Abstract
Background:
Spasticity is a motor disorder that interferes with mobility and affects the quality of life. Different approaches have been utilized to address patients with spastic diplegia, among which is selective dorsal rhizotomy (SDR). Although SDR has been shown to be efficacious in treating spastic patients, many neurologists and neurosurgeons are not well aware of the procedure, its indications, and expected outcomes due to the limited number of centers performing this procedure.
Objectives:
The aim of this study is to describe the collaborative multidisciplinary approach between neurosurgeons, neurophysiologists, and physiotherapists in performing SDR. In addition, we delineate three illustrative cases in which SDR was performed in our patients.
Materials and Methods:
A retrospective review and analysis of the clinical records of our three patients who underwent SDR was conducted and reported. Patients’ outcomes were evaluated and compared to preoperative measurements based on clinical examination of power, tone (Ashworth scale), gait, and range of motion, as well as subjective functional assessment, gross motor function classification system, and gross motor function measure with follow-up at 6, 12, and 24 months postoperatively. A detailed description of our neurosurgical technique in performing SDR in collaboration with neurophysiology and physiotherapy monitoring is provided.
Results:
The three patients who underwent SDR using our multidisciplinary approach improved both functionally and objectively after the procedure. No intraoperative or postoperative complications were encountered. All patients were doing well over a long postoperative follow-up period.
Conclusion:
A multidisciplinary approach to treating spastic diplegia with SDR can provide good short-term and long-term outcomes in select patients suffering from spastic diplegia.
Keywords: Cerebral palsy, intraoperative monitoring, neurophysiology, physiotherapy, selective dorsal rhizotomy, spastic diplegia, spasticity
Introduction
Cerebral palsy (CP) is a group of disorders of the motor system, movement, and/or posture caused by interference with the developing or immature brain.[1] Spasticity, one of the main signs of CP, is a serious issue that affects mobility, causing pain, disability, and interfering with daily activities. Patients affected with CP suffer from abnormalities in gait and posture due to the damage of the motor nerve fibers that control muscle function, leading to unopposed muscular contraction and stiffness.[2,3] This condition is nonprogressive and can occur during the prenatal, perinatal, or postnatal life as the brain/central nervous system continues to develop throughout the first 5 years of life.[3] The most common cause for CP is premature birth with very low birth weight (<1500 g), which is associated with brain hypoperfusion that can lead to brain ischemia, periventricular leukomalacia, and germinal matrix hemorrhage.[4,5]
Spasticity causes impaired bone and muscle development, shortening of the muscles, and deformities in the affected limbs.[6] It most commonly involves the muscles of the lower limbs including the muscles of the thighs, legs, and feet because the motor nerve fibers that innervate the lower limb muscles travel closer in proximity to the ventricles than the nerve fibers involved in the innervation of the upper limbs and thus are more prone to being affected by periventricular insults.[6]
The overall prevalence of CP worldwide is 2.11/1000 live births.[7] There is an established association between CP with spastic diplegia and preterm children with very low birth weight, and the number of patients diagnosed with spastic CP is growing due to advances in the medical field, especially the advances in neonatal intensive care that allowed for greater survival of preterm children with very low birth weight.[8]
Several treatment modalities are available for patients with CP. The choice of treatment modality depends on the age of the patient, severity of the injury, clinical manifestations, and availability of expertise. These modalities are classified into rehabilitative modalities, assistive devices, medical modalities, and surgical modalities. Rehabilitative modalities include physiotherapy, occupational therapy (OT), and others such as speech and language therapy and swallowing therapy. Assistive devices include walkers, braces, wheelchairs, orthotics, and prosthetics.[3] Medical treatment includes oral muscle relaxants, intramuscular botulinum toxin injection, intrathecal/intravenous baclofen, and benzodiazepines. Surgical modalities include orthopedic interventions and surgery, osteotomies, muscle and tendon lengthening, baclofen pump insertion, and selective dorsal rhizotomy (SDR).[3] These modalities have increased the survival rates among CP patients.
SDR is one of the best modalities available for treating CP patients with spasticity, especially mobile spastic diplegia, with positive short-term and long-term outcomes.[9] Although SDR has been shown to be efficacious in treating spastic patients, many neurologists and neurosurgeons are not well aware of the procedure, its indications, and expected outcomes. SDR is performed in a limited number of centers around the world, and it requires effective communication of multidisciplinary teams from various specialties before, during, and after surgery to ensure good patient outcomes. The aim of this study is to describe the collaborative multidisciplinary approach between neurosurgeons, neurophysiologists, and physiotherapists in performing SDR. In addition, we delineate three illustrative cases in which SDR was performed.
Materials and Methods
Patients
We performed a retrospective review and analysis of the clinical records of patients who underwent SDR at the King Fahad Medical City (KFMC). Our inclusion and exclusion criteria are described below and summarized in Tables 1 and 2. All patients were examined and treated in the Department of Neurosciences, KFMC. All patients underwent the same preoperative workup, operation technique, and postoperative care, as described below. The study was approved by the Institutional Review Board and informed consent was obtained from the patients’ guardians involved in the study.
Table 1.
SDR patients’ inclusion criteria

Table 2.
SDR patients’ exclusion criteria
Patient demographics, including age, gender, diagnosis, clinical symptoms, neuroimaging, operation, sectioning rate, and intra- and post-operative complications were assessed. Patients’ outcomes were evaluated and compared to preoperative measurements based on clinical examination of power, tone (Ashworth scale), gait, and range of motion, as well as activities of daily living (ADL), gross motor function classification system (GMFCS), and gross motor function measure (GMFM) at 6, 12, and 24 months.
Selection criteria
Not every CP patient with spasticity is a candidate for SDR. Selection criteria are made in order to choose those that will benefit the most from the surgery. The criteria we follow include, but are not limited to, the items in Tables 1 and 2. In our center, all patients must have strong lower extremity extensors, and patients’ ability to stand should not be dependent on spasticity. This is verified by asking the candidate to stand from squatting position at least 10 times consecutively. Helping the candidate to stand against gravity by supporting the pelvic girdle during the test is permitted.
The procedure is not recommended if any of the criteria elements is not met or in the presence of any contraindication. All SDR patients should be motivated to follow-up and cooperate postoperatively with an intensive rehabilitation program in order to facilitate their regain of muscle function and to attain better outcomes from the surgery. We will only admit the candidate for surgery if an intensive inpatient rehabilitation bed is available for the patient for 6 weeks postoperatively, and if the patient's family is willing to cooperate with the length of stay for the inpatient and the following outpatient rehabilitative programs. The surgery is not indicated if the predominating manifestation of CP is dystonia, athetosis, ataxia, or if the trunk tone is not adequate to support walking. In general, the patient should have an adequate cognitive status, and a stable psychological status.
Preoperative workup
A detailed medical history is obtained from the patient and his/her parents/guardian with focus on the perinatal events (gestational age, multiple or single birth, birth weight, hemorrhagic and ischemic events, infections, seizures). The medical team should specifically inquire about any prior modality of treatment, e.g. intrathecal baclofen or orthopedic surgery. A neurological examination is also performed to confirm the increased muscle tone and hyperactive reflexes. In addition, magnetic resonance imaging (MRI) of the brain and spinal cord, as well as X-rays of the hip and the spine, should be done in order to detect any abnormalities for which the procedure might be contraindicated, and to establish the baseline for the patient, which will aid in anticipating the outcomes. Preoperatively, a comprehensive physiotherapy assessment is performed in order to establish the baseline and anticipate the outcomes for the patient. This includes assessment of muscle tone, muscle length, functional mobility, GMFM, and GMFCS in addition to gait analysis, bracing assessment, and videotaping of the patient's function in different activities. Consent for the photography and videotaping of the patient is first obtained by the physiotherapist. Depending on the patient's ability, the following activities should be videotaped: Long sitting, half kneel to stand, or getting up from the floor by the child's usual maneuver, squatting, walking forward and backward, running, and stair climbing. Finally, the inpatient treating physiotherapist should conduct a preoperative teaching session with the patient's parents/guardian to explain the process of care at the rehabilitation hospital, the importance of commitment to the postoperative regimen of intensive physiotherapy and continuation of care after discharge, rhizotomy precaution and positioning in bed for the first 6 weeks following surgery, and transferring and lifting techniques that should be followed.
The medical team should also explain the procedure to the patient and his/her family/guardian, answer their questions, and inform them about the need for intensive postoperative inpatient rehabilitation sessions, if needed. The team also discusses the possible short- and long-term complications, the goals, and the expected outcomes of the procedure. The anesthesia team explains the possible complications of the general anesthesia given during and after the surgery. Consent should be signed by the child patient's parents/guardian. The anesthesia team would also see the patient on the day preceding the surgery to assess fitness for surgery.
On the day of the operation, the patient is given midazolam orally. He/she is taken to the preparation room, where he/she is evaluated by the anesthesia team. The patient is then transferred to the operation room. The procedure involves neurosurgery, neurophysiology, physiotherapy, and anesthesia teams, and it takes around 4–5 hours to be performed. Communication between the teams is essential during the surgery in order to have the best outcome for the patient.
Anesthesia considerations
In the operating room, the patient will be put into general anesthesia using fentanyl. Endotracheal intubation is performed with the aid of an intermediate acting muscle relaxant like rocuronium. A Foley's catheter is also placed. The patient's general anesthesia is preserved with total intravenous anesthesia (TIVA), propofol, and fentanyl or remifentanyl. Proper communication with anesthesia is extremely important to achieve successful results. Avoidance of bolus doses and achieving a steady-state anesthesia are desired and communicated to the anesthesia team. Long-acting neuromuscular blocking agents should be avoided due to their ability to alter the electromyography (EMG) activity. Mild anesthesia is advantageous since it does not suppress muscular reflex responses. Ankle clonus is a reliable sign to monitor the anesthesia effect. The presence of this sign indicates good neurophysiological monitoring. Additionally, electroencephalograhy (EEG) is used to identify excessive brain suppression by TIVA medications. In our experience, a steady-state anesthesia with optimized doses of TIVA is what works best for the patients.
Intraoperative neurophysiology preparation and monitoring
Neurophysiological monitoring is a critical component in SDR procedures. We have adopted a combination technique derived from Peacock et al.[10,11] as well as Phillips and Park[12] techniques with minor changes, described below, to achieve greater precision and efficiency. Among the most important modalities are the EMG, triggered EMG (TEMG) with standard single pulse method and with 50-Hz train method, transcranial motor evoked potentials (TcMEPs), and somatosensory evoked potentials (SSEPs). Baseline signals are recorded before incision under optimum TIVA anesthetic regimen and communicated to the surgeon.
We utilize Cadwell Cascade Elite 32 Channel intraoperative neurophysiological monitoring device and place 1.5 m, 0.4 mm needle paired electrodes for EMG monitoring bilaterally on one of the upper limb muscles (most commonly brachioradialis), then the lower limbs muscles including, but not limited to, the iliopsoas, one of the adductors of the thigh (adductor longus, adductor brevis, adductor magnus), tibialis anterior, extensor hallucis longus, gastrocnemius, extensor hallucis brevis, hamstrings (HAMS) bilaterally, as well as the anal sphincter [Figure 1]. Most of the electrodes are placed while the patient is supine before switching him/her to the prone position. Caution is always observed, and muscles with evident clinical spasticity are always included in the protocol in addition to the muscles mentioned before. A tap-test is performed on each electrode to produce movement artifacts in order to verify that the electrodes are connected to the monitoring device and functioning properly, and impedance testing must show values under 5 kΩ to be acceptable [Figure 1].
Figure 1.
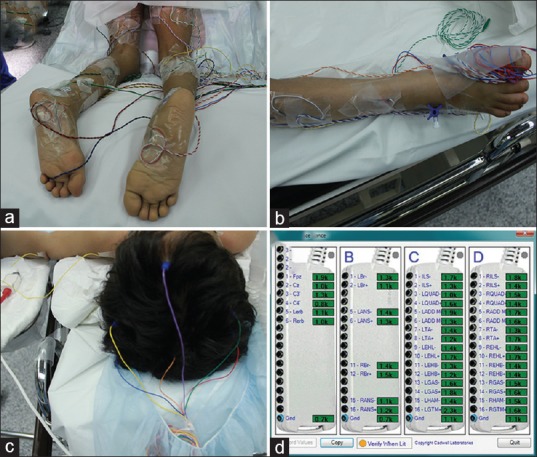
Sensory electrode placement for somatosensory evoked potential from posterior tibial nerve along with electromyography needle placement for various lower limb muscle roots (a and b). Scalp electrode placement for somatosensory evoked potential recording electrodes along with transcranial motor evoked potentials stimulating electrodes (c). Before collecting neurophysiology data, confirmation of good electrical setup with impedance check (all green) showing <5 kΩ impedance should be established (d)
TEMG contains two components (described below): (A) Identification of the threshold of each sensory root (single pulse technique). (B) 50-Hz train stimulation to the rootlets. Grading is performed thoroughly for sectioning only after completing both components of TEMGs.
In TcMEP, the signals are recorded from the muscles upon stimulation of the motor cortex via scalp electrodes placed on the head using the 10/20 EEG electrode placement system [Figures 1 and 2]. Our surgeons separate motor roots from sensory roots on the basis of anatomy, and no motor mapping is performed in SDR. The neurophysiology team stimulates the motor cortex to acquire motor evoked potentials after each sensory rootlet sectioning, and communicates the results to the neurosurgeon, and a comparison is made to the baseline TcMEP responses. Since the stimulation from the cortex is not specific to lower limb muscles only, a bite block should be used during motor stimulation in order to avoid any injury to the patient's tongue due to stimulation of the muscles of mastication. Care is observed to have a prior clinical history of the patient before conducting this test. Certain conditions like a prior history of epilepsy, prior electrodes or grid placement in the brain, or presence of a cardiac pacemaker are clearly documented, and a safety benefit ratio is ascertained and discussed prior to conducting this test. Normally, this test can be performed by experienced neurophysiologists without much concern in most patients.
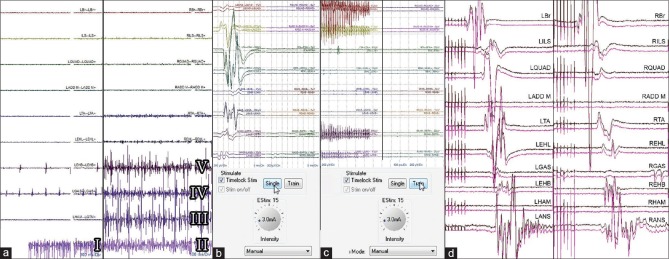
Figure 2.
Free running electromyographies showing root irritation at various cauda equina levels while the surgeon is working on the lower lumbar and sacral roots: anal sphincter irritation (I and II), right hamstring muscles irritation (III), right gastrocnemius muscle irritation (IV), and right extensor hallucis brevis irritation (V), the remaining muscles show no root irritation (a). Identifying root threshold with single pulse technique showing triggered electromyogram responses at 3 mA on the left side (b). Identifying and grading the rootlet with 50-Hz train technique showing sustained discharges on the left side at 3 mA (c). Transcranial motor evoked potentials showing the presence of compound muscle action potentials confirming the integrity of specific motor roots (d)
Finally, recording SSEP is the least important, but still usable, modality used to test the spinal cord function intraoperatively in SDR. We record the SSEPs from the somatosensory cortex through specific electrodes placed on the scalp upon electrical stimulation of peripheral nerves (most commonly the median nerve and the posterior tibial nerve) [Figure 1].
Intraoperative physiotherapist preparation and assessment
The patient is placed in Trendelenburg position in order to decrease the cerebrospinal fluid loss during the procedure. The patient's legs are covered with transparent drapes in order to allow the neurosurgeon and the physiotherapist to see the movements caused by electrical stimulation [Figure 3].
Figure 3.
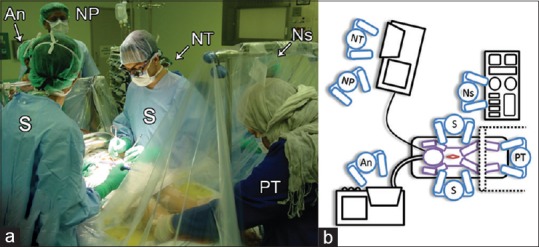
Operating team setup in photographic (a) and schematic (b) representation: Physiotherapist (PT) is positioned behind the screen and transparent drapes (dotted lines) where she can visualize the surgeons (S) stimulating the rootlets and the surgeons are able to see the lower extremities moving. The anesthetist (An) keeping the patient light enough for stimulation without moving or sensing the stimulations. The neurophysiologist (NP) supervising the neurophysiology technicians (NT) as they stimulate and record results. The team will concordantly interpret the results and make concurrent decisions on what nerve rootlets to rhizotomize. The nurse (Ns) is in the lower left side of the patient
A place is arranged to accommodate the physiotherapist to stand comfortably behind the transparent drapes in order to palpate and observe the patient's muscle contractions of the lower extremities during the rootlets electro-stimulation. This increases the reliability of testing and helps in judging the congruity of the results. It also aids in reaching the best decision on how many rootlets have to be cut [Figure 3].
Selective dorsal rhizotomy procedure
A dose of antibiotics is given to the patient before making the skin incision. Afterward, a skin incision is made from just above the second lumbar to just below the first sacral spinal level and narrow laminotomies (L2-S1) are done to reduce the risk of spinal instability postoperatively. The dura is then opened and secured. The arachnoid is also opened. Then, the cauda equina is exposed just at the lower end of the conus down to the second sacral root as it exits the canal [Figure 4]. Saline irrigation should be used with caution due to its ability to alter the EMG activity. The roots are identified grossly through their exit through the transverse foramina of the vertebral segments, as well as by using electrical stimulation. Sometimes, CP patients show alterations in the muscular innervation due to an existing pathology. For example, the HAMS muscles are normally innervated by L5-S2, but in some CP patients, EMG activity is seen in the HAMS muscles upon L3 stimulation. Classical anatomy with the standard known root innervations does not always exist in these patients.
Figure 4.
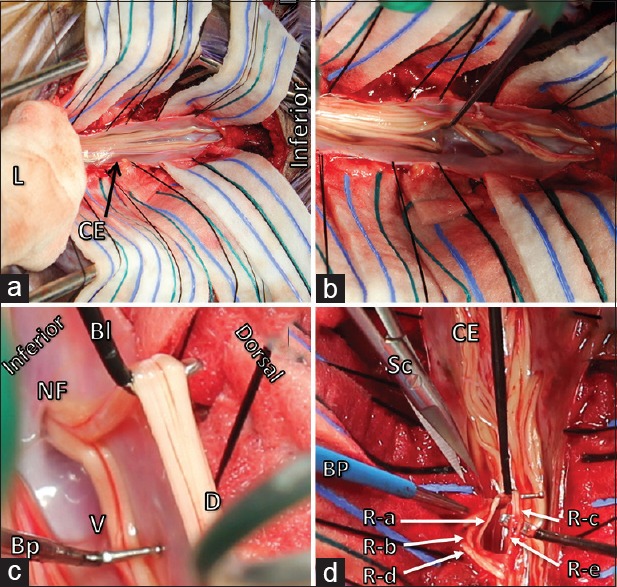
The laminae (L) of L2-S1 are opened, wrapped in gauze and retracted superiorly. Cauda equina (CE) is observed and arachnoid webs are released to identify and separate the ventral from the dorsal roots (a and b). The dorsal sensory root (DR) easily separated from the ventral motor root (VR) using Sabbagh–Bunyan dissecting electrodes (Bl: Blade electrode, Bp: Ball-probe electrode). The roots are uniting as they exit the spinal canal into the dural sleeve through the neural foramen (NF) (c). The dorsal root is divided into five rootlets (R-a, R-b, R-c, R-d and R-e). R-c and R-e (solid arrows) are selected for rhizotomy. Bipolar cautery is performed and micro-scissors (Sc) are used to rhizotomize the selected rootlets. This process is done one level and one side at a time (d)
Once the segmental spinal roots are identified using anatomy and clear visualization, each spinal root is separated into dorsal sensory and ventral motor roots based on their structure and position [Figure 4]. We do not stimulate the motor roots after separating them from the sensory roots in order to avoid exhausting the roots and anterior horns.
The threshold current, usually measured in milli-amperes, for each dorsal sensory root is identified by placing the root over two hooks of rhizotomy probes, Sabbagh–Bunyan Probes (525317 Inomed Medizintechnik GmbH), and stimulating the root by single pulse threshold identification technique. This is better achieved by bipolar stimulation. The threshold will be identified when a first repeatable and reliable response from the muscle(s) innervated by this root is noted. Ideally, motor roots produce a more robust response upon receiving less current when compared to sensory roots. Sensory roots should require higher threshold of electrical current to produce a compound muscle action potential (CMAP). Gradual increase of electrical current with single pulse technique is used. Each sensory root is stimulated, starting with 1 mA and gradually increasing until a reliable CMAP is observed [Figure 2]. Interestingly, we have noticed even < 1 mA threshold occasionally in extremely spastic roots. Each dorsal sensory root is graded upon the criteria described in Table 3, and we interpret the findings in combination with the results of sensory rootlet response grading after 50-Hz train stimulation of the rootlets, as described below. The thresholds are recorded in an internally designed template and the muscles producing the CMAPs upon stimulation for each root are noted. This is done to identify the severity of spasticity at each individual root level and helps in decision-making upon sectioning of rootlets in the steps described later. Each sensory root is then separated into five (sometimes 3–8) sensory rootlets using the Sabbagh–Bunyan (525317 Inomed Medizintechnik GmbH) dissecting electrodes, blade electrode, and ball-probe electrode [Figure 4]. Sometimes, the sectioning process causes irritation to the rootlets and produces false positive EMG activity that may interfere with the assessment of the rootlet and its grading. If this happens, irrigation of the area with body temperature saline will restore proper EMG recordings and enable the neurosurgeon to proceed safely. Afterward, the sensory rootlets are placed, one by one, over the two hooks of the rhizotomy probes, where a train of impulses at 50-Hz is applied, increasing the intensity of the stimulus gradually until the threshold current for each sensory rootlet is established through monitoring the reflex activity from the muscles observed by the physiotherapist as well as a CMAP on the EMG monitoring window [Figure 2]. A train of 50-Hz stimulation is given to each sensory rootlet in order to determine the grade of the response according to the criteria in Table 3. It is highly desirable, but not necessarily recommended, to repeat the train stimulation 2–3 times to confirm the grade of the response before sectioning. Identification of sensory root threshold in the previous step helps here, as most sensory rootlets produce a response in this step at a higher current intensity. For example, if left L4 root produced a response at 3 mA with single pulse stimulation on the left quadriceps femoris, then after sectioning left L4 root into rootlets, the rootlets would likely produce a response higher than 3 mA at 50-Hz train stimulation.
Table 3.
Modified Park and Phillips grading criteria

Putting the patient's clinical presentation into consideration, the rootlets generating a response activity of 0, 1+, or 2+ are left intact, while rootlets generating a response activity of 3+ or 4+ are sectioned. The desirable sectioning percentage at each segment level is 40–60% with the exception of L4 level, where only up to 50% rootlet sectioning is performed in order to avoid causing permanent sensory loss postoperatively and also to minimize complications specifically related to quadriceps femoris muscle function.
Careful observation is required to avoid cutting rootlets with increased sphincter activity, especially if it is coming from sacral rootlets. Lumbar rootlets that show Grade 3+ or 4+ along with sphincter activity are sectioned with the exception of rootlets at L5 level. In the first sacral segment level, the rootlets that show high-grade responses with sphincter coverage are spared, whereas rootlets that show minimal sphincter coverage at Grade 3+ or 4+ are cut.
The neurosurgeon performs the procedure of stimulating, dividing roots into rootlets, re-stimulating each rootlet and cutting the undesired rootlets in all levels on one side before moving to the other. We usually start from right L2-S1 levels then progress upwards all the way to right L2 then progress downwards on the other side to left L2-S1 levels, or vice versa, for the sake of comfort, reliability, and having a systematic surgical technique. Rootlets sectioning percentage should be calculated at each side. Accordingly, an overall sectioning percentage is calculated for each patient. Ankle clonus will be dramatically reduced after performing SDR on one side while it will still be preserved on the other side. Therefore, it is considered a good sign for determining the effectiveness of the procedure intraoperatively. The presence of ankle clonus after performing SDR on one side is an indication for neurophysiological re-assessment of the remaining rootlets for sectioning. Communication between the neurophysiology team, the physiotherapy team, and the neurosurgery team is essential to facilitate precise decisions for rootlet sectioning or sparing, which will lead to the best outcome for the patient.
In case of bleeding from the cut of the rootlet fascicles, bipolar cautery should be used to control the bleeding. 4-0 monofilament nylon is used to close the dura in a running pattern. The Trendelenburg position is reversed and the wound is closed in layers. Shortly before waking up, the patient is given a bolus dose of morphine intrathecally, as well as rectal paracetamol.
Postoperative care
After the operation, the patient should stay at least one night in the Intensive Care Unit. The patient should feel an instant reduction in spasticity and tone of the involved limbs after the surgery. The patient is given fentanyl, intravenous benzodiazepam, intrathecal bupivacaine, and morphine for pain management. If the patient is vitally stable, he/she will be transferred to the ward 1 day after the surgery. The patient will keep taking epidural morphine and bupivacaine, while fentanyl and benzodiazepam are discontinued. The patient should start gentle physiotherapy in the bed and begin wearing ankle-foot orthosis. On the 3rd day after the surgery, the patient should start to sit out of the bed, and the physiotherapy team should follow-up with the patient. The epidural catheter is removed on day three postoperatively. The Foley catheter is removed 24 hours after the removal of the epidural catheter in order to reduce the incidence of transient urinary retention that is usually associated with removing the Foley catheter before the epidural catheter is removed. One week after the surgery, if the patient has no complications, he/she is transferred to the rehabilitation ward to begin a 6-week course of intensive indoor physiotherapy and OT.
Results
Case I
A 9-year-old female diagnosed with CP was refractory to medical treatment and was subsequently selected as a candidate for SDR using the criteria in Tables 1 and 2. Her preoperative assessment showed moderate spasticity (Ashworth II) with a power of 3 bilaterally. The range of motion was 50° at the right popliteal angle, and 70° on the left side. She was walking independently indoors with a low crouch gait with tip-toeing, feet inversion, and left femoral anteversion with fair balance and frequent falls; for outdoors, she was using a walker. She was found to have a moderate dependency on her ADL evaluation. She was GMFCS Level II, with a GMFM score of 85%. She was operated with the surgical procedure described. The percentages of rootlet sectioning were 43% and 48% on the right and left side, respectively, accounting for 46% of rootlets in total. No intra- or post-operative complications were encountered. Postoperatively, she improved and had a normal tone (Ashworth 0) with a power of 3+ bilaterally. The range of motion at the right popliteal angle was normal and was 35° on the left side. She was ambulating with a more upright gait, with a mild degree of crouching and bilateral intoeing, mainly due to femoral anteversion. She had no more restrictions in her ADL and was completely independent, at GMFCS Level I. Physiotherapy follow-up was up to 2 years, showing gradual improvements in her GMFM score, which was found to be 88% at 6 months, 93% at 12 months, and 95% at 24 months follow-up. The patient was doing well on her last follow-up visit 29 months postoperatively.
Case II
An 8-year-old male known to have CP refractory to medical treatment was selected for SDR. His preoperative assessment showed mild spasticity (Ashworth I) with a power of 3+ bilaterally. The range of motion was 55° at the right popliteal angle, and 30° on the left side. He was independent in walking but had a narrow base of support, mild crouching gait with left side intoeing, and no heel strike, with lumbar lordosis. He was independent in his ADL. He was GMFCS Level I, with a GMFM score of 96%. The percentages of rootlet sectioning were 45% and 42% on the right and left side, respectively, accounting for 44% of rootlets in total. No perioperative complications were encountered. Postoperatively, he improved and had a normal tone (Ashworth 0) with a power of 4 and 4+ on the right and left side, respectively. The range of motion at the right popliteal angle was normal bilaterally. His gait improved, showing straight knees with a wider base of support and some degree of ankle dorsiflexion for the heel strike, no left side intoeing and reduced compensatory lumbar lordosis. His ADL and GMFCS level were preserved. Physiotherapy follow-up showed improvement in his GMFM score, which was found to be 98% at 6 months and 12 months follow-up. The patient was lost to follow-up for his 24 months GMFM assessment. On his last follow-up, 23 months postoperatively, he was doing well, with no complications.
Case III
A 6-year-old male diagnosed with CP which was refractory to medical treatment was selected to undergo SDR. His preoperative assessment showed moderate spasticity (Ashworth II) with a power of 3 and 3+ on the right and left side, respectively. The range of motion was 50° at the right and left popliteal angles. He was walking independently but exhibited a mild crouch gait pattern with bilateral intoeing, partial foot contact, and genu valgum. He was found to have a moderate dependency in his ADL. He was GMFCS Level I, with a GMFM score of 93%. The percentages of rootlet sectioning were 55% and 47% on the right and left side, respectively, accounting for 52% of rootlets in total. No perioperative complications were encountered. Postoperatively, he improved and had a normal tone (Ashworth 0) with a power of 4 bilaterally. The range of motion at the right popliteal angle was 20°, and was 30° on the left side. His gait was more upright with full foot contact but still no heel strike. He became fully independent in his ADL, and his GMFCS level was preserved. Physiotherapy follow-up showed overall improvement in his GMFM score, which was found to be 95% at 6 months. At 12 months follow-up, the patient was found to have bilateral HAMS shortening, which greatly affected his gross motor ability and gait pattern. He was crouching and scored 94% in GMFM. This reduction was attributed to the poor compliance of the patient and his family with the home exercise program. The patient underwent bilateral distal HAMS lengthening. He showed significant improvement in his gait pattern and GMFM increased to 99% at 24 months follow-up. The patient was doing well on his last follow-up visit 35 months postoperatively with no complications.
Discussion
Selective dorsal rhizotomy background
SDR is a neurosurgical procedure developed in order to reduce the spasticity and improve the mobility in spastic CP patients. The procedure has evolved throughout history. In 1898, after performing segmental dorsal rhizotomies in decerebrate cats, Sherrington noted that limb rigidity is a consequence of hyperactive reflexes resulting from the decrease in inhibitory signals coming from damaged motor nerve fibers.[13] Abbe and Bennett were the first surgeons to perform dorsal rhizotomies in human patients in 1889.[14,15]
During the years from 1908 to 1912, Dr. Harvey Cushing performed dorsal rhizotomies for a few patients to relieve their spasticity symptoms.[16] In 1913, Foerster used Sherrington's study and other neurosurgeons’ studies as basis for improving the procedure. He sectioned all sensory nerve roots to the lower limbs, which led to improvement in spasticity but also caused severe muscular weakness with complete loss of skin sensation and proprioception.[17]
In 1967, Gros et al., were the first to perform partial nonselective rhizotomies at each segmental level. This led to a decrease in both the muscular weakness and loss of proprioception and skin sensation that were associated with the procedure.[18]
In 1979, Fasano et al., revolutionized the procedure by introducing intraoperative neurophysiological recording, which is still used nowadays.[19,20] They used an electrical stimulator and recorded the EMG responses of the lower limb muscles innervated by the stimulated nerve root. The stimulator is composed of two electrodes (a cathode and an anode) located 1 cm apart from each other. After separating the sensory nerve roots into rootlets and recording the electrical threshold (measured in milli-amperes) for each sensory root, they stimulated the rootlets and sectioned the ones that caused constant muscular activity contributing to the patient's spasticity. This technique enabled them to selectively cut the sensory nerve rootlets involved in the abnormally increased muscular activity, and it was associated with favorable results.[19,20]
During the 1980s, Peacock and Arens refined criteria that helped the surgeons identify which rootlets are to be sectioned, and which ones are to be spared.[10,11] They changed the surgical approach to the lumbosacral roots from the extramedullary approach used by Fasano to the intraforaminal approach. They continued using Fasano's technique of intraoperative neurophysiological monitoring and popularized it in the United States as they published many papers describing the procedure.[10,11]
Farmer et al., at the Montreal Children's Hospital and the Shriners Hospital for Children had most rigorously evaluated the benefit and the short-term and long-term outcomes of this procedure and documented significant positive findings from several functional perspectives.[4,9,21,22,23,24]
Patients selection and surgical approaches
Optimal selection of patients is essential to maximize the benefits and reduce the risk of complications. There are many criteria that have been established by different institutions to select the proper candidates for SDR.[11,25,26] Our selection criteria is optimized to ensure the greatest benefit to the patients. It is generally known that patients who are independent ambulators preoperatively will have better results than those who need assistive devices.[4] In addition, a normal brain MRI may predict better gross motor skills improvement after surgery compared to patients who have periventricular leukomalacia. Patients with hydrocephalus may have a less favorable prognosis after the procedure.[27]
Various surgical techniques have been utilized by neurosurgeons over time. Peacock and Arens applied SDR through complete laminectomy or laminotomy from the second lumbar to the fifth lumbar or first sacral level, and sometimes the second sacral level (L2-L5 or L2-S1/2), which enabled them to have a complete visualization of the cauda equina.[10] This approach was used by others as well.[28]
The group at the Montreal Children's Hospital performed a L1-S2 narrow laminotomy for their patients, which enabled them to visualize the whole cauda equina.[28] The laminotomy is closed at the end of the procedure as an attempt to reconstruct the posterior elements, knowing that there is a risk of developing scoliosis in the long-term follow-up postoperatively.[29]
Fasano et al., and Park et al., used the conus exposure modality, which involves complete laminectomy at the level of T12-L1, enabling them to visualize the conus medullaris.[20,30] This approach requires a smaller surgical incision, and therefore decreases the risks of postsurgical infections, spinal instability, postsurgical pain, time of operation, and probably the surgical trauma to the rootlets.[31] However, this approach does not facilitate the complete visualization of the cauda equina, and therefore does not aid in the selective identification of the nerve roots, which might be substituted for by utilizing electrophysiology.
At our institution, the neurosurgeons follow the lumbosacral laminotomy approach. Narrow laminotomies from L2-S1 are made for better visualization of the cauda equina and the nerve roots exiting the transverse foramina. The laminotomy is closed at the end of the operation.
Neurophysiology monitoring
Intraoperative neurophysiology monitoring is imperative in SDR procedures. Multiple modalities can be employed as we described above. We perform extensive muscle EMG monitoring in order to ensure maximum root coverage, and our EMG recording technique helps us identify the severity of mechanical irritation during exposure and sectioning, besides identifying variations in myotomes. Our surgeons separate motor roots from sensory roots on the basis of anatomy, and no motor mapping is performed in SDRs. Motor roots are usually round, small, and ventral while sensory roots are dorsal, broad, and larger than motor roots. After each sensory rootlet sectioning, the neurophysiology team acquires the TcMEPs, and a comparison is made to the baseline TcMEP responses. This will reassure surgeons about the maintained integrity of pathways after each sectioning, and provide information to the neurosurgeon about any muscular motor loss, which can happen, sometimes, during the roots separation process where some motor fibers are carried along with the sensory ones. Hence, the use of TcMEP monitoring helps the surgeons understand the overall neurophysiological picture and the integrity of the pathways and roots during the procedure. The information gathered through this modality is helpful and more desirable than its absolute avoidance.
We also record SSEPs as an additional monitoring modality in SDR. Having reliable SSEP recordings in spastic diplegia patients is uncommon, but SSEPs can be reliably obtained in some patients and in those cases, it can be of great benefit, especially when combined with the other monitoring modalities. Performing a comparison between the upper limb median nerve SSEPs versus lower limb posterior tibial nerve SSEPs can help the surgeon in identifying and documenting a difference between the upper and lower limb sensory systems. Ideally, the SSEPs show healthy, sharp morphology waves as the signals ascend from the peripheral nerves to the brain, but in CP patients this is not always the case. Low amplitude and poor morphology on SSEP are usually due to the patient's pathology. This can also can be due to the presence of electrical noise caused by the EEG, scalp muscle EMG, or other electrical devices present in the operating room. Most CP patients show poor morphology in this test, especially from the lower limb nerves. Overall, considering the potential benefits of SSEP monitoring in a subset of patients, the ease of attaching SSEP electrodes to the patient, and that it does not hinder the surgeons during the surgery or require any change in anesthesia than already used for the other modalities, this test is performed in our patients but is not always helpful.
The key to successful utilization of intraoperative monitoring to select rootlets for sectioning is to recognize that individual patients differ greatly in the extent to which they demonstrate the different EMG patterns to train stimulation described above. In our patients, sensory root stimulation/mapping with single pulse technique ranged between intensities of 0.20 mA from very spastic muscles/roots to up to 7 mA in relatively less spastic roots. Similarly, 50-Hz train stimulation for rootlets stimulation intensities ranged between 0.5 and 15 mA. Careful dissection was especially followed in Grades 3 and 4. The TcMEP stimulation in our patients resulted in CMAPs at voltages as low as 80–300 V.
Since the original report of Fasano et al.,[20] there have been many attempts to clarify the criteria used to determine which roots or rootlets should be sectioned. Fasano proposed a straightforward criterion; there was either a single response to a 50-Hz train stimulation (normal), or extended response (abnormal). Peacock et al.[10,11] found that this was overly simplified and potentially led to sectioning of more rootlets than necessary to achieve good clinical outcomes. Thus, they identified at least eight different response patterns and gave each pattern a descriptive name, which has proven useful in communication between the surgeons and neurophysiologists. The patterns were classified as decremental, squared, decremental squared, incremental, multiphasic, clonic, irregular, and sustained. The first of these roughly corresponds to Fasano's “normal” response, although rather than requiring only a single response to 50-Hz train stimulation, a decremental response only requires that the response amplitude continues to decline throughout the duration of the stimulus train. The squared and decremental squared categories appear to represent what Fasano et al. would have termed “lack of inhibition,” which they considered abnormal. However, these are considered within the normal range of variation in Peacock's scheme, and would thus be spared from sectioning. The last five categories, which involve either increasing amplitude of the response during the stimulus train, more complex patterns of alternating excitation and inhibition, or discharges after the end of the stimulus, are all termed abnormal by Peacock et al., as are any responses that spread to multiple segmental levels or involve bilateral responses, and would be candidates for sectioning.
As discussed above, we utilize a combination technique where we grade sensory roots response after single pulse stimulation in addition to grading sensory rootlet response after 50-Hz train stimulation, according to Philips and Park's criteria. This criterion does not distinguish among different categories of sustained response, as does Peacock's, but relies primarily on the anatomical distribution of the responses. Generally, nearly all rootlets producing three or four responses are sectioned, although at least one rootlet may be spared at each level.[28] Mittal et al.[32] have studied the reliability of responses to repeated train stimulation, using the grading scheme of Phillips and Park. They found that 93% of dorsal rootlets had either no change or a change of only one grade between repeated stimulation runs. They proposed their own algorithm for determining which rootlets to section, in which whole roots are first tested, and if the response is Grade 0, 1, or 2, the root is not subdivided and they move on to the next level. If the entire root produces a Grade 3 or 4 response, then it is subdivided and each rootlet tested individually. Only the rootlets that consistently produce a Grade 4 response are sectioned. Overall, this produced a lesion rate of 51%. Harper and Nelson[28] also noted that they may section rootlets showing only one or two responses “if they innervate muscles contributing to the patient's spasticity, and all rootlets at that level have a similar grade.” They also note that “the contraction strength perceived by the electrophysiologist may also be used to distinguish rootlets of the same grade. Hence, after reviewing all of this data, a combination technique with grading both sensory roots and rootlets has been adapted.[33] The use of intraoperative physiotherapeutic assessment of motor responses and response grade assignment helps in improving the team's decision-making in terms of what rootlets to lesion.[32]
Benefits and outcomes
Almost all the patients with spastic diplegia and some patients with spastic quadriplegia have benefited from the procedure.[4,9,34,35,36,37,38] Reduction of spasticity is what patients and their families expect as a means to an end, and many studies have documented that, but that does not guarantee functional improvement.[23,34,35] After the surgery, patients have increased strength and range of motion in their affected limbs, which helps them in their daily activities.[34,38,39,40,41] Patients who can ambulate without assistance had more benefit from SDR compared to patients who could ambulate with assistance or could not ambulate before the procedure.[4] The procedure also avoids the progression of hip subluxation or dislocation, which is commonly seen in spastic CP patients.[42] In addition, SDR seems to improve cognitive power, evident by marked improvement in language, attention, temperament, mood, and interaction with people in the affected children who seemed to be mentally challenged before the procedure.[43]
There are validated methods that are useful in attempting to quantify changes in gross motor function in children with CP. GMFM is one of the most widely used scales.[44,45] McLaughlin et al. performed a meta-analysis looking at controlled trials comparing GMFM assessments of the patients who had rhizotomy in addition to physiotherapy with those who had physiotherapy alone, which showed significant improvement in the former group.[46] Randomized controlled trials to date do not exceed 2 years of follow-up, and this may be a reason why no statistical difference was seen. Mittal et al. documented a significant persistent improvement in 1-, 3-, and 5-year follow-up GMFM scores, especially in dimensions reflecting lower extremity motor function.[21] These findings are also consistent with our experience with our patients who underwent SDR in addition to physiotherapy. Our patients demonstrated continuous benefit from the procedure over 24 months of follow-up.
The long-term durability of the procedure was evaluated in a study by the group in Montreal, and the durable benefit was shown in patients as far as 15 years postoperatively.[9] Therefore, SDR is considered to be a great choice for the management of spastic CP patients.
Complications
SDR is generally safe, and permanent complications after the surgery are rare. However, some complications might occur during or after the surgery, and the patient and his/her parents/guardian should be informed about these complications before the surgery. These complications include, but not are limited to, transient dysesthesias, numbness, tingling, transient urinary retention (frequent), back pain, headache, infections, pneumonia, bronchospasm, ileus, hemorrhage, cerebrospinal fluid leak from the surgical side, meningitis, fluid collection or seroma beneath the skin, incomplete reduction of the spasticity, muscle weakness, spinal deformities (scoliosis, kyphosis, hyperlordisis, spondylolisthesis), permanent hypoesthesia (rare), permanent urinary incontinence (rare), impotence, bladder and bowel dysfunction, spinal cord stenosis, risks associated with general and local anesthesia (rare), and the risk of death (extremely rare).[10,23,29,47,48,49] We have not encountered any intraoperative or postoperative complications in our patients, and they were all doing well at their subsequent follow-up assessments.
Conclusion
SDR is a safe and effective procedure for patients with medically refractory spastic diplegia. A multidisciplinary approach collaborating neurosurgery, neurophysiology, physiotherapy, and anesthesiology is needed to ensure good short-term and long-term outcomes in select patients suffering from spastic diplegia. Using our collaborative approach resulted in functional and objective improvement in our patients, which was maintained over a long follow-up time with no complications. Knowledge about the procedure, its indications, patients’ selection, intraoperative monitoring, and outcomes may aid in helping a substantial number of patients that might benefit from such a procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Ayman A. Al Banyan for developing the KFMC SDR-Inclusion criteria, creating the multidisciplinary team, and helping in performing the surgeries. We would like to acknowledge Dr. Reem Bunyan for co-creating the Sabbagh-Bunyan Rhizotomy Electrode Probes (525317 Inomed Medizintechnik GmbH) that were used in all the surgeries. We would like to acknowledge the Rehabilitation hospital at King Fahad Medical City led by Dr. Ahmed Abu Abad for their guidance and for making it possible for patients to be in long-term inpatient rehabilitation.
References
- 1.Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol. 2002;44:633–40. [PubMed] [Google Scholar]
- 2.Cahill-Rowley K, Rose J. Etiology of impaired selective motor control: Emerging evidence and its implications for research and treatment in cerebral palsy. Dev Med Child Neurol. 2014;56:522–8. doi: 10.1111/dmcn.12355. [DOI] [PubMed] [Google Scholar]
- 3.Krigger KW. Cerebral palsy: An overview. Am Fam Physician. 2006;73:91–100. [PubMed] [Google Scholar]
- 4.Farmer JP, Sabbagh AJ. Selective dorsal rhizotomies in the treatment of spasticity related to cerebral palsy. Childs Nerv Syst. 2007;23:991–1002. doi: 10.1007/s00381-007-0398-2. [DOI] [PubMed] [Google Scholar]
- 5.du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15:151–7. doi: 10.1097/00019052-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971;9:751–67. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- 7.Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev Med Child Neurol. 2013;55:509–19. doi: 10.1111/dmcn.12080. [DOI] [PubMed] [Google Scholar]
- 8.Rumeau-Rouquette C, Grandjean H, Cans C, du Mazaubrun C, Verrier A. Prevalence and time trends of disabilities in school-age children. Int J Epidemiol. 1997;26:137–45. doi: 10.1093/ije/26.1.137. [DOI] [PubMed] [Google Scholar]
- 9.Dudley RW, Parolin M, Gagnon B, Saluja R, Yap R, Montpetit K, et al. Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr. 2013;12:142–50. doi: 10.3171/2013.4.PEDS12539. [DOI] [PubMed] [Google Scholar]
- 10.Peacock WJ, Arens LJ. Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J. 1982;62:119–24. [PubMed] [Google Scholar]
- 11.Peacock WJ, Arens LJ, Berman B. Cerebral palsy spasticity. Selective posterior rhizotomy. Pediatr Neurosci. 1987;13:61–6. doi: 10.1159/000120302. [DOI] [PubMed] [Google Scholar]
- 12.Phillips LH, Park TS. Electrophysiological studies of selective posterior rhizotomy patients. In: Park TS, Phillips LH, Peacock WJ, editors. Management of Spasticity in Cerebral Palsy and Spinal Cord Injury. Neurosurgery State of the Art Reviews. Vol. 4. Philadelphia: Hanley and Belfus; 1989. pp. 459–69. [Google Scholar]
- 13.Sherrington CS. Decerebrate rigidity, and reflex coordination of movements. J Physiol. 1898;22:319–32. doi: 10.1113/jphysiol.1898.sp000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbe R. A contribution to surgery of the spine. Med Rec N Y. 1889;35:149–52. [Google Scholar]
- 15.Bennett WH. A case in which acute spasmodic pain in the left lower extremity was completely relieved by sub-dural division of the posterior roots of certain spinal nerves, all other treatment having proved useless. Death from sudden collapse and cerebral hemorrhage on the twelfth day after the operation, at the commencement of apparent convalescence. Med Chir Trans. 1889;72:329–348. doi: 10.1177/095952878907200119. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dasenbrock HH, Pendleton C, McGirt MJ, Sciubba DM, Gokaslan ZL, Quiñones-Hinojosa A, et al. Fulfilling the chief of his duties as a physician: Harvey cushing, selective dorsal rhizotomy and elective spine surgery for quality of life. J Neurosurg Spine. 2011;14:421–7. doi: 10.3171/2010.10.SPINE10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foerster O. On the indications and results of the excision of posterior spinal nerve roots in men. Surg Gynecol Obstet. 1913;16:463–74. [Google Scholar]
- 18.Gros C, Ouaknine G, Vlahovitch B, Frèrebeau P. Selective posterior radicotomy in the neurosurgical treatment of pyramidal hypertension. Neurochirurgie. 1967;13:505–18. [PubMed] [Google Scholar]
- 19.Fasano VA, Barolat-Romana G, Ivaldi A, Sguazzi A. Functional posterior radiculotomy, in the treatment of cerebral spasticity. Peroperative electric stimulation of posterior roots and its use in the choice of the roots to be sectioned. Neurochirurgie. 1976;22:23–34. [PubMed] [Google Scholar]
- 20.Fasano VA, Barolat-Romana G, Zeme S, Squazzi A. Electrophysiological assessment of spinal circuits in spasticity by direct dorsal root stimulation. Neurosurgery. 1979;4:146–51. doi: 10.1227/00006123-197902000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mittal S, Farmer JP, Al-Atassi B, Gibis J, Kennedy E, Galli C, et al. Long-term functional outcome after selective posterior rhizotomy. J Neurosurg. 2002;97:315–25. doi: 10.3171/jns.2002.97.2.0315. [DOI] [PubMed] [Google Scholar]
- 22.Mittal S, Farmer JP, Al-Atassi B, Montpetit K, Gervais N, Poulin C, et al. Functional performance following selective posterior rhizotomy: Long-term results determined using a validated evaluative measure. J Neurosurg. 2002;97:510–8. doi: 10.3171/jns.2002.97.3.0510. [DOI] [PubMed] [Google Scholar]
- 23.Abbott R, Johann-Murphy M, Shiminski-Maher T, Quartermain D, Forem SL, Gold JT, et al. Selective dorsal rhizotomy: Outcome and complications in treating spastic cerebral palsy. Neurosurgery. 1993;33:851–7. doi: 10.1227/00006123-199311000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Houle AM, Vernet O, Jednak R, Pippi Salle JL, Farmer JP. Bladder function before and after selective dorsal rhizotomy in children with cerebral palsy. J Urol. 1998;160(3 Pt 2):1088–91. doi: 10.1097/00005392-199809020-00032. [DOI] [PubMed] [Google Scholar]
- 25.Albright AL, Turner M, Pattisapu JV. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104(4 Suppl):233–9. doi: 10.3171/ped.2006.104.4.233. [DOI] [PubMed] [Google Scholar]
- 26.Grunt S, Fieggen AG, Vermeulen RJ, Becher JG, Langerak NG. Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: A systematic review of the literature. Dev Med Child Neurol. 2014;56:302–12. doi: 10.1111/dmcn.12277. [DOI] [PubMed] [Google Scholar]
- 27.Grunt S, Becher JG, van Schie P, van Ouwerkerk WJ, Ahmadi M, Vermeulen RJ. Preoperative MRI findings and functional outcome after selective dorsal rhizotomy in children with bilateral spasticity. Childs Nerv Syst. 2010;26:191–8. doi: 10.1007/s00381-009-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper CM, Nelson KR. Intraoperative electrophysiological monitoring in children. J Clin Neurophysiol. 1992;9:342–56. doi: 10.1097/00004691-199207010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Golan JD, Hall JA, O’Gorman G, Poulin C, Benaroch TE, Cantin MA, et al. Spinal deformities following selective dorsal rhizotomy. J Neurosurg. 2007;106(6 Suppl):441–9. doi: 10.3171/ped.2007.106.6.441. [DOI] [PubMed] [Google Scholar]
- 30.Park TS, Gaffney PE, Kaufman BA, Molleston MC. Selective lumbosacral dorsal rhizotomy immediately caudal to the conus medullaris for cerebral palsy spasticity. Neurosurgery. 1993;33:929–33. doi: 10.1227/00006123-199311000-00026. [DOI] [PubMed] [Google Scholar]
- 31.Peter JC, Hoffman EB, Arens LJ, Peacock WJ. Incidence of spinal deformity in children after multiple level laminectomy for selective posterior rhizotomy. Childs Nerv Syst. 1990;6:30–2. doi: 10.1007/BF00262263. [DOI] [PubMed] [Google Scholar]
- 32.Mittal S, Farmer JP, Poulin C, Silver K. Reliability of intraoperative electrophysiological monitoring in selective posterior rhizotomy. J Neurosurg. 2001;95:67–75. doi: 10.3171/jns.2001.95.1.0067. [DOI] [PubMed] [Google Scholar]
- 33.Yingling C. Intraoperative Monitoring of Neural Function. 1st ed. Vol. 8. Amsterdam: Elsevier (B.V); 2008. Selective dorsal rhizotomy; pp. 439–54. [Google Scholar]
- 34.Steinbok P, Reiner AM, Beauchamp R, Armstrong RW, Cochrane DD, Kestle J. A randomized clinical trial to compare selective posterior rhizotomy plus physiotherapy with physiotherapy alone in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 1997;39:178–84. doi: 10.1111/j.1469-8749.1997.tb07407.x. [DOI] [PubMed] [Google Scholar]
- 35.Wright FV, Sheil EM, Drake JM, Wedge JH, Naumann S. Evaluation of selective dorsal rhizotomy for the reduction of spasticity in cerebral palsy: A randomized controlled tria. Dev Med Child Neurol. 1998;40:239–47. doi: 10.1111/j.1469-8749.1998.tb15456.x. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin JF, Bjornson KF, Astley SJ, Graubert C, Hays RM, Roberts TS, et al. Selective dorsal rhizotomy: Efficacy and safety in an investigator-masked randomized clinical trial. Dev Med Child Neurol. 1998;40:220–32. doi: 10.1111/j.1469-8749.1998.tb15454.x. [DOI] [PubMed] [Google Scholar]
- 37.Engsberg JR, Olree KS, Ross SA, Park TS. Spasticity and strength changes as a function of selective dorsal rhizotomy. J Neurosurg. 1998;88:1020–6. doi: 10.3171/jns.1998.88.6.1020. [DOI] [PubMed] [Google Scholar]
- 38.Peacock WJ, Staudt LA. Functional outcomes following selective posterior rhizotomy in children with cerebral palsy. J Neurosurg. 1991;74:380–5. doi: 10.3171/jns.1991.74.3.0380. [DOI] [PubMed] [Google Scholar]
- 39.Berman B, Vaughan CL, Peacock WJ. The effect of rhizotomy on movement in patients with cerebral palsy. Am J Occup Ther. 1990;44:511–6. doi: 10.5014/ajot.44.6.511. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery PC. A clinical report of long term outcomes following selective posterior rhizotomy: Implications for selection, follow-up, and research. Phys Occup Ther Pediatr. 1992;12:69–87. [Google Scholar]
- 41.Steinbok P, Gustavsson B, Kestle JR, Reiner A, Cochrane DD. Relationship of intraoperative electrophysiological criteria to outcome after selective functional posterior rhizotomy. J Neurosurg. 1995;83:18–26. doi: 10.3171/jns.1995.83.1.0018. [DOI] [PubMed] [Google Scholar]
- 42.Greene WB, Dietz FR, Goldberg MJ, Gross RH, Miller F, Sussman MD. Rapid progression of hip subluxation in cerebral palsy after selective posterior rhizotomy. J Pediatr Orthop. 1991;11:494–7. doi: 10.1097/01241398-199107000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Craft S, Park TS, White DA, Schatz J, Noetzel M, Arnold S. Changes in cognitive performance in children with spastic diplegic cerebral palsy following selective dorsal rhizotomy. Pediatr Neurosurg. 1995;23:68–74. doi: 10.1159/000120939. [DOI] [PubMed] [Google Scholar]
- 44.Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: A means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31:341–52. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 45.Russell DJ, Rosenbaum PL, Avery LM, Lane M. Gross Motor Function Measure (GMFM-66 and GMFM-88) User's Manual. London: Mac Keith Press; 2002. [Google Scholar]
- 46.McLaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, et al. Selective dorsal rhizotomy: Meta-analysis of three randomized controlled trials. Dev Med Child Neurol. 2002;44:17–25. doi: 10.1017/s0012162201001608. [DOI] [PubMed] [Google Scholar]
- 47.Steinbok P, Schrag C. Complications after selective posterior rhizotomy for spasticity in children with cerebral palsy. Pediatr Neurosurg. 1998;28:300–13. doi: 10.1159/000028668. [DOI] [PubMed] [Google Scholar]
- 48.Abbott R. Complications with selective posterior rhizotomy. Pediatr Neurosurg. 1992;18:43–7. doi: 10.1159/000120640. [DOI] [PubMed] [Google Scholar]
- 49.Steinbok P. Selective dorsal rhizotomy for spastic cerebral palsy: A review. Childs Nerv Syst. 2007;23:981–90. doi: 10.1007/s00381-007-0379-5. [DOI] [PubMed] [Google Scholar]