
- 13.Stitzenberg KB, Chang Y, Louie R, Groves JS, Durham D, Fraher EF. Improving our understanding of the surgical oncology workforce. Ann Surg. 2014;259(3):556–562. doi: 10.1097/SLA.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Falkenberry SS, Chung MA. The surgeon’s role in outcome in contemporary breast cancer. Surg Oncol Clin N Am. 2000;9(1):119–132. viii. [PubMed] [Google Scholar]
- 15.Newman LA. Locoregional control of breast cancer: surgical technique does matter. Ann Surg Oncol. 2004;11(1):11–13. doi: 10.1007/BF02524339. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GF, Giuliano AE, Veronesi U Consensus Conference Committee. Cancer; Proceedings of the Consensus Conference on the role of sentinel lymph node biopsy in carcinoma of the breast; April 19–22, 2001; Philadelphia, Pennsylvania. 2002. pp. 2542–2551. [DOI] [PubMed] [Google Scholar]
- 17.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raut CP, Hunt KK, Akins JS, et al. Incidence of anaphylactoid reactions to isosulfan blue dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis: results of a surgical prospective clinical practice protocol. Cancer. 2005;104(4):692–699. doi: 10.1002/cncr.21226. [DOI] [PubMed] [Google Scholar]
- 19.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 21.Thevarajah S, Huston TL, Simmons RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg. 2005;189(2):236–239. doi: 10.1016/j.amjsurg.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Bleicher RJ, Kloth DD, Robinson D, Axelrod P. Inflammatory cutaneous adverse effects of methylene blue dye injection for lymphatic mapping/sentinel lymphadenectomy. J Surg Oncol. 2009;99(6):356–360. doi: 10.1002/jso.21240. [DOI] [PubMed] [Google Scholar]
- 23.Teknos D, Ramcharan A, Oluwole SF. Pulmonary edema associated with methylene blue dye administration during sentinel lymph node biopsy. J Natl Med Assoc. 2008;100(12):1483–1484. doi: 10.1016/s0027-9684(15)31552-2. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria S, Hoskin TL, Degnim AC. Safety and technical success of methylene blue dye for lymphatic mapping in breast cancer. Am J Surg. 2008;196(2):228–233. doi: 10.1016/j.amjsurg.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 25.Golshan M, Nakhlis F. Can methylene blue only be used in sentinel lymph node biopsy for breast cancer? Breast J. 2006;12(5):428–430. doi: 10.1111/j.1075-122X.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin RC, Edwards MJ, Wong SL, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. For the University of Louisville Breast Cancer Study Group. Surgery. 2000;128(2):139–144. doi: 10.1067/msy.2000.108064. [DOI] [PubMed] [Google Scholar]
- 27.Chung A, Yu J, Stempel M, Patil S, Cody H, Montgomery L. Is the “10% rule” equally valid for all subsets of sentinel-node-positive breast cancer patients? Ann Surg Oncol. 2008;15(10):2728–2733. doi: 10.1245/s10434-008-0050-8. [DOI] [PubMed] [Google Scholar]
- 28.McMasters KM, Wong SL, Martin RC, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001;233(5):676–687. doi: 10.1097/00000658-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed M, Purushotham AD, Horgan K, Klaase JM, Douek M. Meta-analysis of superficial versus deep injection of radioactive tracer and blue dye for lymphatic mapping and detection of sentinel lymph nodes in breast cancer. Br J Surg. 2015;102(3):169–181. doi: 10.1002/bjs.9673. [DOI] [PubMed] [Google Scholar]
- 30.Yi M, Meric-Bernstam F, Ross MI, et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer. 2008;113(1):30–37. doi: 10.1002/cncr.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ban EJ, Lee JS, Koo JS, Park S, Kim SI, Park B-W. How many sentinel lymph nodes are enough for accurate axillary staging in t 1-2 breast cancer? J Breast Cancer. 2011;14(4):296–300. doi: 10.4048/jbc.2011.14.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zakaria S, Degnim AC, Kleer CG, et al. Sentinel lymph node biopsy for breast cancer: how many nodes are enough? J Surg Oncol. 2007;96(7):554–559. doi: 10.1002/jso.20878. [DOI] [PubMed] [Google Scholar]
- 33.Chagpar AB, Scoggins CR, Martin RCG, et al. Are 3 sentinel nodes sufficient? Arch Surg Chic Ill 1960. 2007;142(5):456–459. doi: 10.1001/archsurg.142.5.456. discussion 459-460. [DOI] [PubMed] [Google Scholar]
- 34.Chagpar A, Martin RC, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg Chic Ill 1960. 2004;139(6):614–618. doi: 10.1001/archsurg.139.6.614. discussion 618-620. [DOI] [PubMed] [Google Scholar]
- 35.Manca G, Volterrani D, Mazzarri S, et al. Sentinel lymph node mapping in breast cancer: a critical reappraisal of the internal mammary chain issue. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Radiopharm Chem Biol. 2014;58(2):114–126. [PubMed] [Google Scholar]
- 36.Mansel RE, Goyal A, Newcombe RG ALMANAC Trialists Group. Internal mammary node drainage and its role in sentinel lymph node biopsy: the initial ALMANAC experience. Clin Breast Cancer. 2004;5(4):279–284. doi: 10.3816/cbc.2004.n.031. discussion 285-286. [DOI] [PubMed] [Google Scholar]
- 37.Chagpar AB, Kehdy F, Scoggins CR, et al. Effect of lymphoscintigraphy drainage patterns on sentinel lymph node biopsy in patients with breast cancer. Am J Surg. 2005;190(4):557–562. doi: 10.1016/j.amjsurg.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Harlow S, Krag D, Weaver D, Ashikaga T. Extra-Axillary Sentinel Lymph Nodes in Breast Cancer. Breast Cancer Tokyo Jpn. 1999;6(2):159–165. doi: 10.1007/BF02966925. [DOI] [PubMed] [Google Scholar]
- 39.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242(4):593–599. doi: 10.1097/01.sla.0000184210.68646.77. discussion 599-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


