Abstract
Risk factors for cerebral aneurysms typically include age, hypertension, smoking, and alcohol usage. However, the possible connection of aneurysms with genetic conditions such as Marfan's syndrome, polycystic kidney disease, and neurofibromatosis raises the question of possible genetic risk factors for aneurysm, and additionally, genetic risk factors for rupture. We conducted a literature review using the PubMed database for studies regarding genetic correlation with cerebral aneurysm formation as well as rupture from December 2008 to Jun 2015. Twenty-one studies related to IA formation and 10 concerning IA rupture that met our criteria were found and tabulated. The most studied gene and the strongest association was 9p21/CDKN2, which is involved in vessel wall remodelling. Other possible genes that may contribute to IA formation include EDNRA and SOX17; however, these factors were not studied as robustly as CDKN2. Multiple factors contribute to aneurysm formation and rupture and the contributions of blood flow dynamics and comorbidities as mentioned previously, cannot be ignored. While these elements are important to development and rupture of aneurysms, genetic influence may predispose certain patients to formation of aneurysms and eventual rupture.
Keywords: Formation, genetics, intracranial aneurysm, rupture, sub-arachnoid hemorrhage
Introduction
The prevalence of unruptured intracranial aneurysms (IAs) in the general population is estimated to be approximately 3%.[1] Traditional risk factors for unruptured aneurysms include female gender and older age, hypertension, the diameter of the aneurysm, alcohol use, and tobacco use.[2,3] The most worrisome event in patients harboring an IA is the rupture of the arterial wall and resultant sub-arachnoid hemorrhage (SAH), which occurs at a rate of 30,000 per year in the United States[4] and carries a mortality rate of up to 45%.[2,3,4,5] Given the fact that IA can remain asymptomatic until the time of rupture and that approximately 80–90% of unruptured aneurysms discovered incidentally, it is critical to better understand the variables that may be associated with higher chances of aneurysm formation, growth, and rupture.[2]
Multiple genetic diseases have been identified as having a possible association with IA. Marfan's syndrome involves defects in the gene FBN1 (which codes the extracellular matrix protein fibrillin-1) and is classically associated with aortic dissection, possibly due to structural compromise of arterial cells caused by the mutation.[5,6] Although an association between Marfan's syndrome and IA has also been suggested,[5,6,7] autopsy studies and analysis of a family multiple members suffering from IA and Marfan's syndrome have not always shown an association.[5,8] Neurofibromatosis type I (NF1) is caused by a mutation that affects the neurofibromin gene on chromosome 17q, which not only is tumor suppressor but may also exert some effect on tubulin as well.[9] NF1 manifestations include café-au-lait spots, neurofibromas, and gliomas. The association of NF1 with IA has also been suggested, albeit with mixed evidence.[9,10] Autosomal dominant polycystic kidney disease (ADPKD) is an inherited renal condition which is caused by mutations in two known genes: PKD1 (85–90% of cases) and PKD2 (10–15% of cases). The mutation results in the formation of multiple renal cysts and eventually renal failure. Patients with ADPKD may have an increased risk of IA formation with prevalence rates estimated to be between 3% and 12%.[11,12] It is believed that the affected genes produce flawed polycystin that leads to structural weaknesses in vascular smooth muscle cells.[13] Based on these genetic diseases, there appears to be a connection between certain genetic traits and IA, which may be indicative of a common element in their formation.
In addition to the aforementioned genetic disorders, there are less obvious variations in the genetic code, which may also play a role in the development and rupture of cerebral aneurysms. Increased familial risk indicates a possible genetic risk.[14,15,16] Specific variations in the genetic code (polymorphisms) and certain loci have also been implicated as having a genetic influence on IA formation.[17,18] A better understanding of the genetic influence on aneurysm formation and rupture risk may aid clinicians in the identification of patients at higher risk for SAH. We sought to review the current literature regarding the genetic factors associated with aneurysm formation and rupture.
Materials and Methods
Our study was divided into two sections: Genetic factors related to IA formation and IA rupture. We performed literature searches using the PubMed database to find relevant literature on these topics. Formal searches were conducted using MeSH database advanced search tool with the MeSH phrases “IA” and “genetics,” “IA” and “gene,” “SAH/genetics” and “(IA) and (rupture) and (genetics).” We limited the inclusion to only articles containing human subjects, available in English, and conducted within 6.5 years (December 2008–June 2015).
Articles were excluded if they did not meet any of the aforementioned criteria or were a case report, single-family study, meta-analysis, or were an analysis of inter-genetic relationships rather than relationship with IA formation or rupture. Studies were also excluded if they concerned non IA or included non IA in their analysis.
We recorded the number of patients and controls and the polymorphisms from each study. For each polymorphism, the location of the polymorphism (and associated gene if known), the odds ratio (OR) (and 95% confidence interval [% CI], if available), and the P value were recorded. In cases where a study sampled multiple geographically different populations, the combined results of the populations found in that particular study were collected.
Results
Twenty-one studies related to IA formation and 10 concerning IA rupture that met our criteria were found and are listed in Tables 1 and 2, respectively.
Table 1.
Aneurysm formation
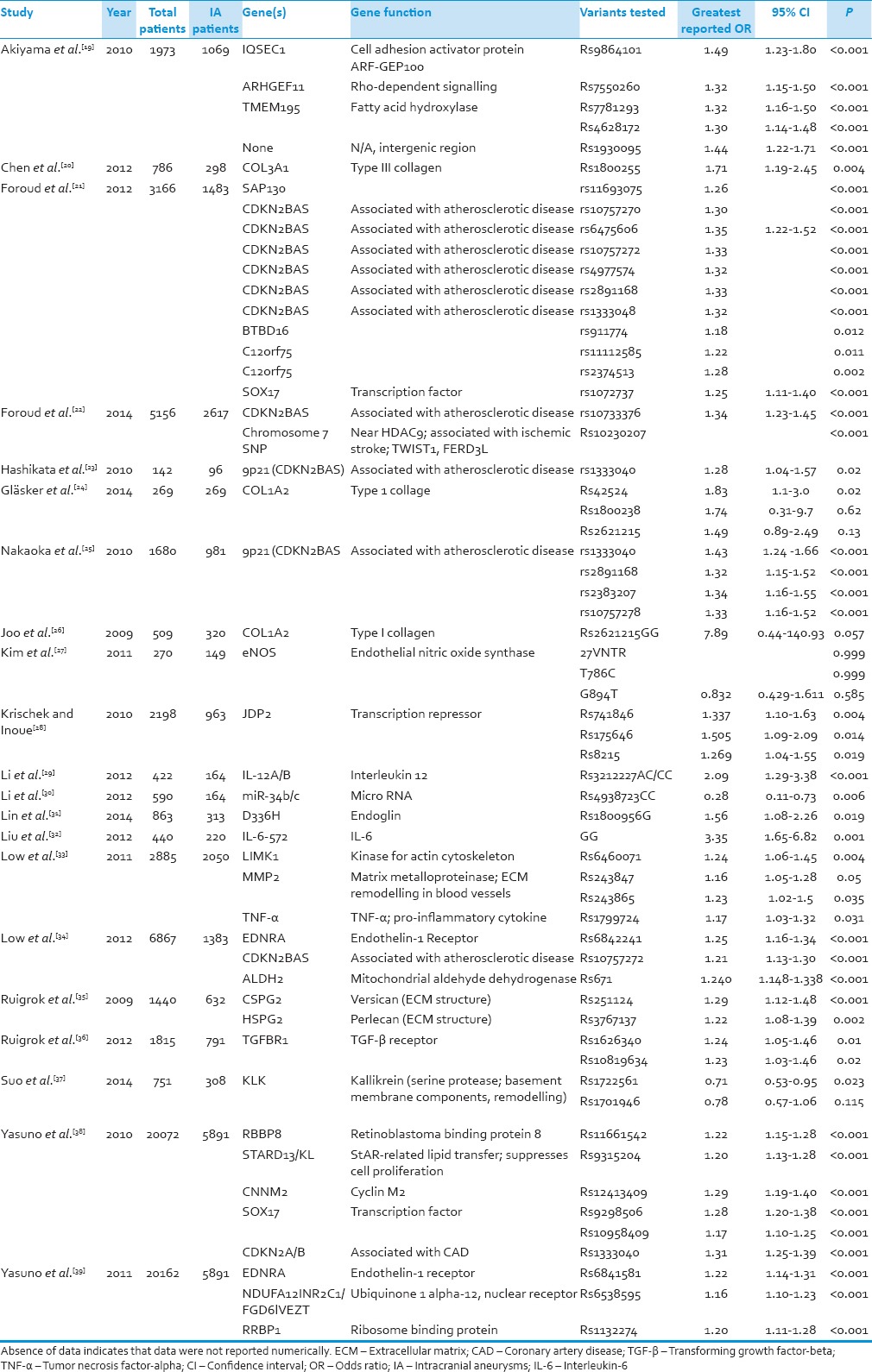
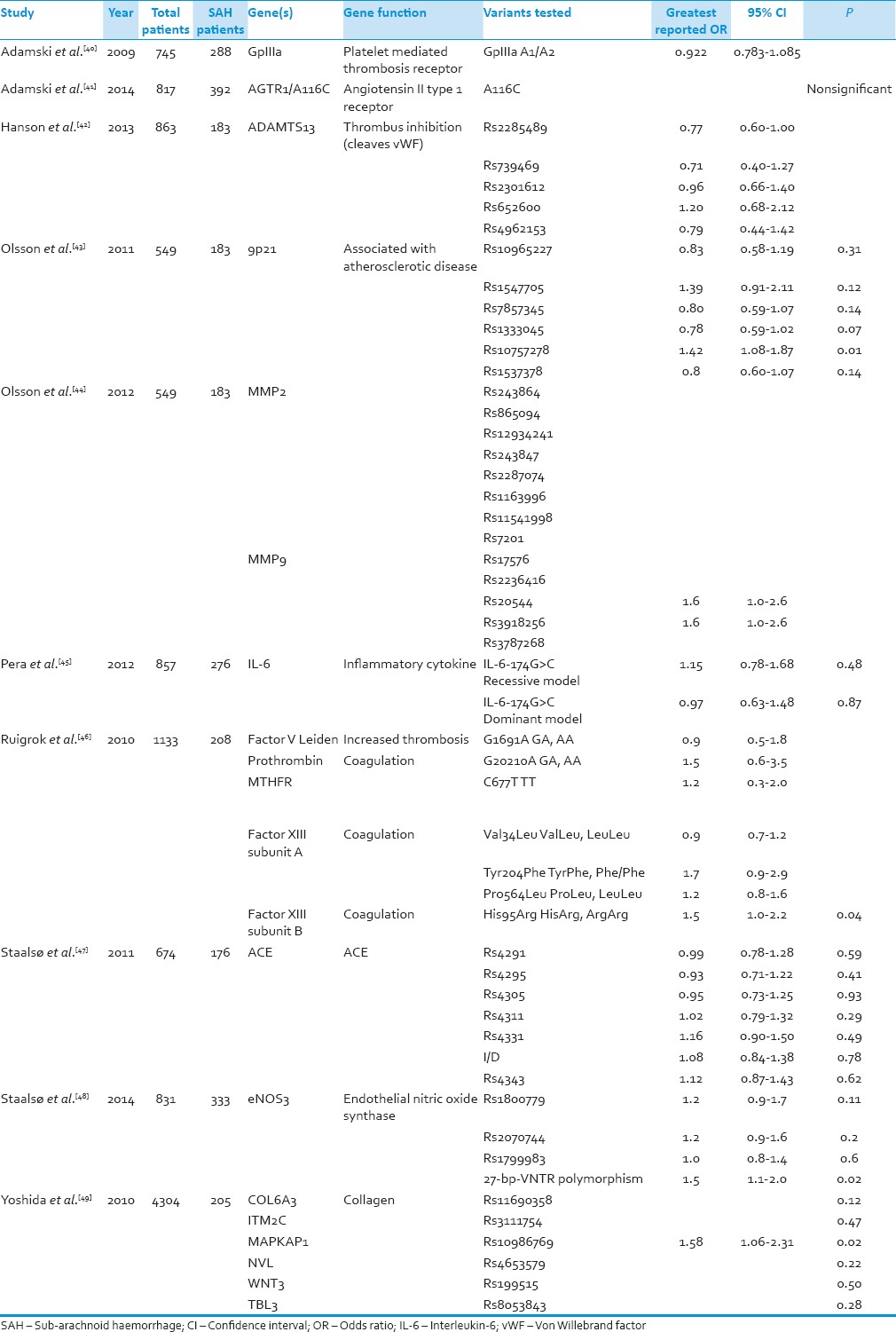
Table 2.
Aneurysm rupture
Aneurysm Formation
The total number of IA patients from these studies was 19,997 while total number of controls was 51,953. Thirty-two different genetic locations were investigated for possible association with IA, as shown in Table 1.
Three genes were consistently shown to have associations with IA formation: CDKN2 (6 studies), EDRNA, (2) and SOX17 (2). Among these genes, only CDKN2 had polymorphisms (rs10757272, rs1333040, rs2891168) found to be associated with IA by multiple studies. Polymorphisms from EDRNA and SOX17, although associated with IA formation, were different between studies.
Of these three genes, the one which showed the most robust association with IA was CDKN2, a gene associated with cyclin-dependent kinase (CDK) inhibitors, polymorphism rs1333040 (OR = 1.43, 95% CI = 1.24–1.66, P < 0.001). However, this same polymorphism was examined in two other studies in our sample and yielded less dramatic although still significant results (OR = 1.31, 95% CI = 1.25–1.39, P < 0.001; OR = 1.28, 95% CI = 1.04–1.57, P = 0.02).[50,51] There were a total of eleven distinct CDKN2 polymorphisms in the sample, all of which showed significant association with IA. The association between CDKN2 rs1333040 and IA was the strongest found in our entire sample. Among the other genes with repeated association with IA, the strongest association of SOX17, a regulator of growth and maintenance of the vascular endothelium, came from rs9298506 (OR = 1.28, 95% CI = 1.20–1.38, P < 0.001) while the most robust association from EDNRA, a gene associated with the Endothelin-1 receptor that controls vascular smooth muscle tone, came from polymorphism rs6842241 (OR = 1.25, 95% CI = 1.16–1.34, P < 0.001).[39,50,52] In our sample, two distinct polymorphisms each for both SOX17 and EDNRA were analyzed and showed significant association with IA. The range of OR for CDKN2 was 1.21–1.43, the range for EDNRA was 1.22–1.25, and the range for SOX17 was 1.17–1.43.
Aneurysm Rupture
There were a total of 2061 IA rupture patients and 10,607 controls. Three studies used the same sample of patients to analyze different genes for association with IA.[43,44] Polymorphisms from 19 different genes were investigated. None of the genes were investigated by more than one study.
Only 5 of the genes had polymorphisms with significant associations. These polymorphisms come from 9p21, coagulation factor XIII, MAPKAP1, and eNOS. The 9p21 polymorphism was found to have an OR of 1.42 (P = 0.01), the Factor XIII subunit B polymorphism had OR = 1.5 (0.04), MAPKAP1 had an OR of 1.58 (P = 0.02), and eNOS had an OR of 1.5 (P = 0.02).
Discussion
This investigation found that multiple studies have shown statistically significant association between IA formation and variants of the genes CDKN2, SOX17, and EDNRA. The association of these genes with IA formation across studies leads to the next possible query of how they are associated. Furthermore, only a few genes are associated with aneurysmal rupture (9p21, coagulation factor XIII, MAPKAP1, and eNOS).
Research into aneurysm wall morphology has shown that IAs display fewer layers of the vessel wall and less cell density than normal vasculature.[52] Challa and Han demonstrated using simulations that decreased vessel wall thickness would lead to greater stress, a known cause of IA.[53,54] Conversely, however, at least, one study has shown that there is a significant association of IA with increased vessel wall thickness through carotid intima-medial thickness testing, with the researchers noting that aneurysm patients had decreased circumferential stress and significantly lower elasticity when compared to controls.[55] This may indicate that aneurysm formation is not necessarily due to either increased or decreased thickness but possibly heterogeneous vessel wall structure. Simulations have also shown that stress was also increased in areas with heterogeneous thickness,[53] an effect may more pronounced in areas such as arterial bifurcations which receive the greatest shear stress.[56] A study by Nakatomi et al. on intracranial fusiform aneurysms noted that IA occurrence can be connected with not only breakdown of the internal elastic lamina, but also to proliferation after initial damage has occurred.[57] A connection between IA formation and CDKN2, SOX17, and EDNRA could genetically link heterogeneous vessel structure and the mechanisms mentioned.
CDKN2 is located at 9p21 and variations may be involved in coronary artery disease or aortic aneurysms.[25] In our literature review, polymorphisms of this gene were found to have associations with IA in multiple studies with a range of OR from 1.21 to 1.43.[21,23,25,34,38] Although the exact relationship is unknown it can be theorized. CDKN2BAS is an antisense region of the DNA bordered by genes for CDK inhibitors, which prevent vascular smooth muscle from proliferating. It is believed that dysfunction of the CDK inhibitors could lead to vascular wall abnormalities and thus to IA aneurysm formation.[58] If the proposed function of CDKN2 is correct, abnormalities could lead to up- or down-regulation of proliferation and, therefore, a heterogeneous wall thickness. Abnormal wall thickness would, in turn, increase the risk of IA according to the previously mentioned work by Nakatomi et al. and Maltete et al., and would explain the relationship between CDKN2 and IA shown by multiple studies in this investigation.[55,57]
EDNRA acts as a receptor for Endothelin-1, which causes both vasoconstriction and proliferation of vascular smooth muscle cells. The theorized purpose of EDNRA is to modulate the effects of hemodynamic stress.[34] The polymorphisms of EDNRA associated with IA formation identified in the present investigation may result in the inability of the vascular smooth muscle to compensate against the shearing forces previously described. Decreased vascular compensation has also been discussed by Maltete et al. in which decreased vascular compliance associated in IA patients was believed to contribute to their formation.[55] SOX17, another gene shown to be associated with IA by two studies in this investigation, also contributes to endothelial maintenance[51] and, therefore, may also be involved in a homeostatic mechanism. While the exact effect of these CDKN2, EDNRA and SOX17 polymorphisms on IA is uncertain, it seems that vessel wall heterogeneity may lead to increased IA formation either through weakening the overall structure of the vessel wall or by limiting its ability to compensate against stress.
When compared to IA formation, our study reveals less information regarding genetics associations of aneurysm rupture and SAH. Only a single study has demonstrated an association between CDKN2 and SAH.[43] Unfortunately, a more definitive link cannot be reached. If aneurysm formation and rupture exist along a continuum of structural change as theorized by Chalouhi et al.,[54] then perhaps this explains the effect of CDKN2 on aneurysm rupture. However, it is important to note that the same study which noted an association between the 9p21 locus and SAH did not find such an association with five other CDKN2 polymorphisms that they tested.[43] A single study noted an association between aneurysm rupture and Factor XIII Subunit B.[46] An association was also found in a different study with a polymorphism for the MAPKAP gene which is involved in cell signaling.[49] The relationship between either of these genes and SAH has not been fully researched, and we were unable to find information about possible mechanisms for aneurysm rupture.[46,49]
Our analysis highlights the possibly critical yet very limited amount of information available on the impact genetic factors may have on IA formation and rupture. A paucity of information exists on the exact mechanisms by which these genes affect aneurysm formation or rupture, and much of the information is theoretical. If the mechanisms of genetic involvement were laid out, perhaps new screening methods would be able to identify individuals at greater risk of aneurysm formation. More importantly, development of a risk stratification tool for aneurysm rupture and SAH would allow for early intervention.
With the identification of a reliable genetic target in IA patients, novel treatment strategies could be devised to exploit a specific genetic locus. In a rabbit model, the progression of abdominal aortic aneurysms was able to be significantly reduced using deoxynucleotides targeted at the specific gene, NFκB.[59] Although this study was conducted in a nonhuman model, and not on IA specifically, it does show the potential of genetically targeted therapy to affect the development of aneurysms. It has been proposed that endovascular treatment of IA could be augmented by gene transfer using vectors embedded in coils.[60] Further studies should be carried out in the future identify a gene or genes strongly associated with IA or SAH to advance treatment strategies.
Conclusion
Our review found that IA formation and SAH was associated with several possible genetic factors. The most studied gene and the strongest association was 9p21/CDKN2, which is involved in vessel wall remodeling. Other possible genes that may contribute to IA formation include EDNRA and SOX17; however, these factors were not studied as robustly as CDKN2. Overall, information regarding genetics of IA formation and SAH is lacking. Genetics are most likely one of many factors contributing to aneurysm formation and rupture. The contributions of blood flow dynamics and comorbidities as mentioned previously, cannot be ignored. While these elements are important to development and rupture of aneurysms, genetic influence may predispose certain patients to the formation of aneurysms and eventual rupture. Further research into the genetic factors responsible for structural remodeling and inflammatory response to endothelial injury that occur in IA and SAH is needed to better identify at-risk patients and develop novel gene-based therapies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011;10:626–36. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 2.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke. 2013;44:2414–21. doi: 10.1161/STROKEAHA.113.001838. [DOI] [PubMed] [Google Scholar]
- 3.Williams LN, Brown RD., Jr Management of unruptured intracranial aneurysms. Neurol Clin Pract. 2013;3:99–108. doi: 10.1212/CPJ.0b013e31828d9f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacigaluppi S, Piccinelli M, Antiga L, Veneziani A, Passerini T, Rampini P, et al. Factors affecting formation and rupture of intracranial saccular aneurysms. Neurosurg Rev. 2014;37:1–14. doi: 10.1007/s10143-013-0501-y. [DOI] [PubMed] [Google Scholar]
- 5.Conway JE, Hutchins GM, Tamargo RJ. Marfan syndrome is not associated with intracranial aneurysms. Stroke. 1999;30:1632–6. doi: 10.1161/01.str.30.8.1632. [DOI] [PubMed] [Google Scholar]
- 6.Schievink WI, Parisi JE, Piepgras DG, Michels VV. Intracranial aneurysms in Marfan's syndrome: An autopsy study. Neurosurgery. 1997;41:866–70. doi: 10.1097/00006123-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 7.ter Berg HW, Bijlsma JB, Veiga Pires JA, Ludwig JW, van der Heiden C, Tulleken CA, et al. Familial association of intracranial aneurysms and multiple congenital anomalies. Arch Neurol. 1986;43:30–3. doi: 10.1001/archneur.1986.00520010026015. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg JS, Limburg M, Hennekam RC. Is Marfan syndrome associated with symptomatic intracranial aneurysms? Stroke. 1996;27:10–2. doi: 10.1161/01.str.27.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Schievink WI, Riedinger M, Maya MM. Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. Am J Med Genet A. 2005;134A:45–8. doi: 10.1002/ajmg.a.30475. [DOI] [PubMed] [Google Scholar]
- 10.Baldauf J, Kiwit J, Synowitz M. Cerebral aneurysms associated with von Recklinghausen's neurofibromatosis: Report of a case and review of the literature. Neurol India. 2005;53:213–5. doi: 10.4103/0028-3886.16415. [DOI] [PubMed] [Google Scholar]
- 11.Romão EA, Moysés Neto M, Teixeira SR, Muglia VF, Vieira-Neto OM, Dantas M. Renal and extrarenal manifestations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006;39:533–8. doi: 10.1590/s0100-879x2006000400014. [DOI] [PubMed] [Google Scholar]
- 12.Ring T, Spiegelhalter D. Risk of intracranial aneurysm bleeding in autosomal-dominant polycystic kidney disease. Kidney Int. 2007;72:1400–2. doi: 10.1038/sj.ki.5002488. [DOI] [PubMed] [Google Scholar]
- 13.Klein JP. On the role of screening for intracranial aneurysms in autosomal dominant polycystic kidney disease. AJNR Am J Neuroradiol. 2013;34:1560–1. doi: 10.3174/ajnr.A3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DH, Van Ginhoven G, Milewicz DM. Familial aggregation of both aortic and cerebral aneurysms: Evidence for a common genetic basis in a subset of families. Neurosurgery. 2005;56:655–61. doi: 10.1227/01.neu.0000156787.55281.53. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg JE, Rinkel GJ, Algra A, van Duyn CM, Greebe P, Ramos LM, et al. Familial subarachnoid hemorrhage: Distinctive features and patterns of inheritance. Ann Neurol. 1995;38:929–34. doi: 10.1002/ana.410380614. [DOI] [PubMed] [Google Scholar]
- 16.Kim CJ, Park SS, Lee HS, Chung HJ, Choi W, Chung JH, et al. Identification of an autosomal dominant locus for intracranial aneurysm through a model-based family collection in a geographically limited area. J Hum Genet. 2011;56:464–6. doi: 10.1038/jhg.2011.27. [DOI] [PubMed] [Google Scholar]
- 17.Ruigrok YM, Rinkel GJ. Genetics of intracranial aneurysms. Stroke. 2008;39:1049–55. doi: 10.1161/STROKEAHA.107.497305. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Wan JQ, Zhou JP, Fan YL, Jiang JY. Gene expression analysis of ruptured and un-ruptured saccular intracranial aneurysm. Eur Rev Med Pharmacol Sci. 2013;17:1374–81. [PubMed] [Google Scholar]
- 19.Akiyama K, Narita A, Nakaoka H, Cui T, Takahashi T, Yasuno K, et al. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J Hum Genet. 2010;55:656–61. doi: 10.1038/jhg.2010.82. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zhu Y, Jiang Y, Yu H, Sun K, Song W, et al. A functional variant of the collagen type III alpha1 gene modify risk of sporadic intracranial aneurysms. Hum Genet. 2012;131:1137–43. doi: 10.1007/s00439-012-1138-6. [DOI] [PubMed] [Google Scholar]
- 21.Foroud T, Koller DL, Lai D, Sauerbeck L, Anderson C, Ko N, et al. Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. Stroke. 2012;43:2846–52. doi: 10.1161/STROKEAHA.112.656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foroud T, Lai D, Koller D, Van’t Hof F, Kurki MI, Anderson CS, et al. Genome-wide association study of intracranial aneurysm identifies a new association on chromosome 7. Stroke. 2014;45:3194–9. doi: 10.1161/STROKEAHA.114.006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashikata H, Liu W, Inoue K, Mineharu Y, Yamada S, Nanayakkara S, et al. Confirmation of an association of single-nucleotide polymorphism rs1333040 on 9p21 with familial and sporadic intracranial aneurysms in Japanese patients. Stroke. 2010;41:1138–44. doi: 10.1161/STROKEAHA.109.576694. [DOI] [PubMed] [Google Scholar]
- 24.Gläsker S, Schatlo B, Klingler JH, Braun V, Spangenberg P, Kim IS, et al. Associations of collagen type I a2 polymorphisms with the presence of intracranial aneurysms in patients from Germany. J Stroke Cerebrovasc Dis. 2014;23:356–60. doi: 10.1016/j.jstrokecerebrovasdis.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 25.Nakaoka H, Takahashi T, Akiyama K, Cui T, Tajima A, Krischek B, et al. Differential effects of chromosome 9p21 variation on subphenotypes of intracranial aneurysm: Site distribution. Stroke. 2010;41:1593–8. doi: 10.1161/STROKEAHA.110.586529. [DOI] [PubMed] [Google Scholar]
- 26.Joo SP, Kim TS, Lee IK, Lee JK, Seo BR, Kim JH, et al. The role of collagen type I alpha2 polymorphisms: Intracranial aneurysms in Koreans. Surg Neurol. 2009;72:48–53. doi: 10.1016/j.surneu.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Kim TG, Kim NK, Baek MJ, Huh R, Chung SS, Choi JU, et al. The relationships between endothelial nitric oxide synthase polymorphisms and the formation of intracranial aneurysms in the Korean population. Neurosurg Focus. 2011;30:E23. doi: 10.3171/2011.2.FOCUS10227. [DOI] [PubMed] [Google Scholar]
- 28.Krischek B, Tajima A, Akagawa H, Narita A, Ruigrok Y, Rinkel G, et al. Association of the Jun dimerization protein 2 gene with intracranial aneurysms in Japanese and Korean cohorts as compared to a Dutch cohort. Neuroscience. 2010;169(1):339–43. doi: 10.1016/j.neuroscience.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Li LJ, Pan XM, Sima X, Li ZH, Zhang LS, Sun H, et al. Interactions of interleukin-12A and interleukin-12B polymorphisms on the risk of intracranial aneurysm. Mol Biol Rep. 2012;39:11217–23. doi: 10.1007/s11033-012-2031-z. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Sima X, Bai P, Zhang L, Sun H, Liang W, et al. Interactions of miR-34b/c and TP53 polymorphisms on the risk of intracranial aneurysm. Clin Dev Immunol 2012. 2012:567586. doi: 10.1155/2012/567586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Yu H, Song W, Zhang Y, Zhang C, Zhu Y, et al. A variant in the endoglin gene is associated with the development of sporadic intracranial aneurysms. Curr Neurovasc Res. 2014;11:294–301. doi: 10.2174/1567202611666140912114450. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Sun J, Wu C, Cao X, He M, You C. The interleukin-6-572G/C gene polymorphism and the risk of intracranial aneurysms in a Chinese population. Genet Test Mol Biomarkers. 2012;16:822–6. doi: 10.1089/gtmb.2012.0004. [DOI] [PubMed] [Google Scholar]
- 33.Low SK, Zembutsu H, Takahashi A, Kamatani N, Cha PC, Hosono N, et al. Impact of LIMK1, MMP2 and TNF-α variations for intracranial aneurysm in Japanese population. J Hum Genet. 2011;56:211–6. doi: 10.1038/jhg.2010.169. [DOI] [PubMed] [Google Scholar]
- 34.Low SK, Takahashi A, Cha PC, Zembutsu H, Kamatani N, Kubo M, et al. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet. 2012;21:2102–10. doi: 10.1093/hmg/dds020. [DOI] [PubMed] [Google Scholar]
- 35.Ruigrok YM, Rinkel GJ, Wijmenga C, Kasuya H, Tajima A, Takahashi T, et al. Association analysis of genes involved in the maintenance of the integrity of the extracellular matrix with intracranial aneurysms in a Japanese cohort. Cerebrovasc Dis. 2009;28:131–4. doi: 10.1159/000223438. [DOI] [PubMed] [Google Scholar]
- 36.Ruigrok YM, Baas AF, Medic J, Wijmenga C, Rinkel GJ. The transforming growth factor-ß receptor genes and the risk of intracranial aneurysms. Int J Stroke. 2012;7:645–8. doi: 10.1111/j.1747-4949.2011.00615.x. [DOI] [PubMed] [Google Scholar]
- 37.Suo M, Lin Y, Yu H, Song W, Sun K, Song Y, et al. Association of Kallikrein gene polymorphisms with sporadic intracranial aneurysms in the Chinese population. J Neurosurg. 2014;120:1397–401. doi: 10.3171/2013.11.JNS131036. [DOI] [PubMed] [Google Scholar]
- 38.Yasuno K, Bilguvar K, Bijlenga P, Low SK, Krischek B, Auburger G, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet. 2010;42:420–5. doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuno K, Bakircioglu M, Low SK, Bilgüvar K, Gaál E, Ruigrok YM, et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A. 2011;108:19707–12. doi: 10.1073/pnas.1117137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adamski MG, Borratynska A, Krupa M, Wloch-Kopec D, Turaj W, Wolkow P, et al. A1/A2 polymorphism of GpIIIa gene and a risk of aneurysmal subarachnoid haemorrhage. Biochem Biophys Res Commun. 2009;383:228–30. doi: 10.1016/j.bbrc.2009.03.156. [DOI] [PubMed] [Google Scholar]
- 41.Adamski MG, Golenia A, Turaj W, Baird AE, Moskala M, Dziedzic T, et al. The AGTR1 gene A1166C polymorphism as a risk factor and outcome predictor of primary intracerebral and aneurysmal subarachnoid hemorrhages. Neurol Neurochir Pol. 2014;48:242–7. doi: 10.1016/j.pjnns.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Hanson E, Olsson S, Bayazit B, Csajbok LZ, Nylén K, Nellgård B, et al. Association between variation in ADAMTS13 and aneurysmal subarachnoid hemorrhage. Thromb Res. 2013;131:99–101. doi: 10.1016/j.thromres.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Olsson S, Csajbok LZ, Jood K, Nylén K, Nellgård B, Jern C. Association between genetic variation on chromosome 9p21 and aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2011;82:384–8. doi: 10.1136/jnnp.2009.187427. [DOI] [PubMed] [Google Scholar]
- 44.Olsson S, Csajbok LZ, Jood K, Nylén K, Nellgård B, Jern C. No evidence for an association between genetic variation at the MMP2 and MMP9 loci and aneurysmal subarachnoid haemorrhage. J Neurol. 2012;259:193–5. doi: 10.1007/s00415-011-6157-z. [DOI] [PubMed] [Google Scholar]
- 45.Pera J, Dziedzic T, Adamski M, Jagiella J, Krupa M, Moskala M, et al. Interleukin 6-174G>C polymorphism and risk of aneurysmal subarachnoid hemorrhage: Case-control study and meta-analysis. Acta Neurol Scand. 2012;125:111–5. doi: 10.1111/j.1600-0404.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruigrok YM, Slooter AJ, Rinkel GJ, Wijmenga C, Rosendaal FR. Genes influencing coagulation and the risk of aneurysmal subarachnoid hemorrhage, and subsequent complications of secondary cerebral ischemia and rebleeding. Acta Neurochir (Wien) 2010;152:257–62. doi: 10.1007/s00701-009-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staalsø JM, Nielsen M, Edsen T, Koefoed P, Springborg JB, Moltke FB, et al. Common variants of the ACE gene and aneurysmal subarachnoid hemorrhage in a Danish population: A case-control study. J Neurosurg Anesthesiol. 2011;23:304–9. doi: 10.1097/ANA.0b013e318225c979. [DOI] [PubMed] [Google Scholar]
- 48.Staalsø JM, Edsen T, Kotinis A, Romner B, Springborg JB, Olsen NV. Association of the NOS3 intron-4 VNTR polymorphism with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2014;121:587–92. doi: 10.3171/2014.5.JNS131572. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, et al. Association of genetic variants with hemorrhagic stroke in Japanese individuals. Int J Mol Med. 2010;25:649–56. doi: 10.3892/ijmm_00000388. [DOI] [PubMed] [Google Scholar]
- 50.Alg VS, Sofat R, Houlden H, Werring DJ. Genetic risk factors for intracranial aneurysms: A meta-analysis in more than 116,000 individuals. Neurology. 2013;80:2154–65. doi: 10.1212/WNL.0b013e318295d751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilguvar K, Yasuno K, Niemelä M, Ruigrok YM, von Und Zu Fraunberg M, van Duijn CM, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40:1472–7. doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abruzzo T, Shengelaia GG, Dawson RC, 3rd, Owens DS, Cawley CM, Gravanis MB. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. AJNR Am J Neuroradiol. 1998;19:1309–14. [PMC free article] [PubMed] [Google Scholar]
- 53.Challa V, Han HC. Spatial variations in wall thickness, material stiffness and initial shape affect wall stress and shape of intracranial aneurysms. Neurol Res. 2007;29:569–77. doi: 10.1179/016164107X164193. [DOI] [PubMed] [Google Scholar]
- 54.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–22. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 55.Maltete D, Bellien J, Cabrejo L, Iacob M, Proust F, Mihout B, et al. Hypertrophic remodeling and increased arterial stiffness in patients with intracranial aneurysms. Atherosclerosis. 2010;211:486–91. doi: 10.1016/j.atherosclerosis.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Alfano JM, Kolega J, Natarajan SK, Xiang J, Paluch RA, Levy EI, et al. Intracranial aneurysms occur more frequently at bifurcation sites that typically experience higher hemodynamic stresses. Neurosurgery. 2013;73:497–505. doi: 10.1227/NEU.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 57.Nakatomi H, Segawa H, Kurata A, Shiokawa Y, Nagata K, Kamiyama H, et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: Insight on the mechanism of growth. Stroke. 2000;31:896–900. doi: 10.1161/01.str.31.4.896. [DOI] [PubMed] [Google Scholar]
- 58.Roberts R, Stewart AF. 9p21 and the genetic revolution for coronary artery disease. Clin Chem. 2012;58:104–12. doi: 10.1373/clinchem.2011.172759. [DOI] [PubMed] [Google Scholar]
- 59.Miyake T, Aoki M, Nakashima H, Kawasaki T, Oishi M, Kataoka K, et al. Prevention of abdominal aortic aneurysms by simultaneous inhibition of NFkappaB and ets using chimeric decoy oligonucleotides in a rabbit model. Gene Ther. 2006;13:695–704. doi: 10.1038/sj.gt.3302704. [DOI] [PubMed] [Google Scholar]
- 60.Ribourtout E, Raymond J. Gene therapy and endovascular treatment of intracranial aneurysms. Stroke. 2004;35:786–93. doi: 10.1161/01.STR.0000117577.94345.CC. [DOI] [PubMed] [Google Scholar]


